Effect of Confining Pressure on CO2-Brine Relative Permeability Characteristics of Sandstone in Ordos Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sample and Conditions
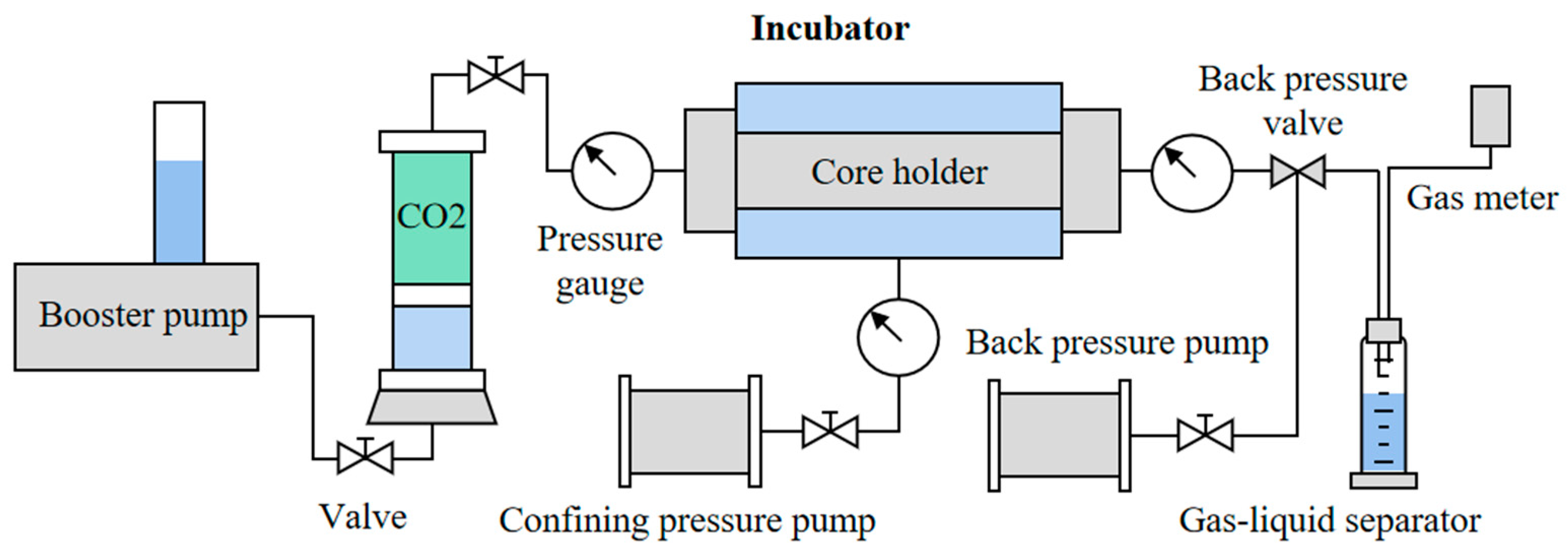
2.2. Experimental Equipment and Process
2.2.1. Experimental Setup
2.2.2. Experimental Process
- (a)
- Dry the core at 60 °C for 24 h and weigh it. Pump to vacuum, then saturate the core with brine. Weigh the rock samples saturated with brine to calculate the effective pore volume of the core.
- (b)
- Before experimenting, CO2 needs to be pressurized. Open the outlet valve of the CO2 cylinder to allow CO2 to enter the piston container. Close the inlet valve of the container and utilize a booster pump to pressurize the CO2 to the required pressure for the displacement experiment.
- (c)
- Load the rock sample saturated with brine into the core holder. Utilize the CP and back pressure device to apply the necessary back pressure and CP to both ends and to the middle section of the core holder. After completing the pressurization, activate the heating device and maintain the instrument at the experiment’s required temperature conditions for 2 h before commencing the experiment.
- (d)
- Activate the booster pump and apply a specific injection pressure to enable the formation brine to pass through the rock sample. Once the pressure difference and flow rate at both ends of the inlet and outlet of the rock sample stabilize, record the flow value.
- (e)
- Utilize the CO2 displacement brine method and record the time, cumulative brine production, CO2 production, and pressure conditions at both ends of the rock sample until no brine is produced (visual observation). Reach the irreducible brine state, establish the irreducible brine saturation of the core sample, and measure the effective permeability of the CO2 in this irreducible brine state.
2.3. Experimental Data Processing Methods
3. Results and Discussion
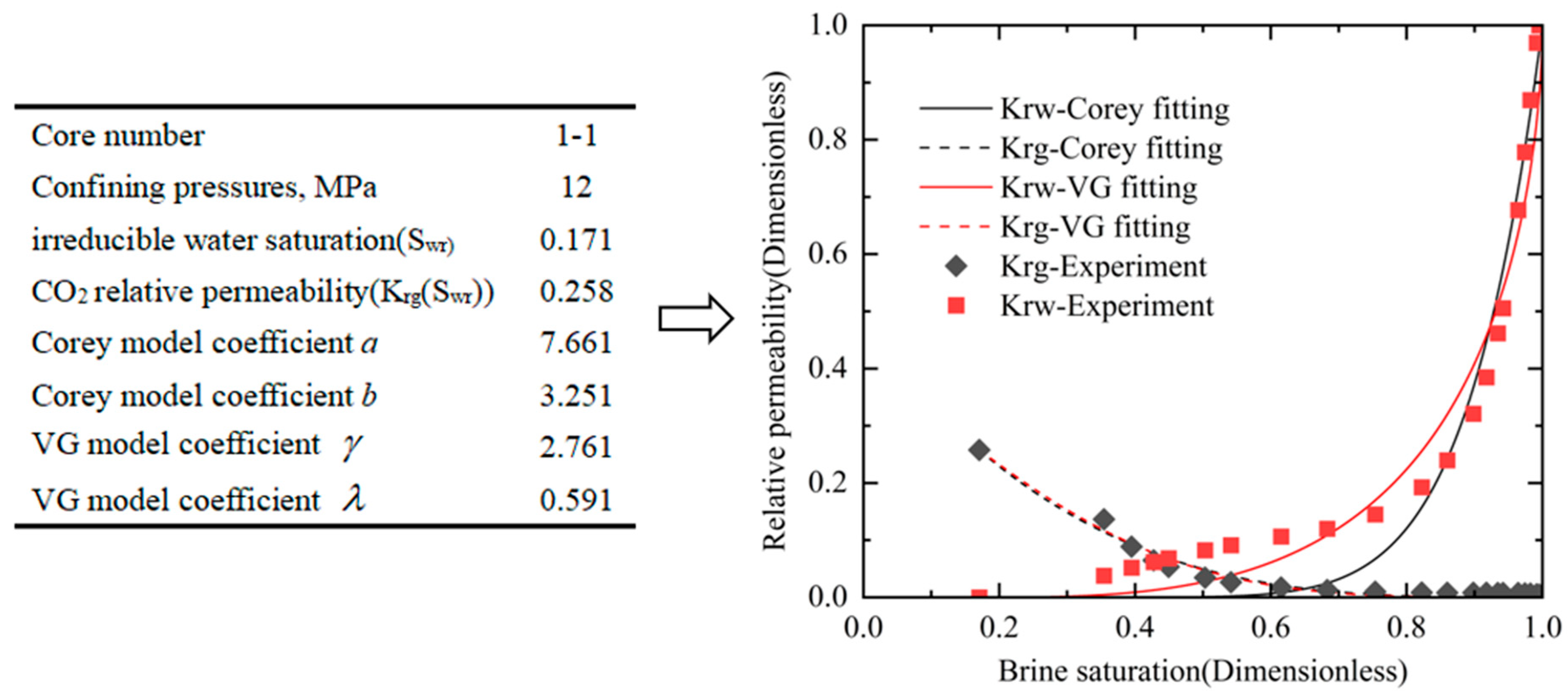
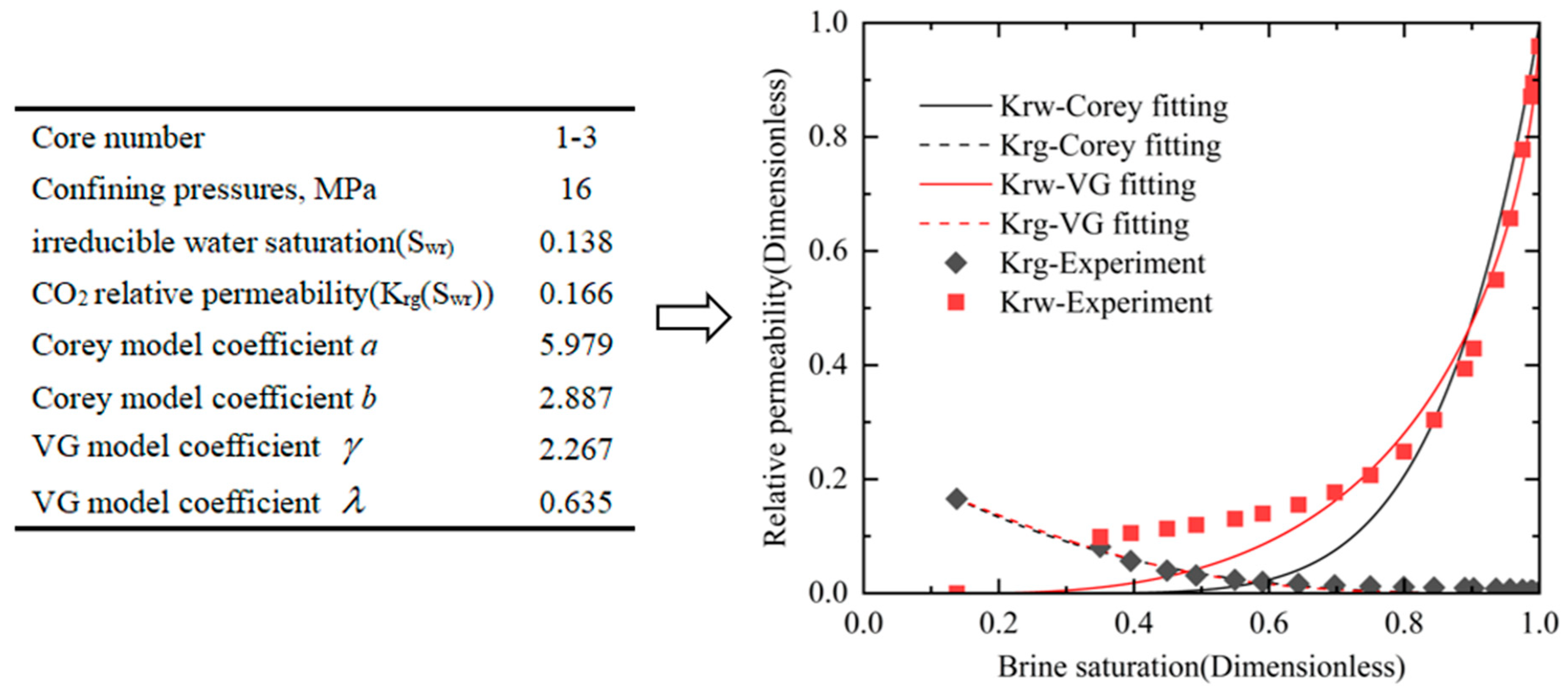
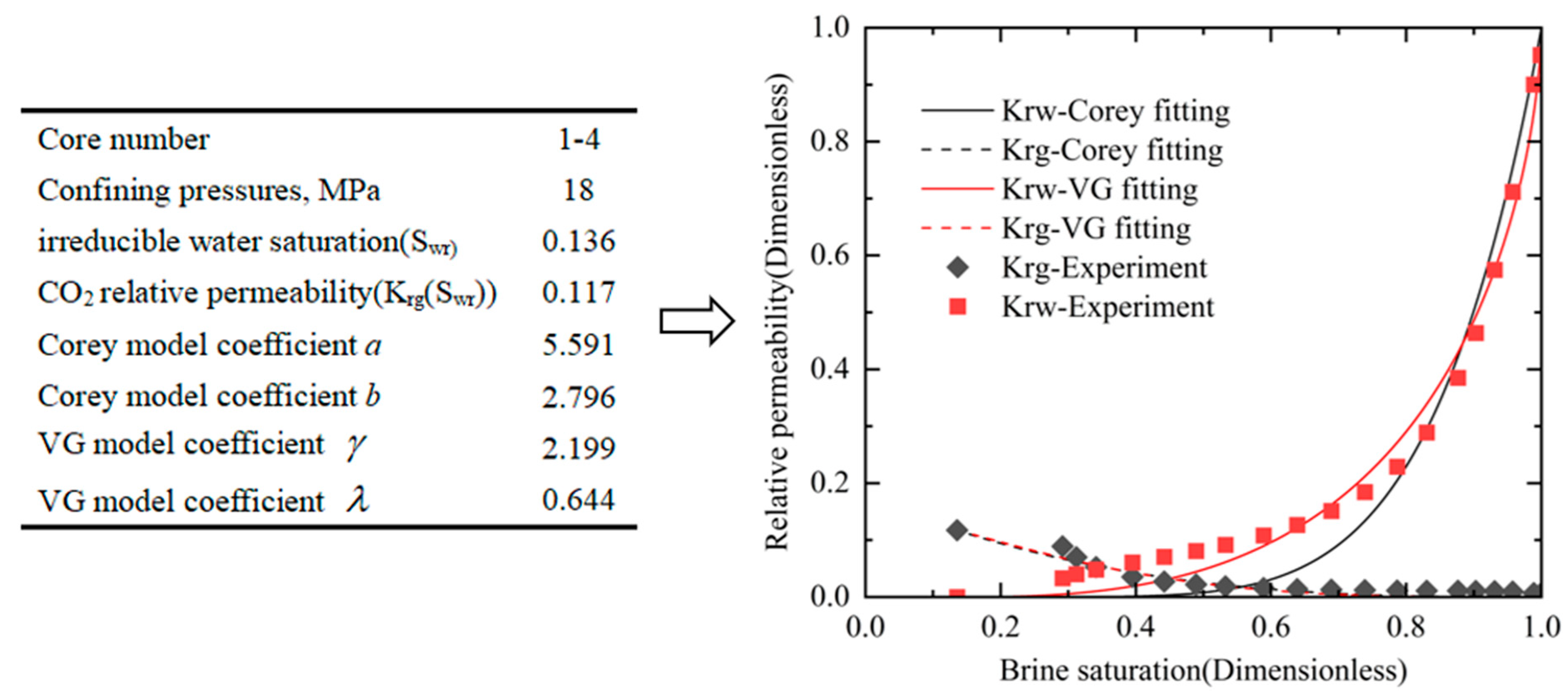
3.1. Relative Permeability Results for Five Sets of Confining Pressure Conditions
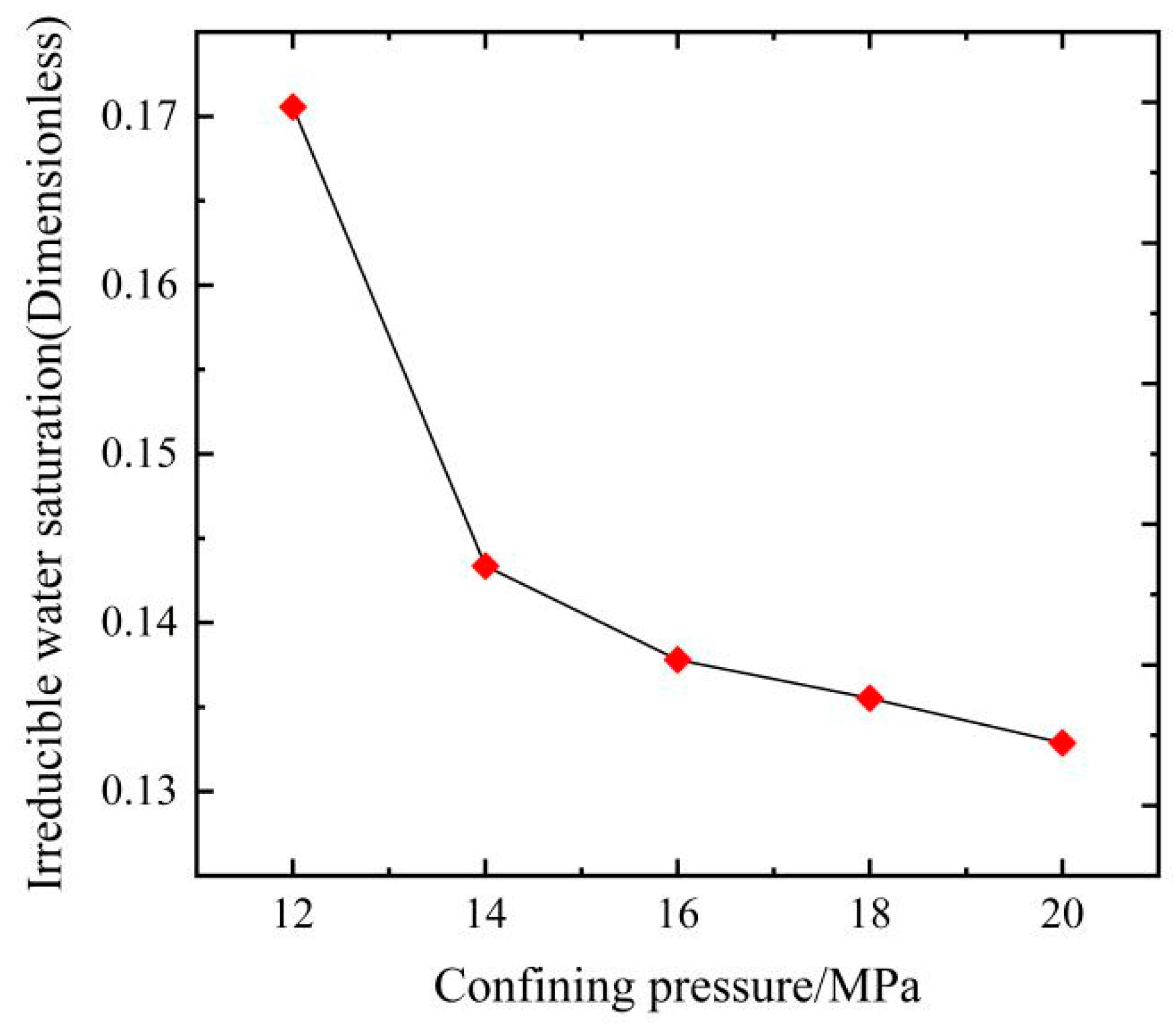
3.2. Effects of Confining Pressures on Irreducible Water Saturation
3.3. Effect of Confining Pressure on CO2 Relative Permeability in the Irreducible Water State
3.4. Effect of Confining Pressure on CO2-Brine Relative Permeability Curve
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hansen, J.; Johnson, D.; Lacis, A.; Lebedeff, S.; Lee, P.; Rind, D.; Russell, G. Climate impact of increasing atmospheric carbon dioxide. Science 1981, 213, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar]
- Kumar, S.; Foroozesh, J.; Edlmann, K.; Rezk, M.G.; Lim, C.Y. A comprehensive review of value-added CO2 sequestration in subsurface saline aquifers. J. Nat. Gas Sci. Eng. 2020, 81, 103437. [Google Scholar] [CrossRef]
- Cui, G.; Wang, Y.; Rui, Z.; Chen, B.; Ren, S.; Zhang, L. Assessing the combined influence of fluid-rock interactions on reservoir properties and injectivity during CO2 storage in saline aquifers. Energy 2018, 155, 281–296. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, J.; Yang, H.; Wang, J.; Liu, L.; Song, H. Relative permeability curve prediction from digital rocks with variable sizes using deep learning. Phys. Fluids 2023, 35, 096605. [Google Scholar] [CrossRef]
- Honarpour, M.M. Relative Permeability of Petroleum Reservoirs; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Al-Menhali, A.; Niu, B.; Krevor, S. Capillarity and wetting of carbon dioxide and brine during drainage in b erea sandstone at reservoir conditions. Water Resour. Res. 2015, 51, 7895–7914. [Google Scholar] [CrossRef]
- Bachu, S.; Bennion, B. Effects of in-situ conditions on relative permeability characteristics of CO2-brine systems. Environ. Geol. 2008, 54, 1707–1722. [Google Scholar] [CrossRef]
- Chen, X. Experimental Studies on CO2-Brine-Decane Relative Permeabilities in Berea Sandstone with New Steady-State and Unsteady-State Methods. Doctoral Dissertation, UT Austin, Austin, TX, USA, 2016. [Google Scholar]
- Levine, J.S.; Goldberg, D.S.; Lackner, K.S.; Matter, J.M.; Supp, M.G.; Ramakrishnan, T.S. Relative permeability experiments of carbon dioxide displacing brine and their implications for carbon sequestration. Environ. Sci. Technol. 2014, 48, 811–818. [Google Scholar] [CrossRef]
- Krevor, S.C.; Pini, R.; Zuo, L.; Benson, S.M. Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Manceau, J.C.; Ma, J.; Li, R.; Audigane, P.; Jiang, P.X.; Xu, R.N.; Tremosa, J.; Lerouge, C. Two-phase flow properties of a sandstone rock for the CO2/water system: Core-flooding experiments, and focus on impacts of mineralogical changes. Water Resour. Res. 2015, 51, 2885–2900. [Google Scholar] [CrossRef]
- Pini, R.; Krevor, S.C.; Benson, S.M. Capillary pressure and heterogeneity for the CO2/water system in sandstone rocks at reservoir conditions. Adv. Water Resour. 2012, 38, 48–59. [Google Scholar] [CrossRef]
- Akbarabadi, M.; Piri, M. Relative permeability hysteresis and capillary trapping characteristics of supercritical CO2/brine systems: An experimental study at reservoir conditions. Adv. Water Resour. 2013, 52, 190–206. [Google Scholar] [CrossRef]
- Chen, X.; DiCarlo, D.A. A new unsteady-state method of determining two-phase relative permeability illustrated by CO2-brine primary drainage in berea sandstone. Adv. Water Resour. 2016, 96, 251–265. [Google Scholar] [CrossRef]
- Bakhshian, S.; Hosseini, S.A.; Lake, L.W. CO2-brine relative permeability and capillary pressure of Tuscaloosa sandstone: Effect of anisotropy. Adv. Water Resour. 2020, 135, 103464. [Google Scholar] [CrossRef]
- Ruprecht, C.; Pini, R.; Falta, R.; Benson, S.; Murdoch, L. Hysteretic trapping and relative permeability of CO2 in sandstone at reservoir conditions. Int. J. Greenh. Gas Control 2014, 27, 15–27. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, K.H.; Lee, T.H.; Sung, W.M.; Park, Y.C.; Lee, J.H. Analysis of CO2 endpoint relative permeability and injectivity by change in pressure, temperature, and phase in saline aquifer. Energy Sources Recovery Util. Environ. Eff. 2009, 32, 83–99. [Google Scholar]
- Farokhpoor, R.; Lindeberg, E.G.B.; Torsæter, O.; Mørk, M.B.; Mørk, A. Permeability and relative permeability measurements for CO2-brine system at reservoir conditions in low permeable sandstones in Svalbard. Greenh. Gases Sci. Technol. 2014, 4, 36–52. [Google Scholar] [CrossRef]
- Ren, X.; Li, A.; Memon, A. Experimental Study on Gas–Water Relative Permeability Characteristics of Tight Sandstone Reservoir in Ordos Basin. Geofluids 2022, 2022, 1521837. [Google Scholar] [CrossRef]
- Reynolds, C.; Blunt, M.; Krevor, S. Impact of reservoir conditions on CO2-brine relative permeability in sandstones. Energy Procedia 2014, 63, 5577–5585. [Google Scholar] [CrossRef]
- Raghavan, R.; Chin, L.Y. Productivity changes in reservoirs with stress-dependent permeability. In SPE Annual Technical Conference and Exhibition? SPE: Houston, TX, USA, 2002; p. SPE-77535. [Google Scholar]
- Alam, A.B.; Niioka, M.; Fujii, Y.; Fukuda, D.; Kodama, J.I. Effects of confining pressure on the permeability of three rock types under compression. Int. J. Rock Mech. Min. Sci. 2014, 65, 49–61. [Google Scholar] [CrossRef]
- Gobran, B.D.; Brigham, W.E.; Ramey, H.J. Absolute permeability as a function of confining pressure, pore pressure, and temperature. SPE Form. Eval. 1987, 2, 77–84. [Google Scholar] [CrossRef]
- Dong, J.J.; Hsu, J.Y.; Wu, W.J.; Shimamoto, T.; Hung, J.H.; Yeh, E.C.; Wu, Y.H.; Sone, H. Stress-dependence of the permeability and porosity of sandstone and shale from TCDP Hole-A. Int. J. Rock Mech. Min. Sci. 2010, 47, 1141–1157. [Google Scholar] [CrossRef]
- Farrell NJ, C.; Healy, D.; Taylor, C.W. Anisotropy of permeability in faulted porous sandstones. J. Struct. Geol. 2014, 63, 50–67. [Google Scholar] [CrossRef]
- Fatt, I. The effect of overburden pressure on relative permeability. J. Pet. Technol. 1953, 5, 15–16. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, C.; Liu, S. Characteristic analysis and fractal model of the gas–water relative permeability of coal under different confining pressures. J. Pet. Sci. Eng. 2017, 159, 488–496. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Zhen, X. Influence of rock stress sensitivity in tight gas reservoir on characteristics of gas/water two phase flows. Complex Hydrocarb. Reserv. 2013, 6, 36–39. [Google Scholar]
- Lai, B.; Miskimins, J.L. A new technique for accurately measuring two-phase relative permeability under non-Darcy flow conditions. J. Pet. Sci. Eng. 2015, 127, 398–408. [Google Scholar] [CrossRef]
- Thomas, R.D.; Ward, D.C. Effect of overburden pressure and water saturation on gas permeability of tight sandstone cores. J. Pet. Technol. 1972, 24, 120–124. [Google Scholar] [CrossRef]
- Esmaeili, S.; Modaresghazani, J.; Sarma, H.; Harding, T.; Maini, B. Effect of temperature on relative permeability—Role of viscosity ratio. Fuel 2020, 278, 118318. [Google Scholar] [CrossRef]
- Owens, W.W.; Archer, D. The effect of rock wettability on oil–water relative permeability relationships. J. Pet. Technol. 1971, 23, 873–878. [Google Scholar] [CrossRef]
- Safaei-Farouji, M.; Thanh, H.V.; Dashtgoli, D.S.; Yasin, Q.; Radwan, A.E.; Ashraf, U.; Lee, K.K. Application of robust intelligent schemes for accurate modelling interfacial tension of CO2 brine systems: Implications for structural CO2 trapping. Fuel 2022, 319, 123821. [Google Scholar] [CrossRef]
- Lian, P.; Cheng, L. The characteristics of relative permeability curves in naturally fractured carbonate reservoirs. J. Can. Pet. Technol. 2012, 51, 137–142. [Google Scholar] [CrossRef]
- Zheng, X.; Zhigang, C.; Weichuan, L. Gas/water flowing ability influence experimental study of permeability stress sensibility in tight gas reservoir. Well Logging Technol. 2013, 4, 360–363. [Google Scholar]
- Adenutsi, C.D.; Li, Z.; Xu, Z.; Sun, L. Influence of net confining stress on NMR T2 distribution and two-phase relative permeability. J. Pet. Sci. Eng. 2019, 178, 766–777. [Google Scholar] [CrossRef]
- Lei, G.; Mo, S.; Dong, Z.; Wang CA, I.; Li, W. Theoretical and experimental study on stress-dependency of oil–water relative permeability in fractal porous media. Fractals 2018, 26, 1840010. [Google Scholar] [CrossRef]
- Simion, A.I.; Grigoraş, C.G.; Roşu, A.M.; Gavrilă, L. Mathematical modelling of density and viscosity of NaCl aqueous solutions. J. Agroaliment. Process. Technol 2015, 21, 41–52. [Google Scholar]
- Kestin, J.; Khalifa, H.E.; Correia, R.J. Tables of the dynamic and kinematic viscosity of aqueous NaCl solutions in the temperature range 20–150 C and the pressure range 0.1–35 MPa. J. Phys. Chem. Ref. Data 1981, 10, 71–88. [Google Scholar] [CrossRef]
- Honarpour, M.; Mahmood, S.M. Relative-permeability measurements: An overview. J. Pet. Technol. 1988, 40, 963–966. [Google Scholar] [CrossRef]
- Müller, N. Supercritical CO2-brine relative permeability experiments in reservoir rocks—Literature review and recommendations. Transp. Porous Media 2011, 87, 367–383. [Google Scholar] [CrossRef]
- Flett, M.; Gurton, R.; Taggart, I. The function of gas-water relative permeability hysteresis in the sequestration of carbon dioxide in saline formations. In SPE Asia Pacific Oil and Gas Conference and Exhibition; OnePetro: Richardson, TX, USA, 2004. [Google Scholar]
- Welge, H.J. A simplified method for computing oil recovery by gas or water drive. J. Pet. Technol. 1952, 4, 91–98. [Google Scholar] [CrossRef]
- Johnson, E.F.; Bossler, D.P.; Bossler, V.N. Calculation of relative permeability from displacement experiments. Trans. AIME 1959, 216, 370–372. [Google Scholar] [CrossRef]
- Corey, A.T. The interrelation between gas and oil relative permeabilities. Prod. Mon. 1954, 19, 38–41. [Google Scholar]
- Brooks, R.H.; Corey, A.T. Hydraulic properties of porous media. Hydrol. Pap. 1964, 3, 1–27. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, C.; Zhu, L.Q. A fractal irreducible water saturation model for capillary tubes and its application in tight gas reservoir. J. Pet. Sci. Eng. 2017, 159, 731–739. [Google Scholar] [CrossRef]
- Chilingarian, G.V.; Torabzadeh, J.; Rieke, H.H.; Metghalchi, M.; Mazzullo, S.J. Interrelationships among surface area, permeability, porosity, pore size, and residual water saturation. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 1992; Volume 30, pp. 379–397. [Google Scholar]
- Luo, N.; Suo, Y.; Fan, X.; Yuan, Y.; Zhai, C.; Sun, W. Research on confining pressure effect of pore structure of coal-rich in coalbed methane under cyclic impact. Energy Rep. 2022, 8, 7336–7348. [Google Scholar] [CrossRef]
- Blanton, T.L. Deformation of chalk under confining pressure and pore pressure. Soc. Pet. Eng. J. 1981, 21, 43–50. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Z.; Sun, W.; Li, Y.; Zhang, X.; Zhu, L.; Wen, M. A multiscale comprehensive study on pore structure of tight sandstone reservoir realized by nuclear magnetic resonance, high pressure mercury injection and constant-rate mercury injection penetration test. Mar. Pet. Geol. 2019, 109, 208–222. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Y.; Liu, N.; Han, Y.; Li, D.; Deng, H. Investigation of pore structure changes in Mesozoic water-rich sandstone induced by freeze-thaw process under different confining pressures using digital rock technology. Cold Reg. Sci. Technol. 2019, 161, 137–149. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Qiao, W.; Zhang, S.; Li, X. The Permeability Evolution of Sandstones with Different Pore Structures under High Confining Pressures, High Pore Water Pressures and High Temperatures. Appl. Sci. 2023, 13, 1771. [Google Scholar] [CrossRef]
- Alizadeh, A.H.; Piri, M. Three-phase flow in porous media: A review of experimental studies on relative permeability. Rev. Geophys. 2014, 52, 468–521. [Google Scholar] [CrossRef]
- Beltrán, A.; Hernández-Díaz, D.; Chávez, O.; García, A.; Mena, B.; Zenit, R. Experimental study of the effect of wettability on the relative permeability for air–water flow through porous media. Int. J. Multiph. Flow 2019, 120, 103091. [Google Scholar] [CrossRef]
- Broseta, D.; Tonnet, N.; Shah, V. Are rocks still water-wet in the presence of dense CO2 or H2S? Geofluids 2012, 12, 280–294. [Google Scholar] [CrossRef]
- Iglauer, S. CO2–water–rock wettability: Variability, influencing factors, and implications for CO2 geostorage. Acc. Chem. Res. 2017, 50, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Bennion, D.B.; Bachu, S. Drainage and imbibition relative permeability relationships for supercritical CO2/brine and H2S/brine systems in intergranular sandstone, carbonate, shale, and anhydrite rocks. SPE Reserv. Eval. Eng. 2008, 11, 487–496. [Google Scholar] [CrossRef]
- Pan, L.; Jones, S.J.; Wang, X.; Guan, W.; Li, L. Re-evaluation of the porosity measurements under different confining pressures: A better appraisal of reservoir porosity. AAPG Bull. 2019, 103, 515–526. [Google Scholar] [CrossRef]
- Handin, J.; Hager, R.V., Jr.; Friedman, M.; Feather, J.N. Experimental deformation of sedimentary rocks under confining pressure: Pore pressure tests. Aapg Bull. 1963, 47, 717–755. [Google Scholar]
- Anderson, W.G. Wettability literature survey part 5: The effects of wettability on relative permeability. J. Pet. Technol. 1987, 39, 1453–1468. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability literature survey-part 6: The effects of wettability on waterflooding. J. Pet. Technol. 1987, 39, 1605–1622. [Google Scholar] [CrossRef]
- Lei, G.; Liao, Q.; Lin, Q.; Zhang, L.; Xue, L.; Chen, W. Stress dependent gas-water relative permeability in gas hydrates: A theoretical model. Adv. Geo-Energy Res. 2020, 4, 326–338. [Google Scholar] [CrossRef]
- Gharbi, O.; Blunt, M.J. The impact of wettability and connectivity on relative permeability in carbonates: A pore network modeling analysis. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Berg, S.; Rücker, M.; Ott, H.; Georgiadis, A.; Van der Linde, H.; Enzmann, F.; Kersten, M.; Armstrong, R.T.; de With, S.; Becker, J.; et al. Connected pathway relative permeability from pore-scale imaging of imbibition. Adv. Water Resour. 2016, 90, 24–35. [Google Scholar] [CrossRef]
- He, M.; Zhou, Y.; Wu, K.; Hu, Y.; Feng, D.; Zhang, T.; Liu, Q.; Li, X. Pore network modeling of thin water film and its influence on relative permeability curves in tight formations. Fuel 2021, 289, 119828. [Google Scholar] [CrossRef]
- Kimmel, G.A.; Stevenson, K.P.; Dohnalek, Z.; Smith, R.S.; Kay, B.D. Control of amorphous solid water morphology using molecular beams. I. Experimental results. J. Chem. Phys. 2001, 114, 5284–5294. [Google Scholar] [CrossRef]


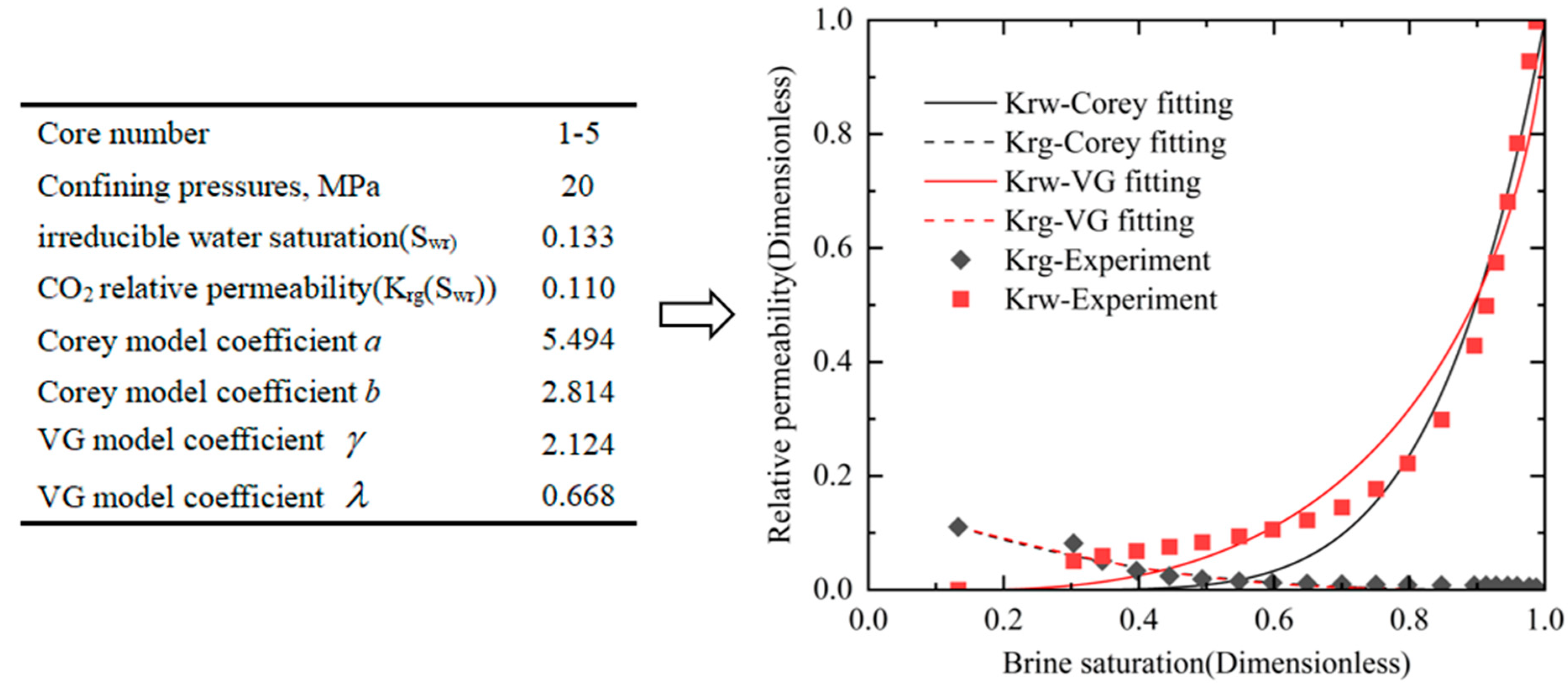



| Core Number | Length/cm | Diameter/cm | Porosity/% | Dry Weight/g | Permeability/mD |
|---|---|---|---|---|---|
| 1-1 | 5.965 | 2.502 | 8.9 | 69.89 | 97 |
| 1-2 | 5.977 | 2.506 | 8.4 | 70.95 | 101 |
| 1-3 | 5.965 | 2.502 | 8.4 | 70.28 | 103 |
| 1-4 | 5.965 | 2.502 | 8.1 | 70.33 | 97 |
| 1-5 | 5.977 | 2.506 | 8.4 | 70.95 | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Ding, G.; Song, S.; Wang, H.; Xie, W.; Zhou, Y.; Song, Z.; Xie, C.; Song, H. Effect of Confining Pressure on CO2-Brine Relative Permeability Characteristics of Sandstone in Ordos Basin. Water 2023, 15, 4235. https://doi.org/10.3390/w15244235
Tang L, Ding G, Song S, Wang H, Xie W, Zhou Y, Song Z, Xie C, Song H. Effect of Confining Pressure on CO2-Brine Relative Permeability Characteristics of Sandstone in Ordos Basin. Water. 2023; 15(24):4235. https://doi.org/10.3390/w15244235
Chicago/Turabian StyleTang, Ligen, Guosheng Ding, Shijie Song, Huimin Wang, Wuqiang Xie, Yiyang Zhou, Zhiyong Song, Chiyu Xie, and Hongqing Song. 2023. "Effect of Confining Pressure on CO2-Brine Relative Permeability Characteristics of Sandstone in Ordos Basin" Water 15, no. 24: 4235. https://doi.org/10.3390/w15244235






