Abstract
Ultraviolet advanced oxidation processes (UV-AOPs) were compared using sodium perborate (UV/NaBO3 AOP) or hydrogen peroxide (UV/H2O2 AOP) for 1,4-dioxane removal from tertiary wastewater effluent. Both UV-AOPs were also tested with the addition of acetic acid. Results revealed that sodium perborate performed similarly to hydrogen peroxide. The UV/NaBO3 AOP with 6 milligrams per liter (mg/L) as H2O2 resulted in 43.9 percent 1,4-dioxane removal, while an equivalent UV/H2O2 AOP showed 42.8 percent removal. Despite their similar performance, NaBO3 is approximately 3.3 times more expensive than H2O2. However, the solid form of NaBO3 can provide a major benefit to remote and mobile operations. Unlike H2O2 solution, which degrades over time and requires repeated costly shipments, NaBO3 is a convenient source of H2O2, and a long-term supply can be shipped at once and mixed into solution as needed. The addition of acetic acid to a UV/H2O2 AOP was found to enhance 1,4-dioxane removal, increasing treatment effectiveness by 5.7%.
Keywords:
1,4-dioxane; ultraviolet; advanced oxidation; perborate; peroxide; acetic acid; cost; wastewater; water reuse 1. Introduction
Use of reclaimed water is becoming increasingly common in the United States of America (USA) as communities realize the pertinence of conserving and protecting water supplies. Water reclamation and reuse are currently not regulated on a federal level in the USA. Thus, existing reclaimed water regulations and guidelines vary by each state, intended reclaimed water use, and impacts on potable water supplies. With the likelihood of reclaimed water having potable quality equivalent criteria under certain circumstances, the need for research on enhanced wastewater treatment for reclamation purposes is important.
One parameter of emerging concern in reclaimed water use is 1,4-dioxane. 1,4-Dioxane is a heterocyclic organic used as an industrial stabilizer for chlorinated solvents and can be found in textiles, cosmetics, adhesives, spray polyurethane foam, and dyes [1,2,3]. 1,4-Dioxane can also be found in groundwater, surface waters, and wastewaters [4]. Detection of 1,4-dioxane in wastewater is not well documented, but published values range from undetected to 97 μg/L [1,4,5,6]. The United States’ Environmental Protection Agency (USEPA) classifies 1,4-dioxane as “likely to be carcinogenic to humans by all routes of exposure” and although is unregulated on a national level, some guidelines have been established [7]. While the USEPA has a health advisory level of 0.35 μg/L for potable water, some states have lower guidelines: for example, the state of California has a notification level of 1 ng/L issued for 1,4-dioxane in potable reuse applications [8,9]. The state of California also lists 0.5-log removal of 1,4-dioxane as a performance metric for potable reuse treatment trains, including groundwater recharge with reclaimed water [10].
Treatment techniques considered for 1,4-dioxane removal from municipal water and wastewater streams generally includes adsorption onto granular activated carbon media, advanced oxidation processes, and in some cases, bioremediation through metabolic and co-metabolic pathways [6,11,12]. Membrane treatment using nanofiltration and reverse osmosis membranes has also been explored and met with moderate success [11,13], however, issues related to concentrate disposal persist. Advanced oxidation processes can break down 1,4-dioxane; if 1,4-dioxane is fully degraded, the end products are carbon dioxide and water. Therefore, advanced oxidation processes that degrade 1,4-dioxane are of particular interest.
Ultraviolet and hydrogen peroxide (UV/H2O2) based advanced oxidation processes (AOPs) have been extensively studied in literature for water reclamation and reuse applications [14,15,16]. UV/H2O2 can effectively degrade a variety of trace organic contaminants [14,17] and natural organic matter (NOM) [18], with effectiveness depending on water quality, UV strength, and hydrogen peroxide dose. Despite benefits of UV/H2O2 processes for water reclamation and reuse, alternative sources of hydrogen peroxide have not been thoroughly investigated. Two such chemicals that have been cited in literature for alternative use in AOPs are sodium percarbonate and far less commonly, sodium perborate. Both sodium percarbonate and sodium perborate are crystalline solids that form hydrogen peroxide when added to water. While sodium percarbonate has been documented in literature to provide benefit to UV-AOPs, sodium perborate offers a unique advantage with intermediate peroxoborate species adding to the reactivity in some cases [19,20]. Sodium perborate’s documented uses range from laundry stain and tooth bleaching to use in membrane-less fuel cells [19,21,22]. Its wide range of applications can be attributed to the chemical’s release of H2O2 upon reacting with water, ease of availability, non-toxic nature, and convenient option for transporting and storing H2O2 sources [19,20].
Use of sodium perborate in an ultraviolet advanced oxidation process (UV/NaBO3 AOP) for water or wastewater treatment is rarely found in literature. Sindelar, Brown and Boyer [18] showed that it is feasible to reduce NOM concentrations in surface/storm water using UV-AOP with hydrogen peroxide, sodium perborate, or sodium percarbonate. Despite successful reduction of dissolved organic carbon (DOC) and UV absorbance at 254 nm (UV254), Sindelar, Brown and Boyer [18] found that the three chemical UV-AOP options were not economically feasible for surface water treatment due to the high NOM content (DOC of 26.4 to 36.2 mg/L), and a high UV fluence (21.8–26.1 J/cm2) and oxidant dose (100 mg/L as H2O2) required for degradation. Yuan, et al. [23] similarly studied a UV/NaBO3 advanced oxidation process for its impacts on organic matter. Yuan, Zhai, Zhu, Liu, Jiao and Tang [23] reported 88.8% removal of humic acids from synthetic water at pH 3 using 1 mmol/L sodium perborate (100 mg/L) with 60 min of exposure time to a UV lamp with fluence of 35.2 mJ/cm2. An analogous experiment with an equivalent molecular weight of hydrogen peroxide in place of the sodium perborate resulted in 40.2% removal of humic acids. Overall, literature agrees that the UV/NaBO3 AOP can degrade organic compounds.
Acetic acid is another potential chemical of interest for use in UV-AOP applications. Acetic acid (CH3C(=O)OH, or C2H4O2) is typically present in discussions of advanced oxidation studies in one of two contexts: (1) as a compound used to react with hydrogen peroxide to generate peracetic acid (CH3C(=O)O2H) for use in the AOP, or (2) as an organic byproduct of AOPs, generally discussed along with other byproducts such as formic and oxalic acids. In the case of peracetic acid use in AOPs, the radicals most prevalent are the hydroxyl radical (•OH), the acetoxyl radical CH3C(=O)O•, and the acetyl peroxyl radical CH3C(=O)OO• [24]. Each of the three radicals have been shown to contribute to the oxidation of contaminants of emerging concern [24]. This includes the acetoxyl radical, which can be formed from acetic acid. However, the specific impact of the acetic acid concentration in the peracetic acid mixture is not clear [24]. It appears that acetic acid additions to UV-AOPs without the pre-formation of peracetic acid has not been explored.
Another appearance of acetic acid in advanced oxidation literature is as an intermediate or byproduct. The generation of acetic acid as a byproduct of the UV/H2O2 AOP was discovered by Stefan and Bolton [25] while investigating the mechanisms of 1,4-dioxane degradation. Final intermediates of the hydrogen peroxide degradation of 1,4-dioxane include glycolic, acetic, and oxalic acids. The degradation mechanisms of acetic acid in a UV/H2O2 AOP have been proposed in literature [25,26]. Acetic acid is often seen as an unwanted byproduct of UV/H2O2 AOPs [27]. Contrarily, acetic acid as a supplement to some UV-AOPs may offer benefits that have not yet been probed.
The use of acetic acid in combination with sodium perborate is also not commonly studied. The synthesis of peracetic acid with NaBO3 as a hydrogen peroxide source has been evaluated but with regards to simple oxidation rather than for advanced oxidation [28,29]. McKillop and Sanderson [19] discussed the use of acetic acid with sodium perborate and sodium percarbonate as alternative hydrogen peroxide sources in non-aqueous chemistry but did not delve into advanced oxidation applications. However, Karunakaran and Kamalam [28] noted that sodium percarbonate does not form peracetic acid when mixed with acetic acid, thus furthering interest in sodium perborate. Burgess and Hubbard [20] explained that the mixture of sodium perborate and acetic acid results in species with greater oxidative power than either sodium perborate or acetic acid alone. Furthermore, Burgess and Hubbard [20] point out that sodium perborate and acetic acid chemistry is complex and involves intermediates that can react with organic substrates. Non-aqueous chemistry applications with sodium perborate and acetic acid has been explored, such as by Gupton, et al. [30], yet UV-AOPs with the aforementioned chemicals remain to be investigated.
In this research, an advanced oxidation process with sodium perborate with ultraviolet light, which has rarely been documented in literature, was assessed and compared to the advanced oxidation process with hydrogen peroxide and ultraviolet light. Both UV/H2O2 and UV/NaBO3 AOPs were tested with and without the addition of acetic acid, which is also not well-documented in literature. The UV-AOPs were investigated for 1,4-dioxane removal from a tertiary wastewater effluent to address the three main research questions:
- UV/NaBO3 vs. UV/H2O2 AOP for 1,4-dioxane breakdown—Does a UV/NaBO3 AOP perform equivalent to or better than the UV/H2O2 AOP for the removal of 1,4-dioxane from tertiary wastewater effluent?
- Acetic acid impacts of UV-AOPs on 1,4-dioxane—Does acetic acid improve performance of UV/H2O2 or UV/NaBO3 AOPs for the removal of 1,4-dioxane from tertiary wastewater effluent?
- UV/NaBO3 vs. UV/H2O2 AOP conceptual economic consideration—How do sodium perborate tetrahydrate and hydrogen peroxide compare in a conceptual economic evaluation?
2. Materials and Methods
2.1. Source Water
The source water for the AOP investigation was collected from the tertiary effluent from the City of Sarasota’s (Sarasota, FL, USA) municipal wastewater treatment plant (WWTP). The City of Sarasota (CITY) owns and operates an advanced wastewater treatment plant that utilizes a five-stage Bardenpho® process to produce high-quality reclaimed water. The wastewater passes through headworks to remove large debris, then flows into an equalization basin prior to the five-stage Bardenpho® process. After treatment with the Bardenpho® process, wastewater effluent enters a rapid mix integrated flocculation basin followed by automatic backwash cloth filters prior to final chlorination. Air stripping and dechlorination are also employed depending on the disposal method. The high-quality effluent produced after final chlorination is referred to as final reuse or reclaimed water. Reclaimed water is used for residential and agricultural irrigation, otherwise sent to a deep injection well or surface water discharge as needed for back-up. For the work presented herein, high-quality tertiary wastewater effluent was sampled from downstream of the cloth filters, prior to final chlorination. Tertiary wastewater effluent was transported to the University of Central Florida (UCF) environmental engineering laboratories in 15-gallon drums; tertiary effluent water quality is summarized in Table 1.

Table 1.
Tertiary Effluent Water Quality.
2.2. Reagent Preparation
Prior to each experiment, solutions were prepared for 1,4-dioxane, hydrogen peroxide, sodium perborate tetrahydrate, and acetic acid. 1,4-Dioxane was prepared using serial dilutions: 99.8% 1,4-dioxane (Sigma Aldrich, St. Louis, MO, USA) was first diluted to a 1% solution, then further diluted to 0.04%. 1,4-Dioxane was diluted and spiked one day prior to each experiment. For the acetic acid solution, 0.4 mL of glacial acetic acid (Fisher Scientific, Hampton, NH, USA) was added to a 100 mL volumetric flask with deionized water to approximately a 3980 mg/L solution. The stock solution was created the day before each experiment and was stored in a sealed glass bottle in the dark until use. Calculations for acetic acid strengths are based on the conservative assumption that the glacial acetic acid purity by weight was 95%, though reported by the manufacturer as >95%.
For the hydrogen peroxide solution, 35% hydrogen peroxide (Brenntag Mid-South, Inc., West Henderson, KY, USA) was diluted into an approximately 1000 mg/L solution with deionized water and stored in a sealed amber glass bottle covered with aluminum foil to prevent impacts from light. Sodium perborate tetrahydrate (Acros Organics, now part of Thermo Fisher Scientific, Pittsburgh, PA, USA) was used to make NaBO3 solutions. The sodium perborate tetrahydrate granules were dissolved in deionized water in a 250 mL volumetric flask for a solution of 5 g/L. A magnetic stir bar and stir plate set to a medium-low heat setting were utilized to aid in the dissolving process. The solution temperature remained below 60 °C. The perborate solution was made within the week prior to experimentation and was stored in a sealed amber glass bottle covered with aluminum foil to prevent impacts from light.
The hydrogen peroxide content of both the diluted hydrogen peroxide solution and the sodium perborate solution was by acidifying and titrating with potassium permanganate solution until a faint pink color remained in the peroxide solution [31]. Hydrogen peroxide and sodium perborate solution strengths were also checked with the Hach Company Hydrogen Peroxide Test Kit, Model HYP-1 (Hach Company, Loveland, CO, USA). Average values from both solution strength tests were used for experimental dose calculations. It was determined that each 1.0 mg/L sodium perborate solution offered 0.23 mg/L H2O2.
2.3. Equipment Description
The bench-scale UV pilot that was assembled for this work is shown in Figure 1a. The UV pilot consisted of a 5-gallon high density polyethylene storage tank, magnetic drive pump, flowmeter, pressure gauge, and ultraviolet irradiation system. The UV-AOP treatment process utilized three low-pressure high output amalgam UV lamps operated in series at constant output. The first UV lamp in series was a Vitapur VUV-S375B (Vitapur, El Paso, TX, USA), which is a 17 watt (W) lamp that can provide 30,000 μW·sec/cm2 exposure at a flow rate of 7 gallons per minute (gpm). The second and third UV lamps in series were Pur PUV15H (Pur, El Paso, TX, USA) units, which are 40 W lamps that deliver approximately twice the UV dose as the Vitapur VUV-S375B. With the combined three lamps using 2.5 gallons at a flow rate of 2.5 gpm with a water with UV transmittance of approximately 60%, a UV dose of 1800 mJ/cm2 was achieved in approximately 7.2 min. The UV exposure time required was estimated using the manufacturer value provided by their technical division [32]. The relatively short recirculation time through the UV pilot to meet the desired UV dose allowed the study to provide more realistic results compared to bench-scale systems that require longer contact times. For example, the graph in Figure 1b suggests that the use of the single Vitapur lamp would require recirculation for approximately 36 min to achieve the same UV dose, water quality, and experimental conditions.

Figure 1.
Bench-scale UV pilot.
The UV lamp quartz sleeves were inspected periodically and cleaned with either deionized water and Kimwipes or, if needed, with dilute hydrochloric acid followed by a DI rinse. Quartz sleeves were allowed to dry prior to re-installing in the UV system.
2.4. 1,4-Dioxane Removal Screening and Experimentation
Wastewater plants typically see 50 μg/L or less of 1,4-dioxane, and more commonly 10 μg/L or less [1,4,5,6]. As such, initial spikes of 10 μg/L were tested in an initial screening with UV/H2O2, UV/NaBO3, or UV/NaBO3 with acetic acid AOPs. Two UV doses were tested (900 and 1800 mJ/cm2) along with 2 or 6 mg/L of hydrogen peroxide or an equivalent dose of sodium perborate. A small amount (0.02 or 0.06 mg/L acetic acid) was added to the UV/NaBO3 AOPs to screen for impacts of acetic acid on UV-AOPs. The two UV doses were also tested as standalone UV treatment.
Further experimentation was conducted with initial 1,4-dioxane spikes between 50 and 170 μg/L. Experimental 1,4-dioxane concentrations in the drum varied from 50 to 170 μg/L to (1) represent a high-level contamination event, and (2) to aim for statistical significance in percent removal evaluations and comparisons. The higher-level 1,4-dioxane experimentation tested at a UV dose of 1800 mJ/cm2 with 2 or 6 mg/L of H2O2 or an equivalent dose of NaBO3. UV/H2O2 and UV/NaBO3 AOPs were tested with acetic acid supplementation at a low level (up to 0.06 mg/L) and a higher level comparable to H2O2 and NaBO3 doses (up to 6 mg/L). The same acetic acid doses were tested with UV instead of as a supplement to another UV-AOP, and UV was tested as a standalone treatment at 1800 mJ/cm2. An experimental outline is included in the supplementary materials. UV/H2O2, UV/NaBO3, and standalone UV treatment were evaluated in triplicate. Experiments involving acetic acid at the higher level (up to 6.0 mg/L) were evaluated in duplicate. Select 1,4-dioxane samples were also evaluated in replicate.
Tertiary effluent was collected from the post-filter sampling point and stored in 15-gallon low-density polyethylene (LDPE), opaque drums at 0 °C until use in the 1,4-dioxane removal experiments. The day before each experiment day, the drum was removed from cold storage, measured to a predetermined volume (generally 15 gallons plus three liters), and dosed with 1,4-dioxane. The drum was then closed tightly, mixed well, and allowed to continue warming up to room temperature overnight. The next morning, the drum was mixed well again, and then samples were collected for water quality analysis. Meanwhile, the UV lamps were allowed to warm up for 20 min prior to use and left on until the end of the experiments conducted that day.
The UV pilot was flushed with deionized water prior to the start of experiments. Each experiment required 2.5 gallons of sample. The drum was mixed prior to transferring each set of 2.5 gallons of sample into the UV pilot’s storage tank. An UV dose of either 900 mJ/cm2 or 1800 mJ/cm2 was used in conjunction with the predetermined dose of H2O2, NaBO3, C2H4O2, or a combination of the oxidants. The oxidant was dosed into the storage tank in the UV bench-scale pilot and allowed to stir with a magnetic stir bar for 30 seconds prior to opening the valve to allow the wastewater effluent to fully enter the UV bench-scale pilot, and subsequently starting the pump. For experiments with acetic acid supplementing UV/H2O2 or UV/NaBO3 AOPs, acetic acid was added to the UV pilot’s storage tank allowed to mix well for 30 seconds prior to adding H2O2 or NaBO3. To end each experiment, the pump was stopped, the sample water was given approximately 30 seconds to accumulate in the storage tank and mix well. Samples were then collected, and the UV bench-scale pilot was flushed with deionized water between experiments.
2.5. Water Quality Analysis and Statistics
1,4-Dioxane was analyzed at an external, National Laboratory Accreditation Conference (NELAC) certified laboratory with method SW-846 8260B with a detection limit of 1.0 μg/L. Several other parameters were monitored prior to each experiment to document variations in wastewater effluent that may impact UV-AOP performance. This includes, but was not limited to, general water quality (pH, temperature, conductivity, oxidative reduction potential (ORP), dissolved oxygen (DO), and alkalinity), select anions and cations, turbidity, UV transmittance at 254 nm (UVT254), UV absorbance at 254 nm (UV254), total organic carbon (TOC), total dissolved solids (TDS), and total suspended solids (TSS). Samples were analyzed at UCF laboratories in accordance with Standard Methods for the Examination of Water and Wastewater (SM), as applicable [33]. Total organic carbon (TOC) samples were analyzed with SM 5130C with a detection limit of 0.25 mg/L using a Teledyne Tekmar TOC Fusion UV/Persulfate Analyzer (Teledyne Tekmar, Mason, OH, USA). Turbidity was analyzed using SM 2130B using the Hach 2100N; TDS and TSS were quantified with SM 2540C and 2540D, respectively; select cations were measured via SM 3120B with a Perkin Elmer Avio 200 Inductively Coupled Plasma Spectrometer (Perkin Elmer, Waltham, MA, USA); select anions were quantified with SM 4110B with a Dioxex ICS-1100 (Dionex, Sunnyvale, CA, USA); UV transmittance and absorbance were read with a Hach DR6000; and total chlorine was monitored Hach Method 8167 with a Hach DR900. Statistics tools implemented from Microsoft® Excel® include means, standard deviation, 95% confidence intervals (assuming normal distributions), analysis of variance (ANOVA) tests, and t-tests. Further information regarding water quality analysis and statistics is included in the supplementary materials.
3. Results and Discussion
3.1. Initial Screening
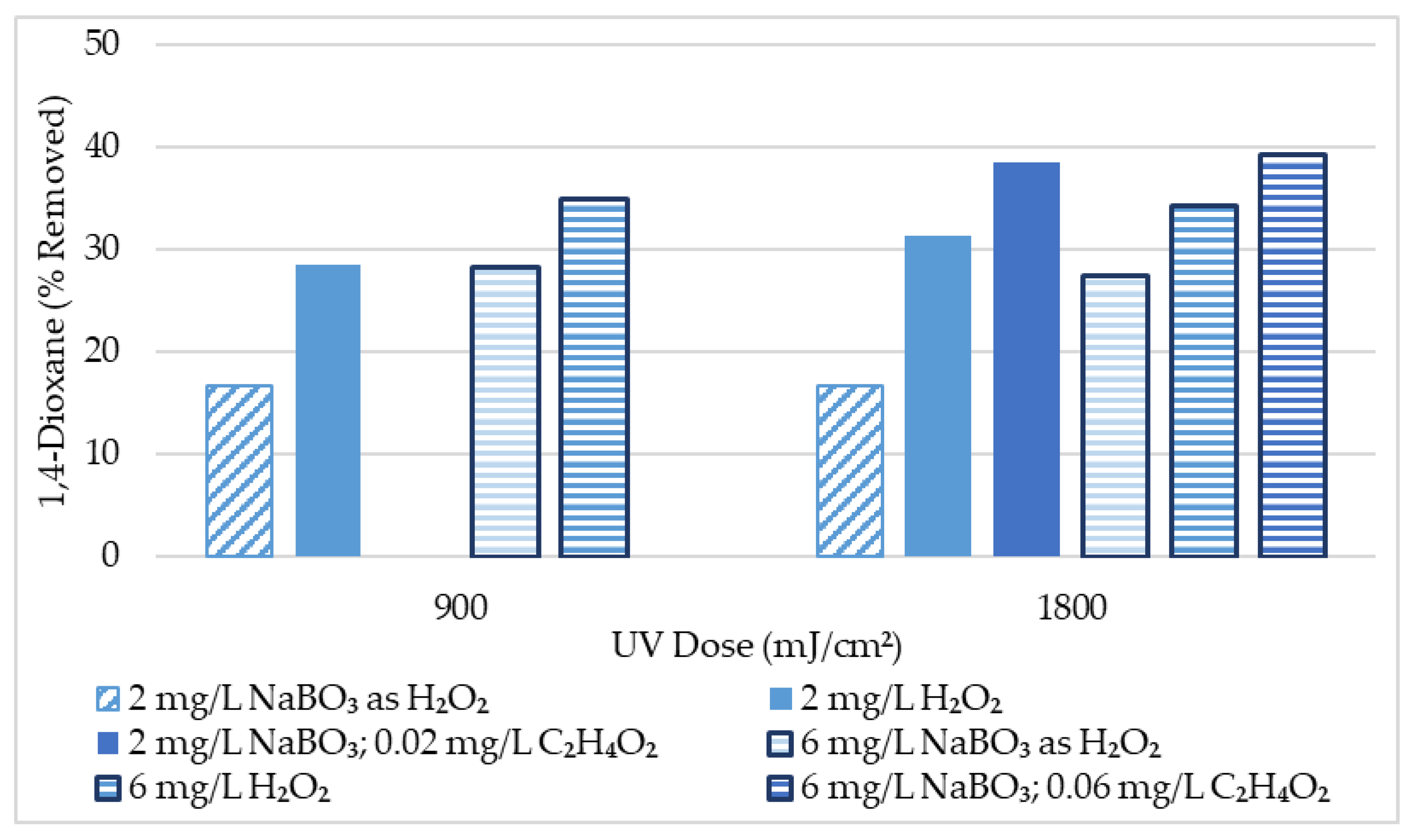
An initial screening was conducted to screen for 1,4-dioxane removal from tertiary wastewater effluent with approximately 10 μg/L initial 1,4-dioxane at various experimental conditions. Results of this initial screening are summarized in Table 2 and Figure 2. Results suggest that UV-AOP performed better for 1,4-dioxane removal when H2O2 is the oxidant rather than sodium perborate alone. However, the addition of acetic acid to the UV/NaBO3 AOP allowed for greater removal of 1,4-dioxane than the UV/H2O2 AOP could accomplish. This data indicated that the UV/NaBO3 AOP is promising, and the addition of even a small amount of acetic acid (≤0.06 mg/L) may improve the AOP’s ability to break down 1,4-dioxane. Nevertheless, lower-level results such as these should be interpreted with caution. Removal of just 1 μg/L of 1,4-dioxane indicates 10% removal from an initial concentration of 10 μg/L. Scrutiny of quality assurance and quality control (QAQC), included in the supplementary materials, reveals up to 4 μg/L difference between replicate 1,4-dioxane samples. Analytical results for the 1,4-dioxane samples in this research conform to the most current NELAC standards, where applicable. Even so, an uncertainty of up to 4 μg/L is increasingly significant with the lower-level samples in this initial screening. Therefore, further experimentation was performed with higher 1,4-dioxane initial concentrations.

Table 2.
1,4-Dioxane Initial Screening Results Summary (10 μg/L initial 1,4-dioxane conc.).
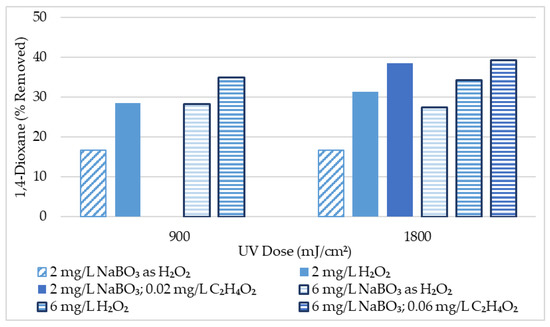
Figure 2.
1,4-Dioxane Initial Screening Results Summary (10 μg/L initial 1,4-dioxane conc.).
3.2. Experimentation
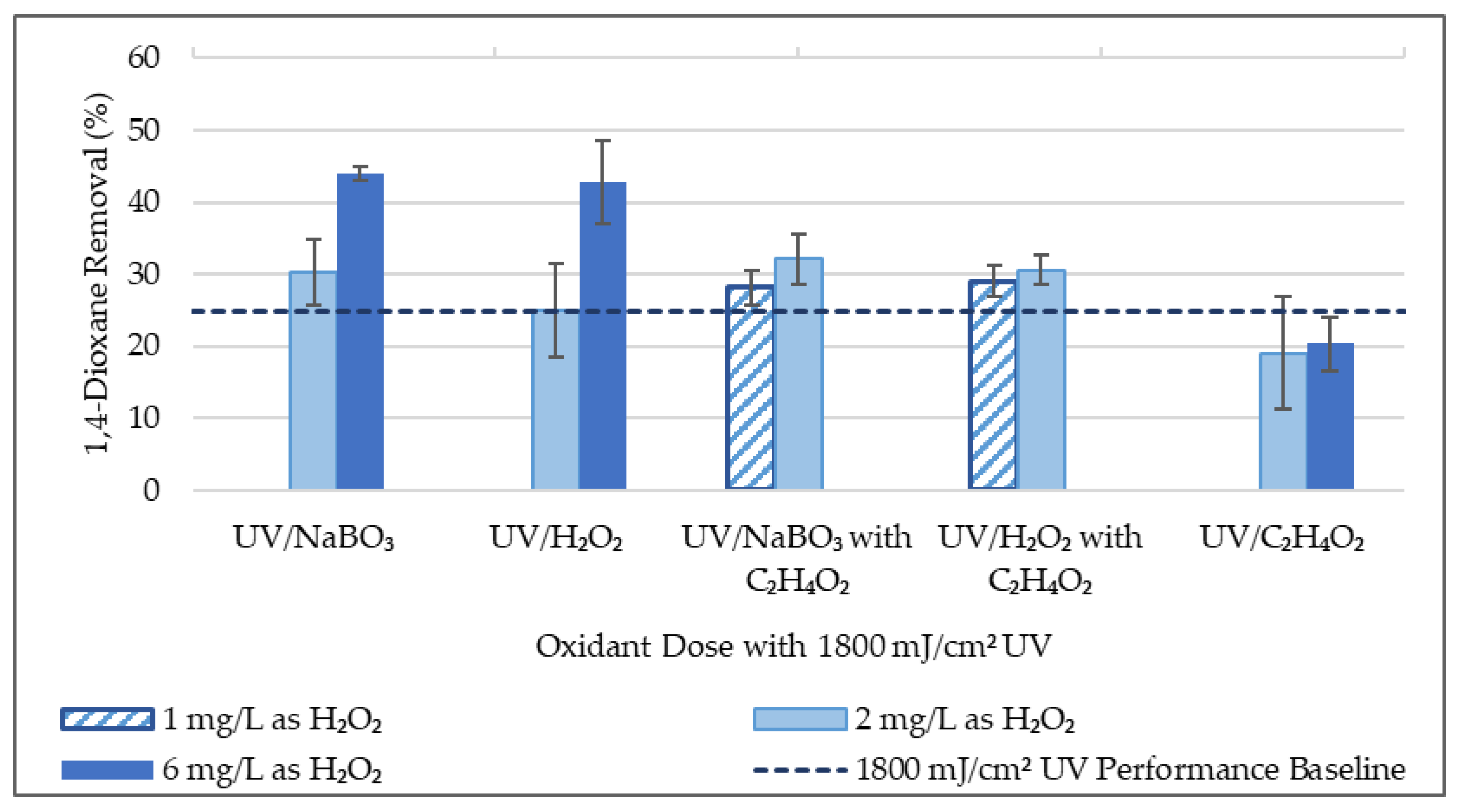
Experimentation was conducted with higher 1,4-dioxane concentrations (50–170 μg/L) to aim for greater significance of percent removal calculations. Results of this experimentation are summarized in Table 3 and Figure 3, and further detailed in the supplementary materials. Experimental results involving acetic acid encompass both the lower doses (0.01–0.06 mg/L) and higher doses (1.0–6.0 mg/L). Varying acetic acid doses are grouped together due to comparable results. H2O2 and NaBO3 are measured in mg/L as H2O2, while acetic acid is measured in mg/L as C2H4O2.

Table 3.
1,4-Dioxane Experimental Results Summary (50 μg/L initial 1,4-dioxane conc.).
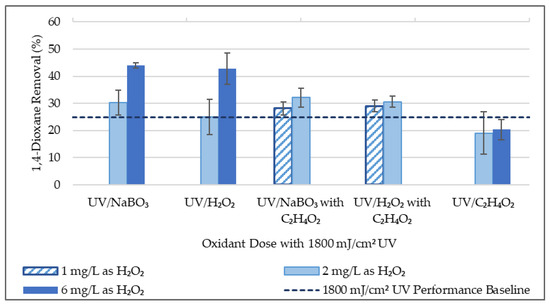
Figure 3.
1,4-Dioxane Experimental Results Summary (50 μg/L initial 1,4-dioxane conc.).
Results suggest that UV-AOP performs similarly for 1,4-dioxane removal when either sodium perborate or H2O2 is the oxidant. A two-factor ANOVA test showed a p-value of 0.31, which suggests that there is not a statistically significant difference in 1,4-dioxane percent removal between UV/H2O2 and UV/NaBO3 AOPs. Furthermore, two-sample t-tests on the UV/H2O2 and UV/NaBO3 AOPs with 2 mg/L and 6 mg/L each as H2O2 resulted in two-tail p-values of 0.99 and 0.98, respectively. These two-tail p-values provide additional support in accepting the hypothesis that there is no statistically significant difference between the effectiveness between 1,4-dioxane percent removal of the UV/H2O2 and UV/NaBO3 AOPs. However, a comparison of the mean 1,4-dioxane percent removal values for the aforementioned AOPs suggests that the UV/NaBO3 AOP may perform better. Regardless of whether sodium perborate or H2O2 were more successful in removing 1,4-dioxane, it can be concluded that sodium perborate is worth considering as an alternative to H2O2 for 1,4-dioxane removal from wastewater effluent.
The benefit of adding acetic acid to UV-AOPs for 1,4-dioxane removal was similarly statistically assessed. The mean 1,4-dioxane percent removal values suggest that the addition of acetic acid to the UV/H2O2 and UV/NaBO3 AOPs improved effectiveness. Two-sample t-tests were used to compare UV-AOPs with and without acetic acid addition to the UV/H2O2 and UV/NaBO3 AOPs with 2 mg/L as H2O2. Two-tail p-values are 0.08 for the UV/H2O2 AOP and 0.13 for the UV/NaBO3 AOP. With an alpha value of 0.10, the null hypothesis that acetic acid does not improve 1,4-dioxane removal can be rejected for the UV/H2O2 AOP and accepted for the UV/NaBO3 AOP. Further details on statistical analysis are included in the supplementary materials. Overall, results of two-sample t-tests and two-tail p-values do not provide sufficient statistical evidence to claim that acetic acid improves 1,4-dioxane removal in a UV/NaBO3 AOP. However, further research with larger sample size and less variability in wastewater effluent quality would provide additional opportunity to assess the impact of acetic acid supplementation on 1,4-dioxane removal in an UV/NaBO3 AOP.
Contrarily, results suggest that acetic acid provides benefit to the UV/H2O2 AOP. Mean 1,4-dioxane percent removal values for the UV/C2H4O2 processes are clearly lower than even UV treatment alone, so it seems that the acetic acid provides benefit when used in conjunction with an UV-AOP involving hydrogen peroxide. According to Shi, et al. [34] and Correa-Sanchez and Penuela [24], UV irradiation of acetic acid produces the acetoxyl radical (CH3C(=O)O•) and UV irradiation of hydrogen peroxide produces the hydroxyl radical (•OH). Thus, results of this work lead to the conclusion that the two radicals cohesively degrade contaminants in a UV/H2O2 AOP with acetic acid. Due to this interaction, the use of both hydrogen peroxide and acetic acid with UV irradiation may be more accurately described as a UV/H2O2/C2H4O2 AOP. In short, evidence supports the hypothesis that acetic acid improves 1,4-dioxane removal in a UV/H2O2 AOP, thus creating an UV/H2O2/C2H4O2 AOP. This novel AOP is distinct from the UV/peracetic acid AOP because it avoids the time-consuming and costly pre-formation of peracetic acid [24,35].
The mechanisms of 1,4-dioxane degradation were considered, however, this research was focused on engineering performance considerations and not in research of fundamental mechanistic approaches. UV/H2O2 AOP mechanisms are more well-documented in literature than those involved in the UV/NaBO3 AOP. For example, reactions in the UV/H2O2 AOP have been summarized in literature [36,37]. Degradation of 1,4-dioxane with various AOPs have also been proposed [11,38,39]. Organic contaminant degradation via the UV/NaBO3 AOP is similar to that of the UV/H2O2 AOP [19]. In some cases, however, borate is the released adduct instead of hydroxide [40]. There is also evidence that peroxoborate species are involved when NaBO3 is used as an oxidant [19,20]. For the acetic acid supplemented UV/NaBO3 AOP, McKillop and Sanderson [19] state that the reaction may be more complex than the conversion of carboxylic acid to a peracid. Instead, the intermediate peracetoxyboron species may be involved in oxidation of organic matter [19]. Specific mechanisms for the degradation of 1,4-dioxane with the UV-AOPs discussed herein should be evaluated for future research. In particular, mechanisms and radical species involved in the UV/NaBO3 and UV/H2O2/C2H4O2 AOPs are worthy of further investigation due to their relative effectiveness at 1,4-dioxane degradation, as shown in this research.
Possible toxicity from boron compounds was also considered with regards to the UV/NaBO3 AOP. Hadrup, et al. [41] conducted a literature review which indicated that boron-containing compounds are not genotoxic or carcinogenic. The United States’ Environmental Protection Agency (USEPA), however, implemented a long-term health advisory level of 2 mg/L for boron in drinking water. Although this research did not necessary aim for potable reuse, boron contributions from sodium perborate were quantified. In this work, it was found that 5.00 g sodium perborate tetrahydrate contributes approximately 1.12 g hydrogen peroxide residual. This mass ratio is equivalent to 1.0:0.23 grams of hydrogen peroxide to grams of sodium perborate. Guhl and Berends [42] similarly noted a ratio of 1.0:0.22. Stoichiometrically, a 2 mg/L of NaBO3 as H2O2 contributes 0.64 mg/L of boron, which is well below the USEPA health advisory level. Even in non-potable applications, boron remains an important factor in the UV/NaBO3 AOP due to impacts on crops. Sensitive crops often permit up to 1.25 mg/L boron, whereas certain tolerant crops can handle upwards of 3.75 mg/L [43]. Stoichiometric calculations reveal that a sodium perborate dose of 6 mg/L as H2O2 contributes 1.92 mg/L boron, which remains acceptable for irrigation of tolerant crops. Therefore, boron addition from sodium perborate is not a limiting factor in the use of the UV/NaBO3 AOP.
3.3. Conceptual Economic Consideration
Sodium perborate tetrahydrate was compared to 50% hydrogen peroxide on material cost estimations and non-cost considerations, assuming that there is a consistent relationship of hydrogen peroxide to sodium perborate in a solution. The cost evaluation in this study was limited to chemical cost considerations only.
The City of Sarasota has an average daily flow of 6.2 million gallons per day (MGD) based on data from 2014–2020. This information was used to calculate chemical costs in $/day for hydrogen peroxide and sodium perborate tetrahydrate based on various sources, as detailed in Table 4. Costs in Table 4 vary, but sodium perborate tetrahydrate is generally more expensive than hydrogen peroxide. The 95% confidence values for sodium perborate and hydrogen peroxide costs per day suggest significant variation in cost estimates. This may be due to several factors related to the cost estimate sources. For example, cost estimates may be lower in sources where the chemical use is more common in that geographical location, or if a supplier provides routine shipments to a major customer. Therefore, cost ratios of NaBO3 to H2O2 were calculated from the sources that supplied quotes for both chemicals. This information is included in Table 5.

Table 4.
Calculated Chemical Costs for H2O2 and NaBO3 Bulk Shipments.

Table 5.
Calculated NaBO3 to H2O2 Chemical Cost Ratio using Quotes from Single Sources.
Chemical stability, ease of transportation, shelf-life and degradation rate were also taken into economic consideration. A bulk shipment of sodium perborate tetrahydrate granules is approximately 40,000 lbs [50]. A 40,000-lb shipment of NaBO3 would last a 6.2 MGD plant approximately 28 days if the application dose was 6 mg/L as H2O2. The same weight shipment of 50% H2O2 would last the same plant 64 days. However, the solid state of NaBO3 provides a benefit due to its ease of storage and transportation. On a similar note, NaBO3 takes up less space than H2O2 when comparing equivalent weights, which provides additional benefit for shipments sent to remote locations or island environment operations. The same 40,000-lb shipments of NaBO3 and 50% H2O2 take up 370 ft3 (10.5 m3) and 534 ft3 (15.1 m3), respectively. For example, remote overseas location may prefer receiving one shipment of a year’s supply of solid sodium perborate that can be used to mix into solution as needed instead of requiring repeated costly shipments of hydrogen peroxide. H2O2 solutions naturally degrade, thus requiring users to check the strength and adjust feed rates accordingly. Best practice for hydrogen peroxide solutions involves the minimization of long-term storage due to degradation over time. On the other hand, NaBO3 can be mixed as needed, thereby minimizing strength degradation concerns.
Furthermore, the high cost of sodium perborate compared to hydrogen peroxide may be due to a limited production capacity. In this case, the increased demand for the chemical may increase production capacities, thus lowering cost. Despite the higher cost of sodium perborate tetrahydrate as compared to 50% hydrogen peroxide, there are advantages related to storage considerations and ease of transportation. These benefits may outweigh the higher chemical cost in certain uses, such as remote and mobile operations. The applications of sodium perborate in water and wastewater treatment have also not been extensively explored. Possible future research areas include but are not limited to:
- The use of sodium perborate in solutions at various pH levels. Burgess and Hubbard [20] found that the ratio of perborate species to hydrogen peroxide is maximum at pH of approximately 10.1. At lower pH values, hydrogen peroxide is more prevalent in aqueous sodium perborate solution. At the pH range from 7 to 13, perborate species are more prevalent, and can speed up or inhibit oxidation effects depending on the water quality and specific perborate species [20]. Yuan, Zhai, Zhu, Liu, Jiao and Tang [23] tested the UV/NaBO3 AOP for the removal of humic acids in deionized water at a pH of 3, 7, and 11 and found that humic acid removal increased with decreasing pH. Similar studies at various pH levels could be conducted with different source waters and to target different contaminants to explore UV/NaBO3 AOP treatment effectiveness.
- The use of UV/NaBO3 perborate AOP to target other CECs. It is possible that the chemistry of this AOP is more effective than the UV/H2O2 AOP at removing specific contaminants that were not explored in this research study.
- A combined UV/H2O2/NaBO3 AOP could be attempted. Hydrogen peroxide and sodium perborate are used in certain dental bleaching techniques but has not yet been attempted with UV irradiation for water or wastewater treatment [55,56].
4. Conclusions
The results of this study demonstrated the ability of the oxidant sodium perborate to perform similarly to hydrogen peroxide in an UV-AOP for the degradation of 1,4-dioxane in tertiary wastewater effluent. The UV/NaBO3 AOP with 6 milligrams per liter (mg/L) as H2O2 resulted in 43.9 percent 1,4-dioxane removal, while an equivalent UV/H2O2 AOP showed 42.8 percent removal. Furthermore, experimental data gathered in this work suggested that acetic acid enhanced the UV/H2O2 AOP, increasing treatment effectiveness by 5.7%. A conceptual cost evaluation indicated that the chemical supply cost of NaBO3 is approximately 3.3 times greater than 50% H2O2; however, depending on the ease of transportation and treatment plant location, the cost of H2O2 delivery may outweigh the cost of NaBO3, useful to remote, site-specific conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15071364/s1, Table S1: Initial Screening and Experimentation Outline; Table S2: Methods and Equipment for Water Quality Analysis at UCF Laboratories; Table S3: Initial Drum Water Quality Data; Table S4: Numerical Results for 1,4-Dioxane Removal Initial Screening and Experimentation; Table S5: ANOVA Test Data Input; Table S6: ANOVA Test Data Output; Table S7: Data Input for t-test 1; Table S8: Data Output for t-test 1; Table S9: Data Input for t-test 2; Table S10: Data Output for t-test 2; Table S11: Data Input for t-test 3; Table S12: Data Output for t-test 3; Table S13: Data Input for t-test 4; Table S14: Data Output for t-test 4; Equations (S1) and (S2): QAQC Calculations; Table S15: 1,4-Dioxane QAQC; Equations (S3)–(S8): Example Chemical Cost Calculations.
Author Contributions
T.L.S. conducted the conceptualization, methodology, validation, formal analysis, investigation, data curation, and writing—original draft preparation, and visualization; S.J.D. provided and obtained the resources and funding acquisition for this study and conducted formal analysis, supported data curation, provided technical supervision, conceptualization, validation, and project administration for the research. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the City of Sarasota Utilities (1750 12th St., Sarasota, FL 34236) through UCF project number 1620-8A29. Additional support was received by UCF’s Research Foundation Jones Edmunds Fund 1620-8A22 (Jones Edmunds, 730 NE Waldo Rd, Gainesville, FL 32641).
Data Availability Statement
The data presented in this study are available in the main body of the paper and in the supplementary materials.
Acknowledgments
The help provided by the City of Sarasota’s Wastewater Treatment Plant staff was outstanding; their efforts are greatly appreciated in the facilitation of this research project. The UCF Water Quality Engineering Research Group is also acknowledged for their support with sampling, general laboratory maintenance, and experimental assistance. Article processing charges were provided in part by the UCF College of Graduate Studies Open Access Publishing Fund. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the University of Central Florida or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the City of Sarasota, the University of Central Florida, or any agency thereof.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
A list of acronyms and abbreviations used in this work is included herein:
| μg/L | Micrograms per liter |
| ANOVA | Analysis of variance |
| AOP | Advanced oxidation process |
| C2H4O2 | Acetic acid |
| CITY | The City of Sarasota |
| cm | Centimeters |
| DO | Dissolved oxygen |
| DOC | Dissolved organic carbon |
| FL | Florida |
| gpm | Gallons per minute |
| H2O2 | Hydrogen peroxide |
| J | Joules |
| mg/L | milligrams per liter |
| NaBO3 | Sodium perborate |
| NELAC | National Laboratory Accreditation Conference |
| ng/L | Nanograms per liter |
| NH | New Hampshire |
| PA | Pennsylvania |
| QAQC | Quality assurance and quality control |
| SM | Standard Methods for the Examination of Water and Wastewater [33] |
| TDS | Total dissolved solids |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UCF | University of Central Florida |
| USA | United States of America |
| USEPA | United States’ Environmental Protection Agency |
| UV/H2O2 AOP | Ultraviolet and hydrogen peroxide advanced oxidation process |
| UV/NaBO3 AOP | Ultraviolet and sodium perborate advanced oxidation process |
| UV254 | Ultraviolet absorbance at 254 nanometers |
| UVA | Ultraviolet absorbance |
| UV-AOPs | Ultraviolet advanced oxidation processes |
| UVT | Ultraviolet transmittance |
| W | Watts |
References
- Tanabe, A.; Tsuchida, Y.; Ibaraki, T.; Kawata, K. Impact of 1,4-Dioxane from Domestic Effluent on the Agano and Shinano Rivers, Japan. Bull. Environ. Contam. Toxicol. 2006, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, S.; Smith, K.; Wang, Y.; Hu, H. Light-driven breakdown of 1,4-Dioxane for potable reuse: A review. Chem. Eng. J. 2019, 373, 508–518. [Google Scholar] [CrossRef]
- USEPA. Final Risk Evaluation for 1,4-Dioxane; EPA-740-R1-8007; Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2020.
- Abe, A. Distribution of 1,4-dioxane in relation to possible sources in the water environment. Sci. Total Environ. 1999, 227, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Lee, C.-S.; Asato, C.; Wang, M.; Mao, X.; Gobler, C.J.; Venkatesan, A.K. Removal of 1,4-dioxane during on-site wastewater treatment using nitrogen removing biofilters. Sci. Total Environ. 2021, 771, 144806. [Google Scholar] [CrossRef]
- USEPA. 1,4-Dioxane, CASRN 123-91-1; Integrated Risk Information System (IRIS); National Center for Environmental Assessment: Washington, DC, USA, 2013. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=326 (accessed on 11 January 2023).
- Florida Department of Health. 1,4-Dioxane—Technical Factsheet; Division of Disease Control and Health Protection: Tallahassee, FL, USA, 2021.
- USEPA. Technical Fact Sheet—1,4-Dioxane; EPA 505-F-17-011; Office of Land and Emergency Management: Washington, DC, USA, 2017; 5106p.
- California State Water Boards. Advanced Treatment Criteria; California Code of Regulations Section 60320.201(d)(1); California State Water Boards: Sacramento, CA, USA, 2018.
- Pollitt, K.J.G.; Kim, J.-H.; Peccia, J.; Elimelech, M.; Zhang, Y.; Charkoftaki, G.; Hodges, B.; Zucker, I.; Huang, H.; Deziel, N.C.; et al. 1,4-Dioxane as an emerging water contaminant: State of the science and evaluation of research needs. Sci. Total Environ. 2019, 690, 853–866. [Google Scholar] [CrossRef]
- Zenker, M.J.; Borden, R.C.; Barlaz, M.A. Occurrence and Treatment of 1,4-Dioxane in Aqueous Environments. Environ. Eng. Sci. 2003, 20, 423–432. [Google Scholar] [CrossRef]
- Higgins, C.J.; Duranceau, S.J. Modeling the mass transfer of 1,4-dioxane in a nanofiltration membrane process. Desalination Water Treat. 2020, 191, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Jain, T.; Ishida, K.; Liu, H. A mechanistic understanding of the degradation of trace organic contaminants by UV/hydrogen peroxide, UV/persulfate and UV/free chlorine for water reuse. Environ. Sci. Water Res. Technol. 2017, 3, 128–138. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Ulliman, S.L.; Miklos, D.B.; Hübner, U.; Drewes, J.E.; Linden, K.G. Improving UV/H2O2 performance following tertiary treatment of municipal wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1321–1330. [Google Scholar] [CrossRef]
- Kim, I.H.; Yamashita, N.; Kato, Y.; Tanaka, H. Discussion on the application of UV/H2O2, O3 and O3/UV processes as technologies for sewage reuse considering the removal of pharmaceuticals and personal care products. Water Sci. Technol. 2009, 59, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, H.R.; Brown, M.T.; Boyer, T.H. Evaluating UV/H2O2, UV/percarbonate, and UV/perborate for natural organic matter reduction from alternative water sources. Chemosphere 2014, 105, 112–118. [Google Scholar] [CrossRef] [PubMed]
- McKillop, A.; Sanderson, W.R. Sodium perborate and sodium percarbonate: Cheap, safe and versatile oxidising agents for organic synthesis. Tetrahedron 1995, 51, 6145–6166. [Google Scholar] [CrossRef]
- Burgess, J.; Hubbard, C.D. Catalysis or Convenience? Perborate in Context. In Advances in Onorganic Chemistry; Homogeneous Catalysis; VanEldik, R., Hubbard, C.D., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2013; Volume 65, pp. 217–310. [Google Scholar]
- Tredwin, C.J.; Naik, S.; Lewis, N.J.; Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 2006, 200, 371–376. [Google Scholar] [CrossRef]
- Gowdhamamoorthi, M.; Arun, A.; Kiruthika, S.; Muthukumaran, B. Perborate as novel fuel for enhanced performance of membraneless fuel cells. Ionics 2014, 20, 1723–1728. [Google Scholar] [CrossRef]
- Yuan, D.; Zhai, Z.; Zhu, E.; Liu, H.; Jiao, T.; Tang, S. Humic Acid Removal in Water via UV Activated Sodium Perborate Process. Coatings 2022, 12, 885. [Google Scholar] [CrossRef]
- Correa-Sanchez, S.; Peñuela, G.A. Peracetic acid-based advanced oxidation processes for the degradation of emerging pollutants: A critical review. J. Water Process. Eng. 2022, 49, 102986. [Google Scholar] [CrossRef]
- Stefan, M.I.; Bolton, J.R. Mechanism of the Degradation of 1,4-Dioxane in Dilute Aqueous Solution Using the UV/Hydrogen Peroxide Process. Environ. Sci. Technol. 1998, 32, 1588–1595. [Google Scholar] [CrossRef]
- Mill, T.; Gould, C.W. Free-radical oxidation of organic phosphonic acid salts in water using hydrogen peroxide, oxygen, and ultraviolet light. Environ. Sci. Technol. 1979, 13, 205–208. [Google Scholar] [CrossRef]
- Chitra, S.; Paramasivan, K.; Cheralathan, M.; Sinha, P.K. Degradation of 1,4-dioxane using advanced oxidation processes. Environ. Sci. Pollut. Res. 2012, 19, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, C.; Kamalam, R. Mechanism and reactivity in perborate oxidation of anilines in acetic acid. J. Chem. Soc. Perkin Trans. 2002, 2, 2011–2018. [Google Scholar] [CrossRef]
- Karunakaran, C.; Palanisamy, P. Autocatalysis in the sodium perborate oxidation of anilines in acetic acid–ethylene glycol. J. Mol. Catal. A Chem. 2001, 172, 9–17. [Google Scholar] [CrossRef]
- Gupton, J.T.; Duranceau, S.J.; Miller, J.F.; Kosiba, M.L. The Reaction of α-Methylstyrene Analogs and Related Compounds with Sodium Perborate in Acetic Acid. Synth. Commun. 1988, 18, 937–947. [Google Scholar] [CrossRef]
- Brandhuber, P.; Korshin, G.V. Methods for the Detection of Residual Concentrations of Hydrogen Peroxide in Advanced Oxidation Processes; WateReuse Foundation: Alexandria, VA, USA, 2009. [Google Scholar]
- Martin, K.; (Pur Representative, GHP Group, Guelph, ON, Canada). Personal communication, 2022.
- Baird, B.R.; Eaton, D.A.; Rice, W.E.; Bridgewater, L.L. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Shi, C.; Li, C.; Wang, Y.; Guo, J.; Barry, S.; Zhang, Y.; Marmier, N. Review of Advanced Oxidation Processes Based on Peracetic Acid for Organic Pollutants. Water 2022, 14, 2309. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Hao, J.; Liu, D. Preparation of peracetic acid from hydrogen peroxide, part II: Kinetics for spontaneous decomposition of peracetic acid in the liquid phase. J. Mol. Catal. A Chem. 2008, 284, 58–68. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Fedorov, K.; Rayaroth, M.P.; Shah, N.S.; Boczkaj, G. Activated sodium percarbonate-ozone (SPC/O3) hybrid hydrodynamic cavitation system for advanced oxidation processes (AOPs) of 1,4-dioxane in water. Chem. Eng. J. 2023, 456, 141027. [Google Scholar] [CrossRef]
- Sonawane, S.; Fedorov, K.; Rayaroth, M.P.; Boczkaj, G. Degradation of 1,4-dioxane by sono-activated persulfates for water and wastewater treatment applications. Water Resour. Ind. 2022, 28, 100183. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Shoup, T.M.; Goudgaon, N.M. Sodium perborate: A mild and convenient reagent for efficiently oxidizing organoboranes. J. Org. Chem. 1989, 54, 5930–5933. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef]
- Guhl, W.; Berends, A.G. Degradation of Sodium Perborate in Domestic Wastewater. Tenside Surfactants Deterg. 2001, 38, 98–102. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters; United States Department of Agriculture: Washington, DC, USA, 1955.
- Pesman, E.; Kalyoncu, E.E.; Kirci, H. Sodium Perborate Usage instead of Hydrogen Peroxide for the Reinforcement of Oxygen Delignification. Fibres Text. East. Eur. 2010, 18, 106–109. [Google Scholar]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Aguiar, J.B. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef]
- Rankin, S.; (Chief Operator at Lake Mary Water Treatment Plant, Lake Mary, FL, USA). Personal communication, 2022.
- City of Fort Lauderdale Hydrogen Peroxide & Odor Control Services (Re-Bid). Bid 12245-393. 2019. Available online: https://www.fortlauderdale.gov/Home/ShowDocument?id=36346 (accessed on 25 January 2023).
- Zhang, Z.; Chuang, Y.-H.; Szczuka, A.; Ishida, K.P.; Roback, S.; Plumlee, M.H.; Mitch, W.A. Pilot-scale evaluation of oxidant speciation, 1,4-dioxane degradation and disinfection byproduct formation during UV/hydrogen peroxide, UV/free chlorine and UV/chloramines advanced oxidation process treatment for potable reuse. Water Res. 2019, 164, 114939. [Google Scholar] [CrossRef]
- Gao, J.; Duan, X.; O’Shea, K.; Dionysiou, D.D. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion. Water Res. 2020, 171, 115394. [Google Scholar] [CrossRef]
- Hall, H.; (Sales Representative at Harcros Chemicals Inc., Tampa, FL, USA). Personal communication, 2022.
- Alioth Finance. Inflation Calculator. Available online: https://www.officialdata.org/ (accessed on 24 January 2023).
- U.S. Bureau of Labor Statistics. CPI Inflation Calculator. United States Department of Labor. Available online: https://www.bls.gov/data/inflation_calculator.htm (accessed on 24 January 2023).
- Exchange Rates UK—Euro to US Dollar Spot Exchange Rates for 2015. Available online: https://www.exchangerates.org.uk/EUR-USD-spot-exchange-rates-history-2015.html#:~:text=Average%20exchange%20rate%20in%202015%3A%201.11%20USD (accessed on 24 January 2023).
- Pennacchio, S.; (Sales Associate at Spectrum Chemical Manufacturing Corp., New Brunswick, NJ, USA). Personal communication, 2023.
- Nutting, E.B.; Poe, G.S. Chemical Bleaching of Discolored Endodontically Treated Teeth. Dent. Clin. N. Am. 1967, 11, 655–662. [Google Scholar] [CrossRef]
- Tran, L.; Orth, R.; Parashos, P.; Tao, Y.; Tee, C.W.; Thomas, V.T.; Towers, G.; Truong, D.T.; Vinen, C.; Reynolds, E.C. Depletion Rate of Hydrogen Peroxide from Sodium Perborate Bleaching Agent. J. Endod. 2017, 43, 472–476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).