Comparing Hydrogen Peroxide and Sodium Perborate Ultraviolet Advanced Oxidation Processes for 1,4-Dioxane Removal from Tertiary Wastewater Effluent
Abstract
:1. Introduction
- UV/NaBO3 vs. UV/H2O2 AOP for 1,4-dioxane breakdown—Does a UV/NaBO3 AOP perform equivalent to or better than the UV/H2O2 AOP for the removal of 1,4-dioxane from tertiary wastewater effluent?
- Acetic acid impacts of UV-AOPs on 1,4-dioxane—Does acetic acid improve performance of UV/H2O2 or UV/NaBO3 AOPs for the removal of 1,4-dioxane from tertiary wastewater effluent?
- UV/NaBO3 vs. UV/H2O2 AOP conceptual economic consideration—How do sodium perborate tetrahydrate and hydrogen peroxide compare in a conceptual economic evaluation?
2. Materials and Methods
2.1. Source Water
2.2. Reagent Preparation
2.3. Equipment Description
2.4. 1,4-Dioxane Removal Screening and Experimentation
2.5. Water Quality Analysis and Statistics
3. Results and Discussion
3.1. Initial Screening
3.2. Experimentation
3.3. Conceptual Economic Consideration
- The use of sodium perborate in solutions at various pH levels. Burgess and Hubbard [20] found that the ratio of perborate species to hydrogen peroxide is maximum at pH of approximately 10.1. At lower pH values, hydrogen peroxide is more prevalent in aqueous sodium perborate solution. At the pH range from 7 to 13, perborate species are more prevalent, and can speed up or inhibit oxidation effects depending on the water quality and specific perborate species [20]. Yuan, Zhai, Zhu, Liu, Jiao and Tang [23] tested the UV/NaBO3 AOP for the removal of humic acids in deionized water at a pH of 3, 7, and 11 and found that humic acid removal increased with decreasing pH. Similar studies at various pH levels could be conducted with different source waters and to target different contaminants to explore UV/NaBO3 AOP treatment effectiveness.
- The use of UV/NaBO3 perborate AOP to target other CECs. It is possible that the chemistry of this AOP is more effective than the UV/H2O2 AOP at removing specific contaminants that were not explored in this research study.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| μg/L | Micrograms per liter |
| ANOVA | Analysis of variance |
| AOP | Advanced oxidation process |
| C2H4O2 | Acetic acid |
| CITY | The City of Sarasota |
| cm | Centimeters |
| DO | Dissolved oxygen |
| DOC | Dissolved organic carbon |
| FL | Florida |
| gpm | Gallons per minute |
| H2O2 | Hydrogen peroxide |
| J | Joules |
| mg/L | milligrams per liter |
| NaBO3 | Sodium perborate |
| NELAC | National Laboratory Accreditation Conference |
| ng/L | Nanograms per liter |
| NH | New Hampshire |
| PA | Pennsylvania |
| QAQC | Quality assurance and quality control |
| SM | Standard Methods for the Examination of Water and Wastewater [33] |
| TDS | Total dissolved solids |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UCF | University of Central Florida |
| USA | United States of America |
| USEPA | United States’ Environmental Protection Agency |
| UV/H2O2 AOP | Ultraviolet and hydrogen peroxide advanced oxidation process |
| UV/NaBO3 AOP | Ultraviolet and sodium perborate advanced oxidation process |
| UV254 | Ultraviolet absorbance at 254 nanometers |
| UVA | Ultraviolet absorbance |
| UV-AOPs | Ultraviolet advanced oxidation processes |
| UVT | Ultraviolet transmittance |
| W | Watts |
References
- Tanabe, A.; Tsuchida, Y.; Ibaraki, T.; Kawata, K. Impact of 1,4-Dioxane from Domestic Effluent on the Agano and Shinano Rivers, Japan. Bull. Environ. Contam. Toxicol. 2006, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Smith, K.; Wang, Y.; Hu, H. Light-driven breakdown of 1,4-Dioxane for potable reuse: A review. Chem. Eng. J. 2019, 373, 508–518. [Google Scholar] [CrossRef]
- USEPA. Final Risk Evaluation for 1,4-Dioxane; EPA-740-R1-8007; Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2020.
- Abe, A. Distribution of 1,4-dioxane in relation to possible sources in the water environment. Sci. Total Environ. 1999, 227, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Lee, C.-S.; Asato, C.; Wang, M.; Mao, X.; Gobler, C.J.; Venkatesan, A.K. Removal of 1,4-dioxane during on-site wastewater treatment using nitrogen removing biofilters. Sci. Total Environ. 2021, 771, 144806. [Google Scholar] [CrossRef]
- USEPA. 1,4-Dioxane, CASRN 123-91-1; Integrated Risk Information System (IRIS); National Center for Environmental Assessment: Washington, DC, USA, 2013. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=326 (accessed on 11 January 2023).
- Florida Department of Health. 1,4-Dioxane—Technical Factsheet; Division of Disease Control and Health Protection: Tallahassee, FL, USA, 2021.
- USEPA. Technical Fact Sheet—1,4-Dioxane; EPA 505-F-17-011; Office of Land and Emergency Management: Washington, DC, USA, 2017; 5106p.
- California State Water Boards. Advanced Treatment Criteria; California Code of Regulations Section 60320.201(d)(1); California State Water Boards: Sacramento, CA, USA, 2018.
- Pollitt, K.J.G.; Kim, J.-H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C.; et al. 1,4-Dioxane as an emerging water contaminant: State of the science and evaluation of research needs. Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef]
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Occurrence and Treatment of 1,4-Dioxane in Aqueous Environments. Environ. Eng. Sci. 2003, 20, 423–432. [Google Scholar] [CrossRef]
- Higgins, C.J.; Duranceau, S.J. Modeling the mass transfer of 1,4-dioxane in a nanofiltration membrane process. Desalination Water Treat. 2020, 191, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Jain, T.; Ishida, K.; Liu, H. A mechanistic understanding of the degradation of trace organic contaminants by UV/hydrogen peroxide, UV/persulfate and UV/free chlorine for water reuse. Environ. Sci. Water Res. Technol. 2017, 3, 128–138. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Ulliman, S.L.; Miklos, D.B.; Hübner, U.; Drewes, J.E.; Linden, K.G. Improving UV/H2O2 performance following tertiary treatment of municipal wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1321–1330. [Google Scholar] [CrossRef]
- Kim, I.H.; Yamashita, N.; Kato, Y.; Tanaka, H. Discussion on the application of UV/H2O2, O3 and O3/UV processes as technologies for sewage reuse considering the removal of pharmaceuticals and personal care products. Water Sci. Technol. 2009, 59, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, H.R.; Brown, M.T.; Boyer, T.H. Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources. Chemosphere 2014, 105, 112–118. [Google Scholar] [CrossRef] [PubMed]
- McKillop, A.; Sanderson, W.R. Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis. Tetrahedron 1995, 51, 6145–6166. [Google Scholar] [CrossRef]
- Burgess, J.; Hubbard, C.D. Catalysis or Convenience? Perborate in Context. In Advances in Onorganic Chemistry; Homogeneous Catalysis; VanEldik, R., Hubbard, C.D., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2013; Volume 65, pp. 217–310. [Google Scholar]
- Tredwin, C.J.; Naik, S.; Lewis, N.J.; Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 2006, 200, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Gowdhamamoorthi, M.; Arun, A.; Kiruthika, S.; Muthukumaran, B. Perborate as novel fuel for enhanced performance of membraneless fuel cells. Ionics 2014, 20, 1723–1728. [Google Scholar] [CrossRef]
- Yuan, D.; Zhai, Z.; Zhu, E.; Liu, H.; Jiao, T.; Tang, S. Humic Acid Removal in Water via UV Activated Sodium Perborate Process. Coatings 2022, 12, 885. [Google Scholar] [CrossRef]
- Correa-Sanchez, S.; Peñuela, G.A. Peracetic acid-based advanced oxidation processes for the degradation of emerging pollutants: A critical review. J. Water Process. Eng. 2022, 49, 102986. [Google Scholar] [CrossRef]
- Stefan, M.I.; Bolton, J.R. Mechanism of the Degradation of 1,4-Dioxane in Dilute Aqueous Solution Using the UV/Hydrogen Peroxide Process. Environ. Sci. Technol. 1998, 32, 1588–1595. [Google Scholar] [CrossRef]
- Mill, T.; Gould, C.W. Free-radical oxidation of organic phosphonic acid salts in water using hydrogen peroxide, oxygen, and ultraviolet light. Environ. Sci. Technol. 1979, 13, 205–208. [Google Scholar] [CrossRef]
- Chitra, S.; Paramasivan, K.; Cheralathan, M.; Sinha, P.K. Degradation of 1,4-dioxane using advanced oxidation processes. Environ. Sci. Pollut. Res. 2012, 19, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, C.; Kamalam, R. Mechanism and reactivity in perborate oxidation of anilines in acetic acid. J. Chem. Soc. Perkin Trans. 2002, 2, 2011–2018. [Google Scholar] [CrossRef]
- Karunakaran, C.; Palanisamy, P. Autocatalysis in the sodium perborate oxidation of anilines in acetic acid–ethylene glycol. J. Mol. Catal. A Chem. 2001, 172, 9–17. [Google Scholar] [CrossRef]
- Gupton, J.T.; Duranceau, S.J.; Miller, J.F.; Kosiba, M.L. The Reaction of α-Methylstyrene Analogs and Related Compounds with Sodium Perborate in Acetic Acid. Synth. Commun. 1988, 18, 937–947. [Google Scholar] [CrossRef]
- Brandhuber, P.; Korshin, G.V. Methods for the Detection of Residual Concentrations of Hydrogen Peroxide in Advanced Oxidation Processes; WateReuse Foundation: Alexandria, VA, USA, 2009. [Google Scholar]
- Martin, K.; (Pur Representative, GHP Group, Guelph, ON, Canada). Personal communication, 2022.
- Baird, B.R.; Eaton, D.A.; Rice, W.E.; Bridgewater, L.L. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Shi, C.; Li, C.; Wang, Y.; Guo, J.; Barry, S.; Zhang, Y.; Marmier, N. Review of Advanced Oxidation Processes Based on Peracetic Acid for Organic Pollutants. Water 2022, 14, 2309. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Hao, J.; Liu, D. Preparation of peracetic acid from hydrogen peroxide, part II: Kinetics for spontaneous decomposition of peracetic acid in the liquid phase. J. Mol. Catal. A Chem. 2008, 284, 58–68. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Fedorov, K.; Rayaroth, M.P.; Shah, N.S.; Boczkaj, G. Activated sodium percarbonate-ozone (SPC/O3) hybrid hydrodynamic cavitation system for advanced oxidation processes (AOPs) of 1,4-dioxane in water. Chem. Eng. J. 2023, 456, 141027. [Google Scholar] [CrossRef]
- Sonawane, S.; Fedorov, K.; Rayaroth, M.P.; Boczkaj, G. Degradation of 1,4-dioxane by sono-activated persulfates for water and wastewater treatment applications. Water Resour. Ind. 2022, 28, 100183. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Shoup, T.M.; Goudgaon, N.M. Sodium perborate: A mild and convenient reagent for efficiently oxidizing organoboranes. J. Org. Chem. 1989, 54, 5930–5933. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef]
- Guhl, W.; Berends, A.G. Degradation of Sodium Perborate in Domestic Wastewater. Tenside Surfactants Deterg. 2001, 38, 98–102. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters; United States Department of Agriculture: Washington, DC, USA, 1955.
- Pesman, E.; Kalyoncu, E.E.; Kirci, H. Sodium Perborate Usage instead of Hydrogen Peroxide for the Reinforcement of Oxygen Delignification. Fibres Text. East. Eur. 2010, 18, 106–109. [Google Scholar]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Aguiar, J.B. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Rankin, S.; (Chief Operator at Lake Mary Water Treatment Plant, Lake Mary, FL, USA). Personal communication, 2022.
- City of Fort Lauderdale Hydrogen Peroxide & Odor Control Services (Re-Bid). Bid 12245-393. 2019. Available online: https://www.fortlauderdale.gov/Home/ShowDocument?id=36346 (accessed on 25 January 2023).
- Zhang, Z.; Chuang, Y.-H.; Szczuka, A.; Ishida, K.P.; Roback, S.; Plumlee, M.H.; Mitch, W.A. Pilot-scale evaluation of oxidant speciation, 1,4-dioxane degradation and disinfection byproduct formation during UV/hydrogen peroxide, UV/free chlorine and UV/chloramines advanced oxidation process treatment for potable reuse. Water Res. 2019, 164, 114939. [Google Scholar] [CrossRef]
- Gao, J.; Duan, X.; O’Shea, K.; Dionysiou, D.D. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion. Water Res. 2020, 171, 115394. [Google Scholar] [CrossRef]
- Hall, H.; (Sales Representative at Harcros Chemicals Inc., Tampa, FL, USA). Personal communication, 2022.
- Alioth Finance. Inflation Calculator. Available online: https://www.officialdata.org/ (accessed on 24 January 2023).
- U.S. Bureau of Labor Statistics. CPI Inflation Calculator. United States Department of Labor. Available online: https://www.bls.gov/data/inflation_calculator.htm (accessed on 24 January 2023).
- Exchange Rates UK—Euro to US Dollar Spot Exchange Rates for 2015. Available online: https://www.exchangerates.org.uk/EUR-USD-spot-exchange-rates-history-2015.html#:~:text=Average%20exchange%20rate%20in%202015%3A%201.11%20USD (accessed on 24 January 2023).
- Pennacchio, S.; (Sales Associate at Spectrum Chemical Manufacturing Corp., New Brunswick, NJ, USA). Personal communication, 2023.
- Nutting, E.B.; Poe, G.S. Chemical Bleaching of Discolored Endodontically Treated Teeth. Dent. Clin. N. Am. 1967, 11, 655–662. [Google Scholar] [CrossRef]
- Tran, L.; Orth, R.; Parashos, P.; Tao, Y.; Tee, C.W.; Thomas, V.T.; Towers, G.; Truong, D.T.; Vinen, C.; Reynolds, E.C. Depletion Rate of Hydrogen Peroxide from Sodium Perborate Bleaching Agent. J. Endod. 2017, 43, 472–476. [Google Scholar] [CrossRef]


| Parameter | 95% Confidence Interval |
|---|---|
| pH | 7.72 ± 0.076 |
| Temperature (°C) | 19.7 ± 0.61 |
| Conductivity (μS/cm) | 1330 ± 32 |
| ORP (mV) | 223 ± 21 |
| Turbidity (NTU) | 0.69 ± 0.03 |
| Total Chlorine (mg/L) | 0.040 ± 0.0084 |
| Monochloramine (mg/L) | 0.090 ± 0.0083 |
| Free Ammonia (mg/L) | 0.040 ± 0.014 |
| Dissolved Oxygen (mg/L) | 8.05 ± 0.41 |
| TOC (mg/L) | 4.16 ± 0.33 |
| UVA at 254 nm (cm−1) | 0.249 ± 0.014 |
| UVT at 254 nm (%) | 54.7 ± 1.9 |
| Alkalinity (mg/L as CaCO3) | 159 ± 6.8 |
| TDS (mg/L) | 781 ± 27 |
| TSS (mg/L) | 0.73 ± 0.21 |
| Calcium (mg/L) | 59.4 ± 3.1 |
| Magnesium (mg/L) | 23.8 ± 0.41 |
| Sodium (mg/L) | 158 ± 6.8 |
| Potassium (mg/L) | 14.9 ± 0.69 |
| Strontium (mg/L) | 3.17 ± 0.14 |
| Silica (mg/L) | 13.1 ± 1.0 |
| Iron (mg/L) | 0.030 ± 0.0022 |
| Manganese (mg/L) | 0.020 ± 0.0025 |
| Boron (mg/L) | 0.250 ± 0.011 |
| Chloride (mg/L) | 188 ± 8.2 |
| Bromide (mg/L) | 0.340 ± 0.039 |
| Sulfate (mg/L) | 187 ± 6.3 |
| Nitrate (mg/L) | 1.58 ± 0.60 |
| Phosphate (mg/L) | <0.30 |
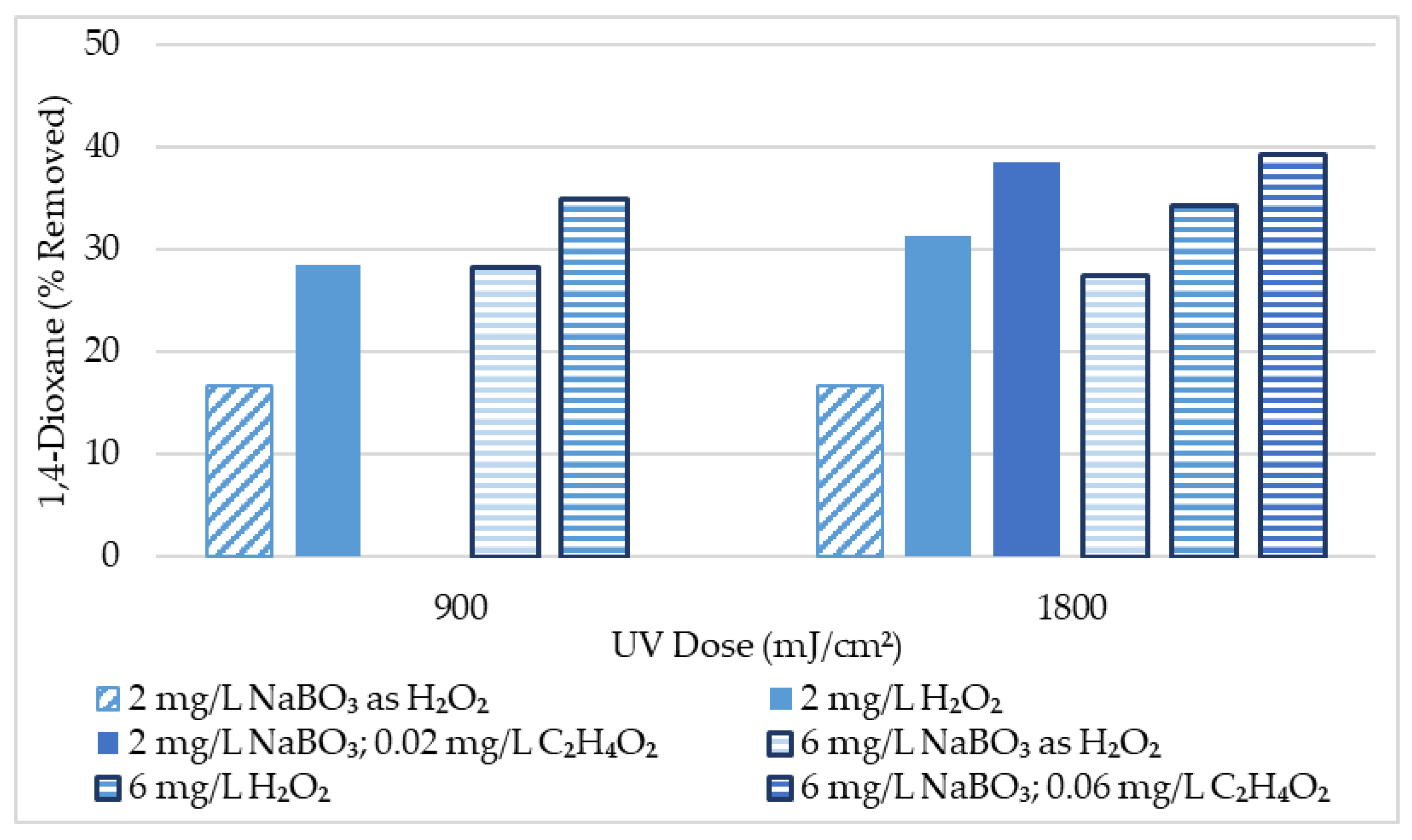
| Oxidant and Dose | 1,4-Dioxane Removal with UV Dose (μg/L) | 1,4-Dioxane Removal with UV Dose (%) | ||
|---|---|---|---|---|
| 900 (mJ/cm2) | 1800 (mJ/cm2) | 900 (mJ/cm2) | 1800 (mJ/cm2) | |
| UV Performance Baseline | NA | 1.0 | NA | 8.3 |
| 2 mg/L NaBO3 as H₂O₂ | 2.0 | 2.0 | 16.7 | 16.7 |
| 6 mg/L NaBO3 as H₂O₂ | 3.4 | 3.3 | 28.3 | 27.5 |
| 2 mg/L H₂O₂ | 4.0 | 4.4 | 28.6 | 31.4 |
| 6 mg/L H₂O₂ | 4.9 | 4.8 | 35.0 | 34.3 |
| 2 mg/L NaBO3; 0.02 mg/L C2H4O2 | NA | 5.0 | NA | 38.5 |
| 6 mg/L NaBO3; 0.06 mg/L C2H4O2 | NA | 5.1 | NA | 39.2 |
| Experiment ID | 1,4-Dioxane Removal with 1800 mJ/cm2 UV Dose (%) | |
|---|---|---|
| Range (min.–max.) | 95% Confidence Interval | |
| 1800 mJ/cm2 UV Baseline | 22.0–28.8 | 24.7 ± 3.30 |
| 1 mg/L NaBO3 with C2H4O2 | 23.5–31.5 | 28.1 ± 2.39 |
| 1 mg/L H2O2 with C2H4O2 | 26.8–31.3 | 29.1 ± 2.06 |
| 2 mg/L NaBO3 | 23.5–37.0 | 30.3 ± 4.66 |
| 2 mg/L NaBO3 with C2H4O2 | 28.0–37.0 | 32.1 ± 3.49 |
| 2 mg/L H2O2 | 17.9–31.9 | 24.9 ± 6.51 |
| 2 mg/L H2O2 with C2H4O2 | 26.8–33.9 | 30.6 ± 2.00 |
| 2 mg/L C2H4O2 | 10.7–27.8 | 19.1 ± 7.89 |
| 6 mg/L NaBO3 | 42.5–45.2 | 43.9 ± 0.96 |
| 6 mg/L NaBO3 | 35.7–50.0 | 42.8 ± 5.87 |
| 6 mg/L C2H4O2 | 17.9–25.0 | 20.3 ± 3.76 |
| Oxidant | Source | Cited Cost | Calculated Cost for Dose as H2O2 ($/day) | ||
|---|---|---|---|---|---|
| Value | Year 1 | 2 mg/L | 6 mg/L | ||
| H2O2 | [44] | $0.35/kg 50% H2O2 | 2006 | 48.30 | 144.00 |
| [45] | 0.98 euros/kg 2 (assumed 3 50% H2O2) | 2015 | 128.11 | 384.36 | |
| [46] | $0.50/lb 50% H2O2 | 2022 | 112.92 | 338.74 | |
| [47] | $0.1971/lb for 50% standard grade H2O2 delivered | 2019 | 51.14 | 153.42 | |
| [48] | $2.75/gal 50% H2O2 | 2018 | 64.86 | 194.59 | |
| [49] | $1100/metric ton (assumed 3 50% H2O2) | 2020 | 118.66 | 355.97 | |
| 95% Confidence Interval: | 87.33 ± 26.61 | 261.85 ± 79.98 | |||
| NaBO3 | [44] | $0.42/kg | 2006 | 129.95 | 389.83 |
| [45] | 1.5 euros/kg 2 | 2015 | 436.67 | 1310.00 | |
| [50] | $0.92/lb | 2022 | 426.88 | 1280.60 | |
| 95% Confidence Interval: | 331.17 ± 161.07 | 993.48 ± 483.20 | |||
| Source | Calculated Cost for 6 mg/L Dose as H2O2 Using Different Oxidants ($/day) | Cost Ratio (NaBO3/H2O2) | |
|---|---|---|---|
| NaBO3 | H2O2 | ||
| [44] | 389.83 | 144.00 | 2.7 |
| [45] | 1310.00 | 384.36 | 3.4 |
| [54] | 7319.18 1 | 1919.78 1 | 3.8 |
| 95% confidence interval of the cost ratio of NaBO3/H2O2: | 3.3 ± 0.52 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, T.L.; Duranceau, S.J. Comparing Hydrogen Peroxide and Sodium Perborate Ultraviolet Advanced Oxidation Processes for 1,4-Dioxane Removal from Tertiary Wastewater Effluent. Water 2023, 15, 1364. https://doi.org/10.3390/w15071364
Shukla TL, Duranceau SJ. Comparing Hydrogen Peroxide and Sodium Perborate Ultraviolet Advanced Oxidation Processes for 1,4-Dioxane Removal from Tertiary Wastewater Effluent. Water. 2023; 15(7):1364. https://doi.org/10.3390/w15071364
Chicago/Turabian StyleShukla, Tulsi L., and Steven J. Duranceau. 2023. "Comparing Hydrogen Peroxide and Sodium Perborate Ultraviolet Advanced Oxidation Processes for 1,4-Dioxane Removal from Tertiary Wastewater Effluent" Water 15, no. 7: 1364. https://doi.org/10.3390/w15071364