Using Clustering, Geochemical Modeling, and a Decision Tree for the Hydrogeochemical Characterization of Groundwater in an In Situ Leaching Uranium Deposit in Bayan-Uul, Northern China
Abstract
:1. Introduction
2. Materials and Methods
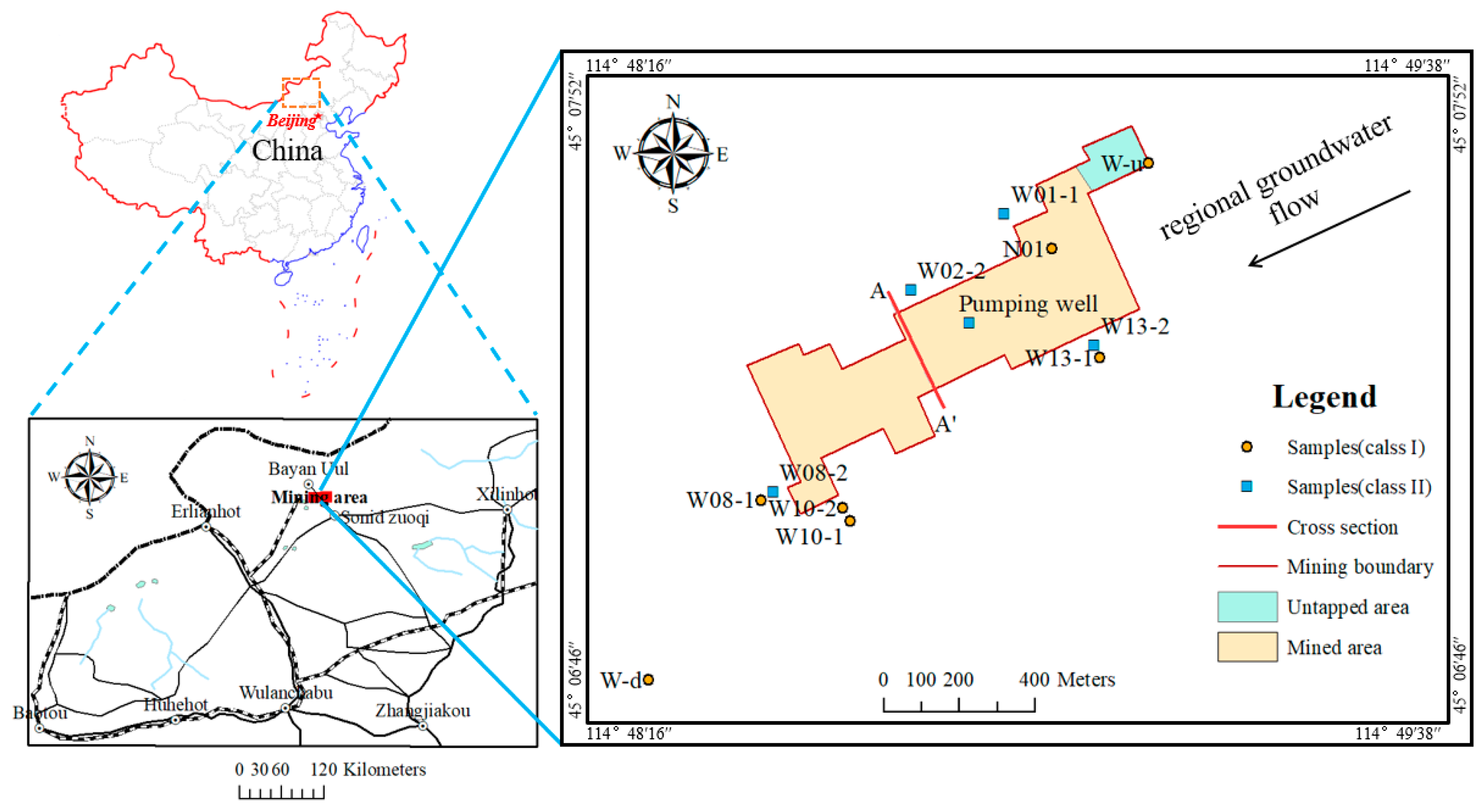
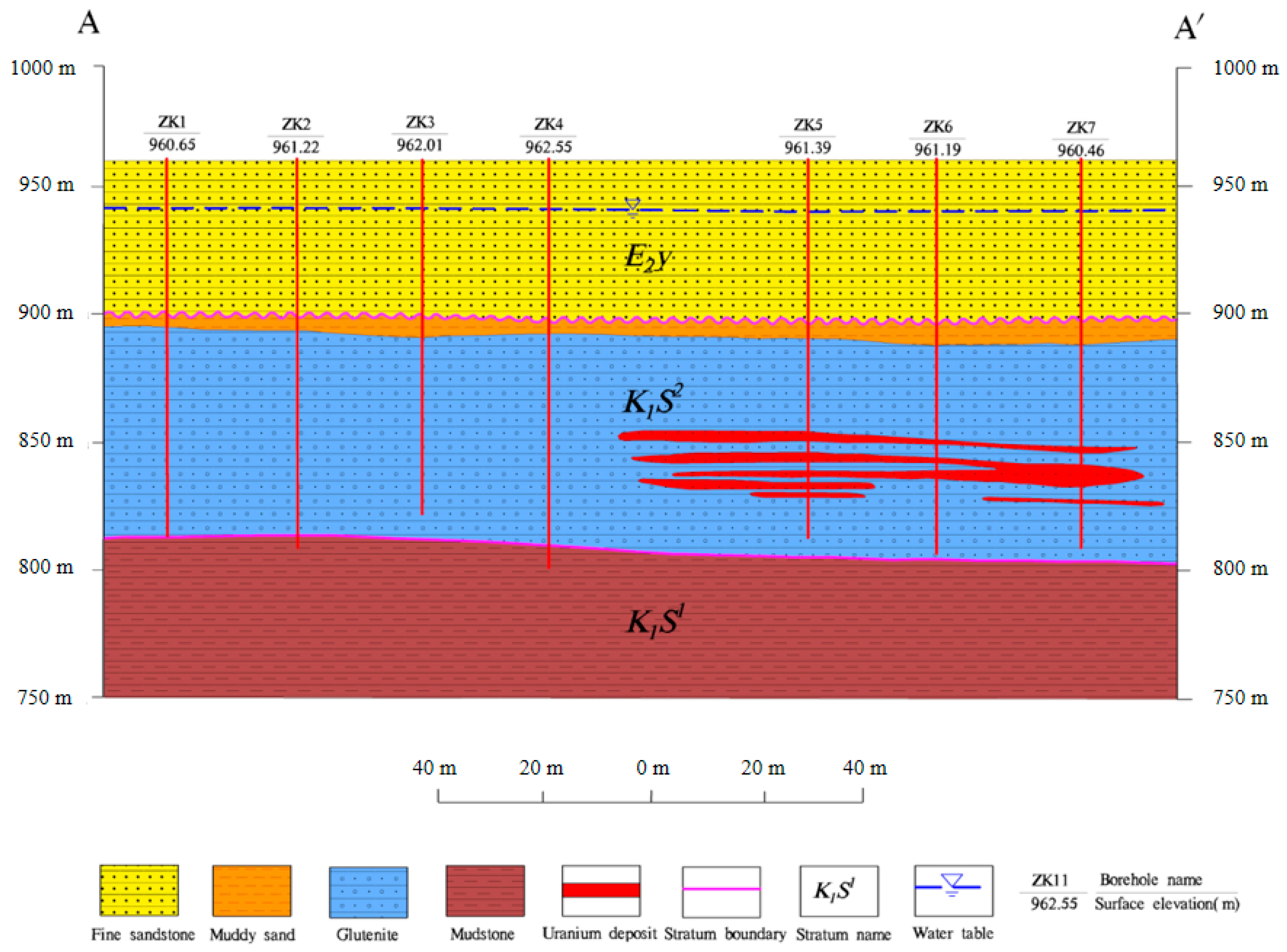
2.1. Study Area
2.2. Sample Collection
2.3. Methodology
2.3.1. Clustering Method
2.3.2. Decision Tree-Based Machine Learning Approach
2.3.3. Geochemical Modeling
3. Results
3.1. Basic Statistical Characteristics and Spatial Distributions of the Hydrochemical Parameters
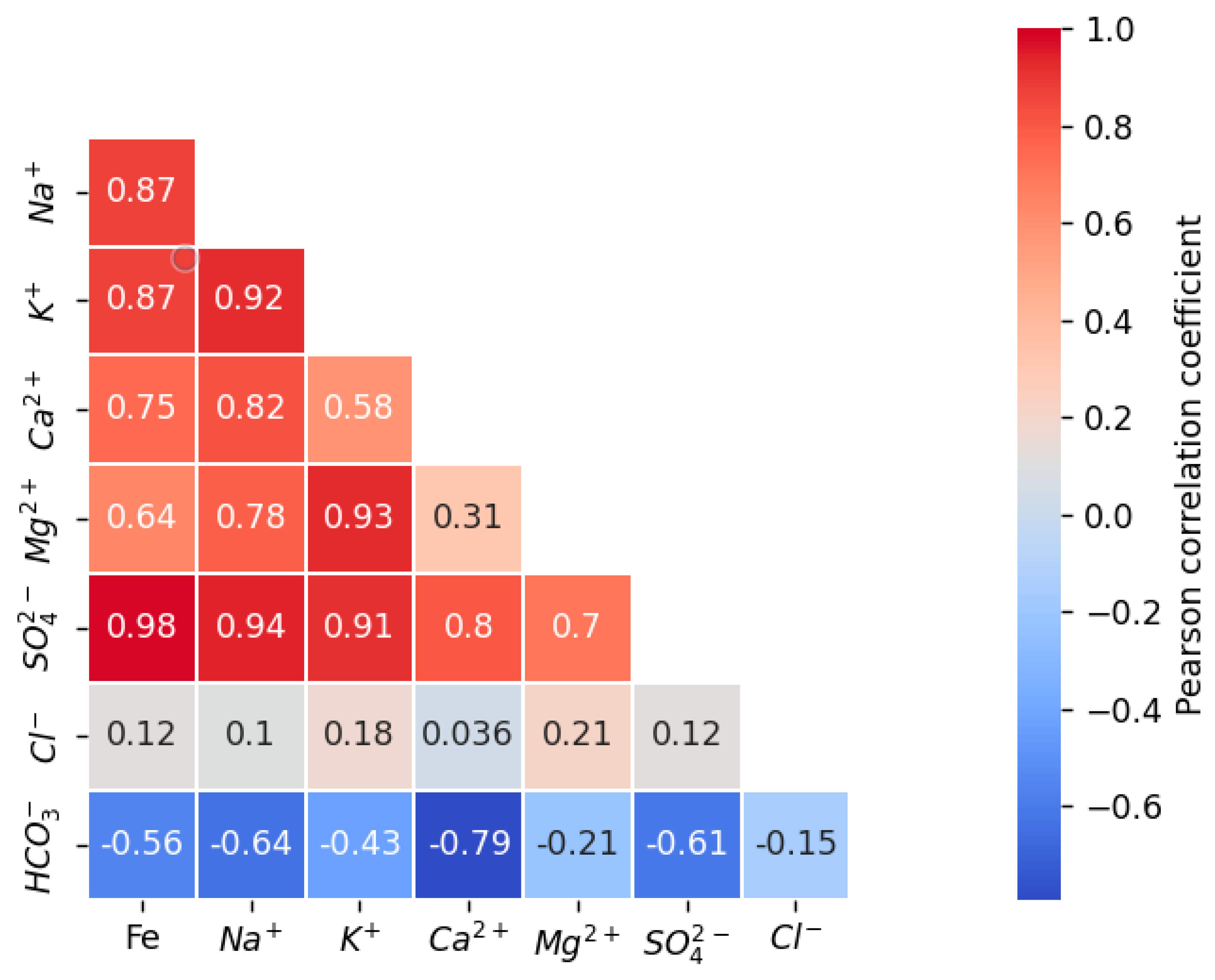
3.2. Pearson’s Correlations between Pairs of Parameters
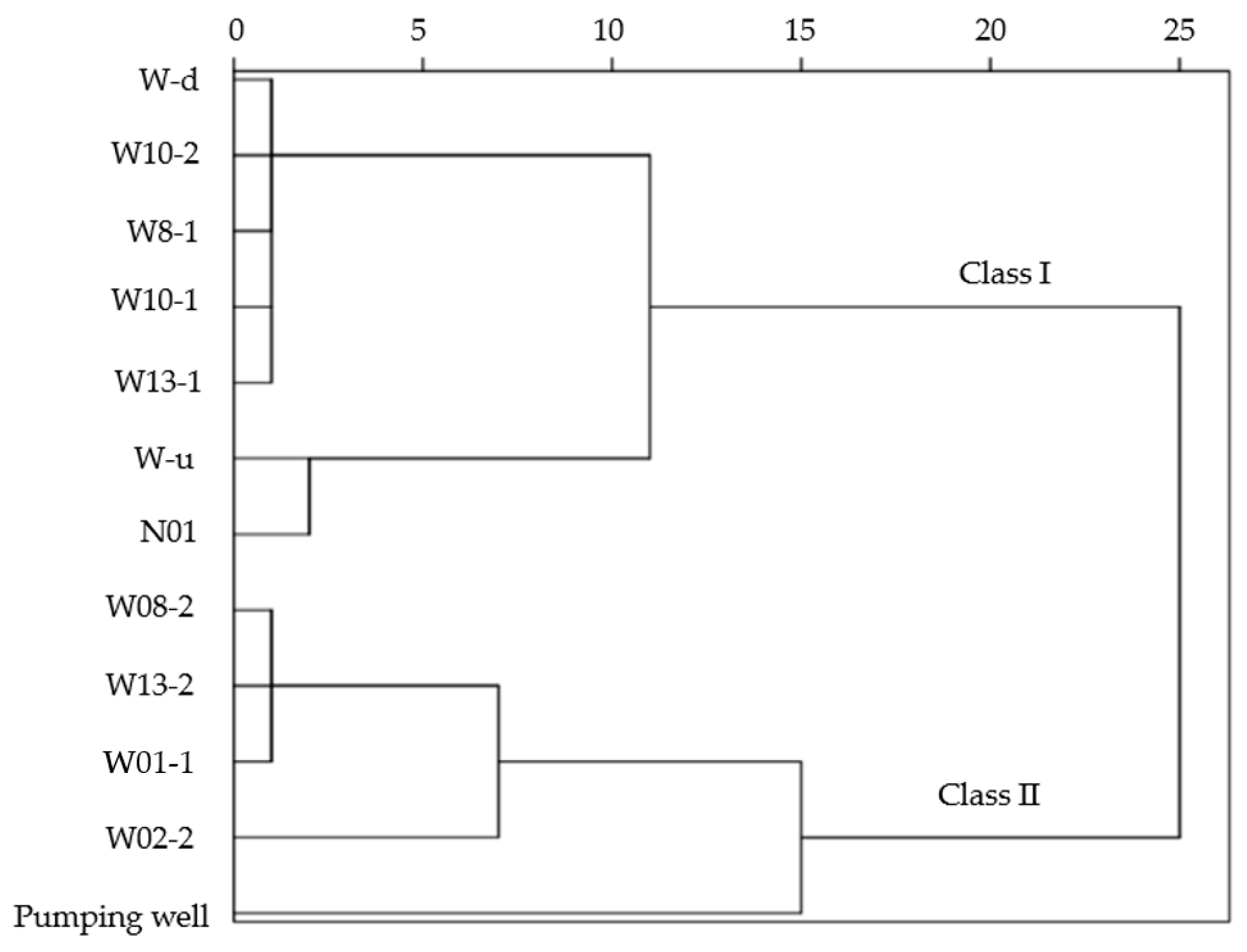
3.3. Classified Two Clusters and Their Main Differences
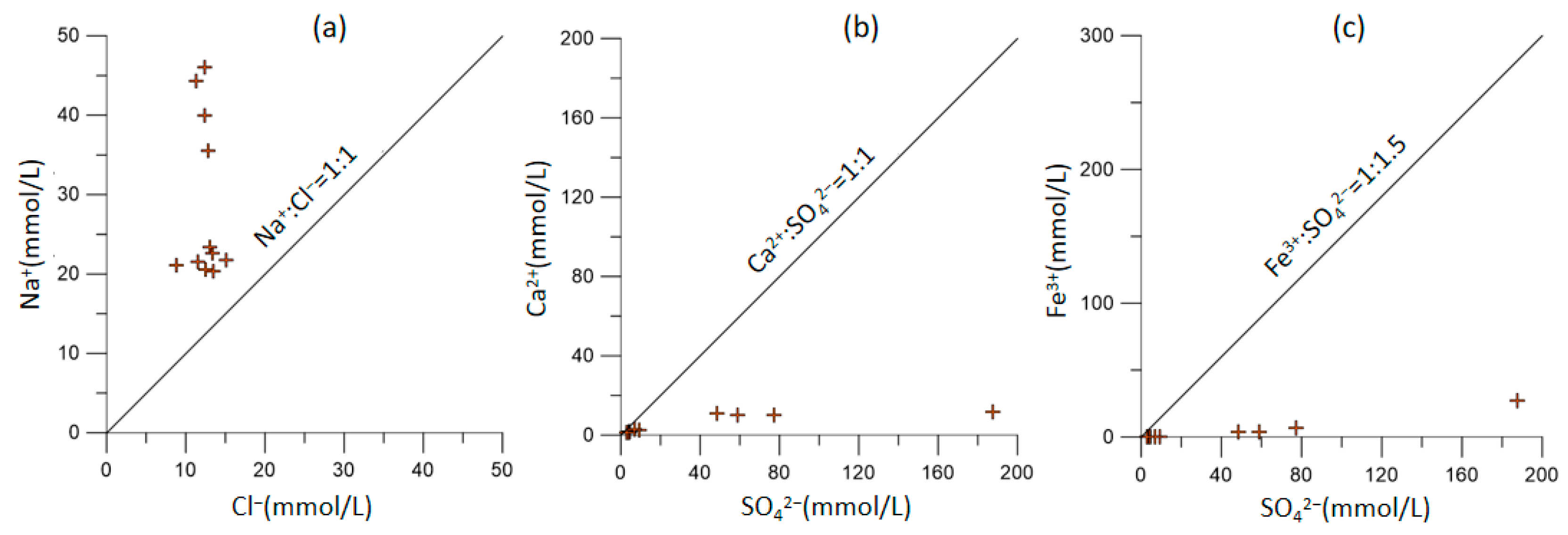
3.4. Hydrogeochemical Process Governing the Hydrogeochemistry
3.5. Saturation Indices of Ca- and Fe-Related Minerals
4. Conclusions
- (1)
- This study highlights that the concentrations of SO42− and total dissolved solids (TDS) are notably high in the groundwater samples. This suggests that the increased salinity in groundwater is primarily attributed to the dissolution of minerals, such as potassium feldspar, sodium feldspar, and calcite in the aquifer, due to the presence of sulfuric acid in the leaching solution. This confirms the significant impact of in situ uranium leaching on groundwater quality.
- (2)
- Correlation analysis indicates a strong positive correlation between key ions such as K+, Na+, Ca2+, and SO42−. This underscores the fact that the leaching solution injected during in situ uranium leaching, which contained substantial sulfuric acid, led to a significant dissolution of minerals and subsequent increases in the concentration of conventional metal cations in groundwater.
- (3)
- The clustering of water samples into two major categories reflects the differential influence of in situ uranium leaching on the surrounding groundwater environment. Samples located closer to the mining area exhibited distinct hydrogeochemical characteristics compared to those located further away. This observation indicates that the proximity to the mining area and the groundwater background flow patterns are pivotal factors influencing the groundwater’s response to uranium leaching. The outcomes of two representative path analyses further substantiate this perspective.
- (4)
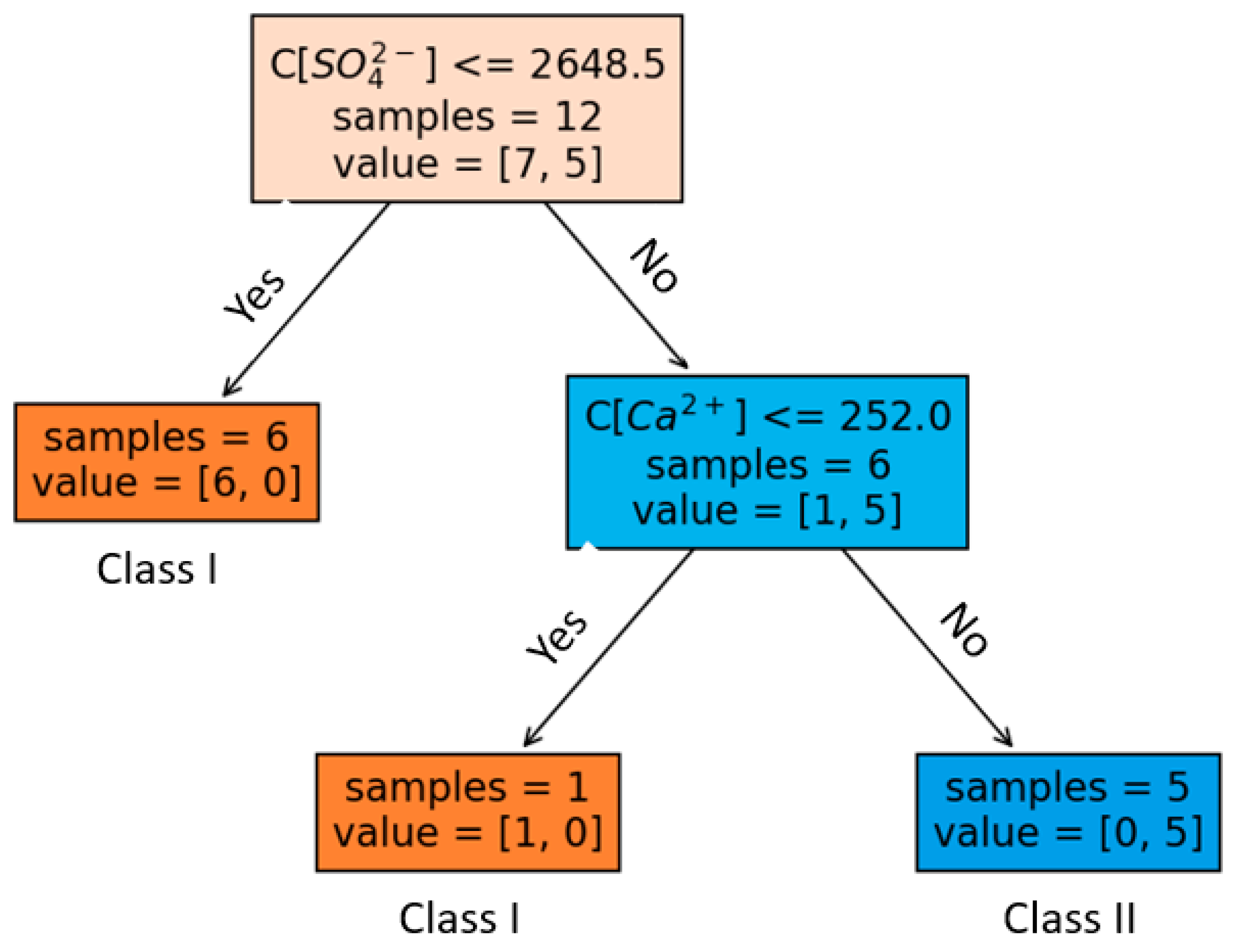
- Decision tree analysis identifies SO42− as the most influential factor, followed by Ca2+. These results provide valuable insights for the classification and prediction of water samples based on their hydrogeochemical characteristics.
- (5)
- The study of mineral saturation indices reveals that calcium-bearing minerals tend to dissolve near the mining area and then precipitate. By contrast, iron-bearing minerals tend to precipitate more gradually. These observations indicate the complex interactions between mineral dissolution and precipitation, emphasizing the impact of in situ uranium leaching on mineral reactions in the aquifer.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Zhang, F.; Niu, Z.; Dong, Z.; Liu, G.; Gao, L. Hydro-chemical characteristics of groundwater in centralized drinking water sources and its quality assessment in northern Anhui Province. J. Univ. Sci. Technol. China 2014, 44, 913–920+925. [Google Scholar]
- Wu, A.; Jin, J.; Song, B. Water Safety Issues of China and Ensuring Roles of Groundwater. Acta Geol. Sin. 2016, 90, 2939–2947. [Google Scholar]
- Sun, C.; Chen, R.; Zhang, Z.; Li, W.; Chen, W. Temporal and spatial variation of hydrochemical characteristics of shallow groundwater in Shanxi Province. Arid. Land. Geogr. 2018, 41, 314–324. [Google Scholar]
- Aliewi, A.; Imseih, N.; Abdulghafour, D. Protecting the Groundwater Environment of Tulkarem City of Palestine from Industrial and Domestic Pollution. J. Environ. Prot. 2013, 04, 27–39. [Google Scholar] [CrossRef]
- Dhillon, M.S.; Kaur, S.; Aggarwal, R. Delineation of critical regions for mitigation of carbon emissions due to groundwater pumping in central Punjab. Groundw. Sustain. Dev. 2019, 8, 302–308. [Google Scholar] [CrossRef]
- Kaur, S.; Aggarwal, R.; Lal, R. Assessment and Mitigation of Greenhouse Gas Emissions from Groundwater Irrigation. Irrig. Drain. 2016, 65, 762–770. [Google Scholar] [CrossRef]
- Sha, C.; Wu, J.; Shen, C.; Wu, J.; Yan, Z.; Wang, M. The ecology of bacterial communities in groundwater of industrial areas: Diversity, composition, network, and assembly. Environ. Pollut. 2023, 322, 121207. [Google Scholar] [CrossRef]
- Nsabimana, A.; Li, P. Hydrogeochemical characterization and appraisal of groundwater quality for industrial purpose using a novel industrial water quality index (IndWQI) in the Guanzhong Basin, China. Geochemistry 2023, 83, 125922. [Google Scholar] [CrossRef]
- Mudd, G.M. The future of Yellowcake: A global assessment of uranium resources and mining. Sci. Total Environ. 2014, 472, 590–607. [Google Scholar] [CrossRef]
- Zeng, S.; Song, J.; Sun, B.; Wang, F.; Ye, W.; Shen, Y.; Li, H. Experimental and theoretical study on the seepage mechanism characteristics coupling with confining pressure. Nucl. Eng. Technol. 2023, 55, 566–574. [Google Scholar] [CrossRef]
- Cary, L.; Petelet-Giraud, E.; Bertrand, G.; Kloppmann, W.; Aquilina, L.; Martins, V.; Hirata, R.; Montenegro, S.; Pauwels, H.; Chatton, E.; et al. Origins and processes of groundwater salinization in the urban coastal aquifers of Recife (Pernambuco, Brazil): A multi-isotope approach. Sci. Total Environ. 2015, 530, 411–429. [Google Scholar] [CrossRef]
- Pittalis, D.; Carletti, A.; Ghiglieri, G.; Celico, F. The influence of hydrogeological properties, seawater intrusion and refreshening on the quality of groundwater used for irrigation in an agricultural coastal plain in North Sardinia, Italy. Environ. Earth Sci. 2016, 75, 963. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; Northey, S.; Yellishetty, M.; Deng, X.; McBride, D. The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner. Eng. 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Hague, N.; Norgate, T. The greenhouse gas footprint of in-situ leaching of uranium, gold and copper in Australia. J. Clean. Prod. 2014, 84, 382–390. [Google Scholar] [CrossRef]
- Islam, A.R.; Ahmed, N.; Bodrud-Doza, M.; Chu, R. Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ. Sci. Pollut. Res. Int. 2017, 24, 26350–26374. [Google Scholar] [CrossRef]
- Bradshaw, P.; Lett, R. Geochemical-exploration for uranium using soils. J. Geochem. Explor. 1980, 13, 305–319. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Hydrogeochemistry and quality assessment of shallow groundwater in the southern part of the Yellow River alluvial plain (Zhongwei Section), northwest China. Earth Sci. Res. J. 2014, 18, 27–38. [Google Scholar] [CrossRef]
- Qian, C.; Wu, X.; Mu, W.P.; Fu, R.Z.; Zhu, G.; Wang, Z.R.; Wang, D.D. Hydrogeochemical characterization and suitability assessment of groundwater in an agro-pastoral area, Ordos Basin, NW China. Environ. Earth Sci. 2016, 75, 1356. [Google Scholar] [CrossRef]
- He, T.; Liu, J.; Yang, Y.; Yao, G.; Xing, Y.; Hu, P.; Wang, R.; Liang, D.; Wang, X.; Zhang, F. Tracer Test of Groundwater in In-situ Leaching Area of Bayanwula Uranium Mine in Inner Mongolia. Nonferrous Met. (Extr. Metall.) 2022, 11, 54–63. [Google Scholar]
- Chang, Y.X.; Tan, K.X.; Zhang, C.; Yao, Y.X.; He, C.Y.; Liu, Y. Simulation study on groundwater dynamics control of leaching range of in-situ uranium well field. J. Univ. South China (Sci. Technol.) 2020, 34, 29–36. [Google Scholar]
- Wang, B.; Luo, Y.; Liu, J.H.; Li, X.; Zheng, Z.H.; Chen, Q.Q.; Li, L.Y.; Wu, H.; Fan, Q.R. Ion migration in in-situ leaching (ISL) of uranium: Field trial and reactivetransport modelling. J. Hydrol. 2022, 615, 128634. [Google Scholar] [CrossRef]
- Biddau, R.; Cidu, R.; Lorrai, M.; Mulas, M.G. Assessing background values of chloride, sulfate and fluoride in groundwater: A geochemical-statistical approach at a regional scale. J. Geochem. Explor. 2017, 181, 243–255. [Google Scholar] [CrossRef]
- Huang, G.; Liu, C.; Sun, J.; Zhang, M.; Jing, J.; Li, L. A regional scale investigation on factors controlling the groundwater chemistry of various aquifers in a rapidly urbanized area: A case study of the Pearl River Delta. Sci. Total Environ. 2018, 625, 510–518. [Google Scholar] [CrossRef]
- Petelet-Giraud, E.; Négrel, P.; Aunay, B.; Ladouche, B.; Bailly-Comte, V.; Guerrot, C.; Flehoc, C.; Pezard, P.; Lofi, J.; Dörfliger, N. Coastal groundwater salinization: Focus on the vertical variability in a multi-layered aquifer through a multi-isotope fingerprinting (Roussillon Basin, France). Sci. Total Environ. 2016, 566, 398–415. [Google Scholar] [CrossRef]
- Santucci, L.; Carol, E.; Kruse, E. Identification of palaeo-seawater intrusion in groundwater using minor ions in a semi-confined aquifer of the Rio de la Plata littoral (Argentina). Sci. Total Environ. 2016, 566, 1640–1648. [Google Scholar] [CrossRef]
- Panaskar, D.B.; Wagh, V.M.; Muley, A.A.; Mukate, S.V.; Pawar, R.S.; Aamalawar, M.L. Evaluating groundwater suitability for the domestic, irrigation, and industrial purposes in Nanded Tehsil, Maharashtra, India, using GIS and statistics. Arab. J. Geosci. 2016, 9, 615. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Li, X.; Qi, J.; Zhang, Q.; Guo, J.; Yu, L.; Zhao, R. Hydrochemical Characteristics and Multivariate Statistical Analysis of Natural Water System: A Case Study in Kangding County, Southwestern China. Water 2018, 10, 80. [Google Scholar] [CrossRef]
- Bam, E.K.P.; Bansah, S. Groundwater chemistry and isotopes reveal vulnerability of granitic aquifer in the White Volta River watershed, West Africa. Appl. Geochem. 2020, 119, 104662. [Google Scholar] [CrossRef]
- Ruiz-Pico, Á.; Pérez-Cuenca, Á.; Serrano-Agila, R.; Maza-Criollo, D.; Leiva-Piedra, J.; Salazar-Campos, J. Hydrochemical characterization of groundwater in the Loja Basin (Ecuador). Appl. Geochem. 2019, 104, 1–9. [Google Scholar] [CrossRef]
- Argamasilla, M.; Barberá, J.A.; Andreo, B. Factors controlling groundwater salinization and hydrogeochemical processes in coastal aquifers from southern Spain. Sci. Total Environ. 2017, 580, 50–68. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, R.H.; Lee, J.; Cheong, T.J.; Yum, B.W.; Chang, H.W. Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol. Process. 2005, 19, 1261–1276. [Google Scholar] [CrossRef]
- Luque-Espinar, J.A.; Chica-Olmo, M. Impacts of Anthropogenic Activities on Groundwater Quality in a Detritic Aquifer in SE Spain. Expo. Health 2020, 12, 681–698. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Wang, D.; Ren, X.; Wei, M. Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. 2020, 26, 1603–1621. [Google Scholar] [CrossRef]
- Barzegar, R.; Asghari Moghaddam, A.; Tziritis, E.; Adamowski, J.; Bou Nassar, J.; Noori, M.; Aalami, M.T.; Kazemian, N. Exploring the hydrogeochemical evolution of cold and thermal waters in the Sarein-Nir area, Iran using stable isotopes (δ18O and δD), geothermometry and multivariate statistical approaches. Geothermics 2020, 85, 101815. [Google Scholar] [CrossRef]
- Singh, C.K.; Kumar, A.; Shashtri, S.; Kumar, A.; Kumar, P.; Mallick, J. Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J. Geochem. Explor. 2017, 175, 59–71. [Google Scholar] [CrossRef]
- Kazakis, N.; Mattas, C.; Pavlou, A.; Patrikaki, O.; Voudouris, K. Multivariate statistical analysis for the assessment of groundwater quality under different hydrogeological regimes. Environ. Earth Sci. 2017, 76, 349. [Google Scholar] [CrossRef]
- Sappa, G.; Ergul, S.; Ferranti, F. Geochemical modeling and multivariate statistical evaluation of trace elements in arsenic contaminated groundwater systems of Viterbo Area, (Central Italy). SpringerPlus 2014, 3, 237. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Li, W.; Zhengwen, J.; Khan, U.; Wang, L.; Wang, F.-Y. Machine learning strategies for lithostratigraphic classification based on geochemical sampling data: A case study in area of Chahanwusu River, Qinghai Province, China. J. Cent. South. Univ. 2021, 28, 1422–1447. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Z.; Wang, G.; Yan, R.; Zhang, Z. Groundwater radon precursor anomalies identification by decision tree method. Appl. Geochem. 2020, 121, 104696. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Wang, G.; Ning, Z.; Lian, B.; Li, S.; Tsangaratos, P.; Ilia, I.; Xue, W. Optimizing Rotation Forest-Based Decision Tree Algorithms for Groundwater Potential Mapping. Water 2023, 15, 2287. [Google Scholar] [CrossRef]
- Duan, H.; Deng, Z.; Deng, F.; Wang, D. Assessment of Groundwater Potential Based on Multicriteria Decision Making Model and Decision Tree Algorithms. Math. Probl. Eng. 2016, 2016, 2064575. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Qian, C.; Zhu, G. Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl. Geochem. 2018, 98, 404–417. [Google Scholar] [CrossRef]
- Zhang, J. Applied Multivariate Statistical Analysis; Tongji University Press: Shanghai, China, 2016. [Google Scholar]
- Li, H. Statistical Learning Method; Tsinghua University Press: Beijing, China, 2011. [Google Scholar]
- Jabal, M.S.A.; Abustan, I.; Rozaimy, M.R.; Al-Najar, H. Fluoride enrichment in groundwater of semi-arid urban area: Khan Younis City, southern Gaza Strip (Palestine). J. Afr. Earth Sci. 2014, 100, 259–266. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey: Reston, VA, USA, 2013; pp. 2328–7055. [Google Scholar]
- GB/T 14848-2017; General Administration of Quality Supervision. Standard for Groundwater Quality (2017 Edition). Inspection and Quarantine of the People’s Republic of China and Standardization Administration: Beijing, China, 2017.
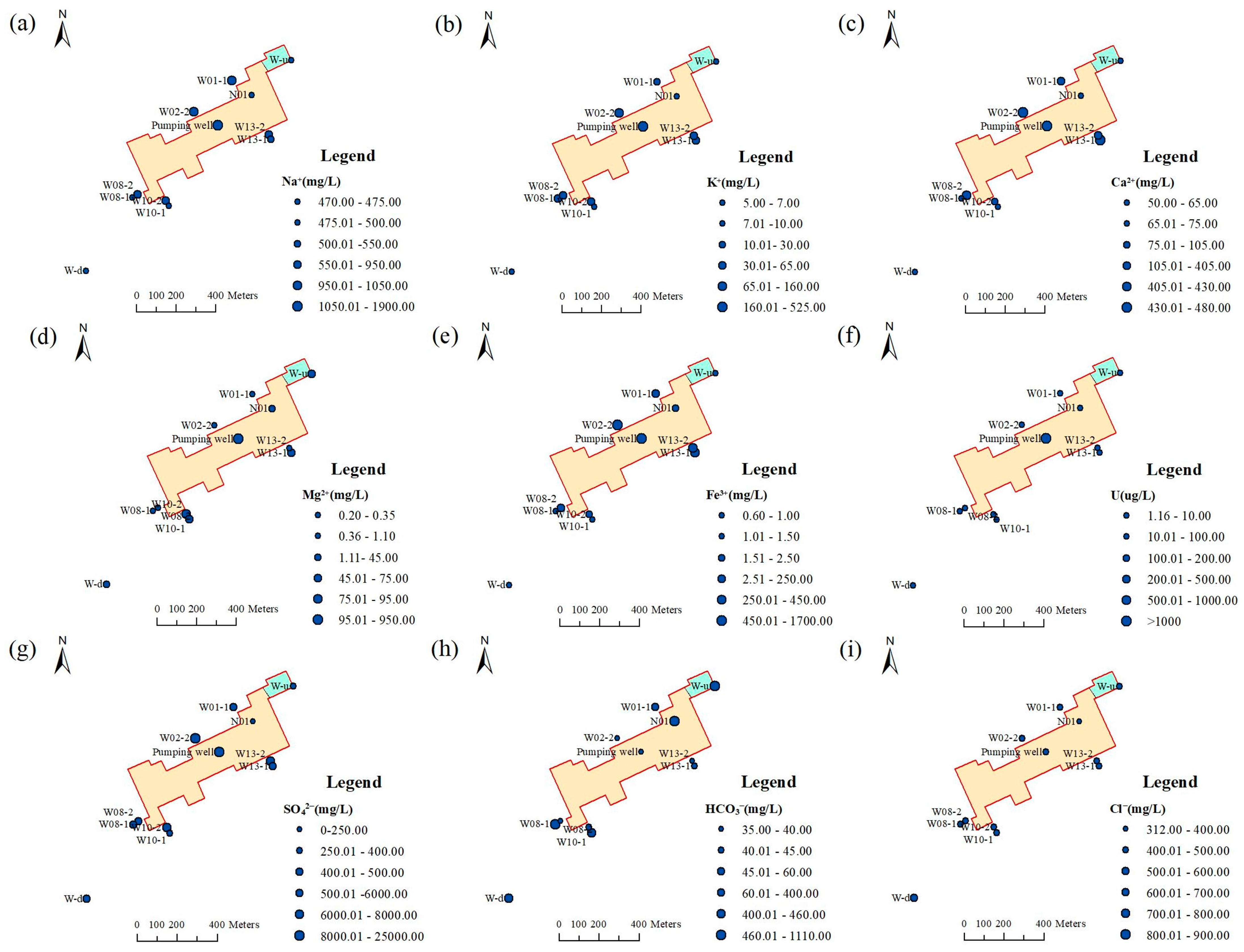
| Parameter | Max | Min | Average | Standard Deviation | Permissible Limits * |
|---|---|---|---|---|---|
| Total Fe | 1530 | 0.63 | 217.05 | 454.77 | 0.3 |
| Na+ | 1060 | 470 | 663.82 | 238.44 | 200 |
| K+ | 162 | 5.55 | 30.80 | 46.05 | / |
| Ca2+ | 472 | 53.2 | 204.84 | 177.84 | / |
| Mg2+ | 94.80 | 0.33 | 36.41 | 32.35 | / |
| U | 30.1 | 1.16 | 12.18 | 10.75 | / |
| SO42− | 18000 | 269 | 3556.82 | 5434.31 | 250 |
| HCO3− | 1110 | 35 | 358.27 | 322.95 | / |
| CI− | 536 | 312 | 440.91 | 55.76 | 250 |
| TDS | 25200 | 1340 | 6011.82 | 7258.67 | 1000 |
| Cluster | Sample | SI (Anhydrite) | SI (Calcite) | SI (Dolomite) | SI (Gypsum) |
|---|---|---|---|---|---|
| Class I | W-d | −1.73 | −1.45 | −3.02 | −1.22 |
| W-u | −0.14 | 3.1 | 5.26 | 0.33 | |
| W08-1 | −1.92 | 1.38 | 2.57 | −1.41 | |
| W10-1 | −1.91 | 1.38 | 2.54 | −1.4 | |
| W10-2 | −1.39 | 1.47 | 2.88 | −0.88 | |
| W13-1 | −1.45 | 1.54 | 2.76 | −0.96 | |
| N01 | −1.98 | 0.47 | 0.9 | −1.5 | |
| Class II | W01-1 | −0.39 | 0.45 | −1.83 | 0.11 |
| W02-2 | −0.2 | −0.48 | −3.5 | 0.3 | |
| W08-2 | −0.4 | 0.24 | −2.55 | 0.11 | |
| W13-2 | −0.36 | 0.01 | −2.67 | 0.13 |
| Cluster | Sample | SI (Fe(OH)3) | SI (Hematite) | SI (Goethite) | SI (Siderite) | SI (Melanterite) |
|---|---|---|---|---|---|---|
| Class I | W-d | −1.51 | 9.3 | 3.69 | −0.86 | −5.14 |
| W-u | 0.54 | 13.46 | 5.76 | −16.13 | −23.49 | |
| W08-1 | 0.99 | 14.28 | 6.18 | −11.07 | −18.37 | |
| W10-1 | 0.83 | 13.95 | 6.02 | −11.2 | −18.48 | |
| W10-2 | 2.79 | 17.9 | 7.99 | −8.18 | −15.05 | |
| W13-1 | 0.9 | 14.22 | 6.15 | −11.32 | −18.36 | |
| N01 | 0.58 | 13.7 | 5.88 | 1.08 | −5.45 | |
| Class II | W01-1 | 5.25 | 22.84 | 10.46 | 2.37 | −2.5 |
| W02-2 | 3.91 | 20.18 | 9.13 | 2.33 | −1.41 | |
| W08-2 | 4.29 | 20.86 | 9.47 | 2.22 | −2.42 | |
| W13-2 | 4.13 | 20.68 | 9.38 | 2.26 | −2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, M.; Jiao, T.; Xiang, D.; Yan, X.; Tang, Z.; Yang, J. Using Clustering, Geochemical Modeling, and a Decision Tree for the Hydrogeochemical Characterization of Groundwater in an In Situ Leaching Uranium Deposit in Bayan-Uul, Northern China. Water 2023, 15, 4234. https://doi.org/10.3390/w15244234
Li H, Liu M, Jiao T, Xiang D, Yan X, Tang Z, Yang J. Using Clustering, Geochemical Modeling, and a Decision Tree for the Hydrogeochemical Characterization of Groundwater in an In Situ Leaching Uranium Deposit in Bayan-Uul, Northern China. Water. 2023; 15(24):4234. https://doi.org/10.3390/w15244234
Chicago/Turabian StyleLi, Haibo, Mengqi Liu, Tian Jiao, Dongjin Xiang, Xiaofei Yan, Zhonghua Tang, and Jing Yang. 2023. "Using Clustering, Geochemical Modeling, and a Decision Tree for the Hydrogeochemical Characterization of Groundwater in an In Situ Leaching Uranium Deposit in Bayan-Uul, Northern China" Water 15, no. 24: 4234. https://doi.org/10.3390/w15244234




