Spatial and Temporal Variations in Phytoplankton Community in Dianchi Lake Using eDNA Metabarcoding
Abstract
:1. Introduction
2. Methods
2.1. Survey Region and Sampling
2.2. Environmental Factors Analysis
2.3. eDNA Extraction, Polymerase Chain Reaction (PCR) Amplification and Sequencing
2.4. Bioinformatics
3. Results
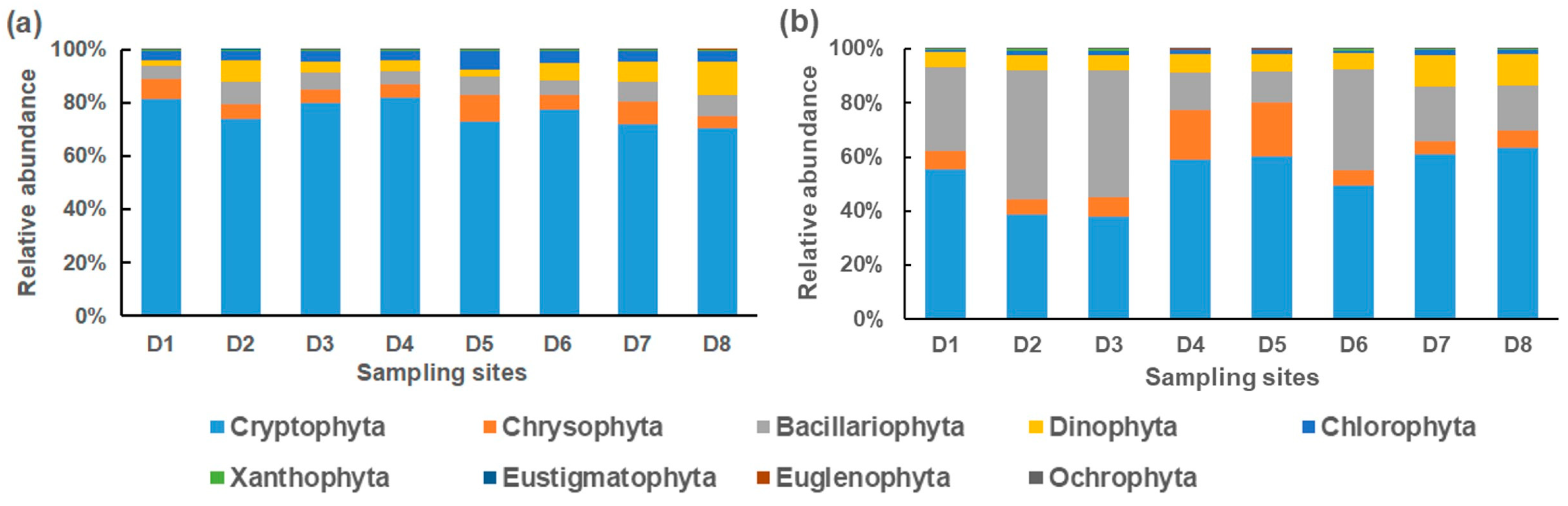
3.1. Sequencing Results and Phytoplankton Community Profile
3.2. Spatial and Temporal Distributions of Phytoplankton Community in Dianchi Lake
3.3. Temporal Variation of Phytoplankton Diversity
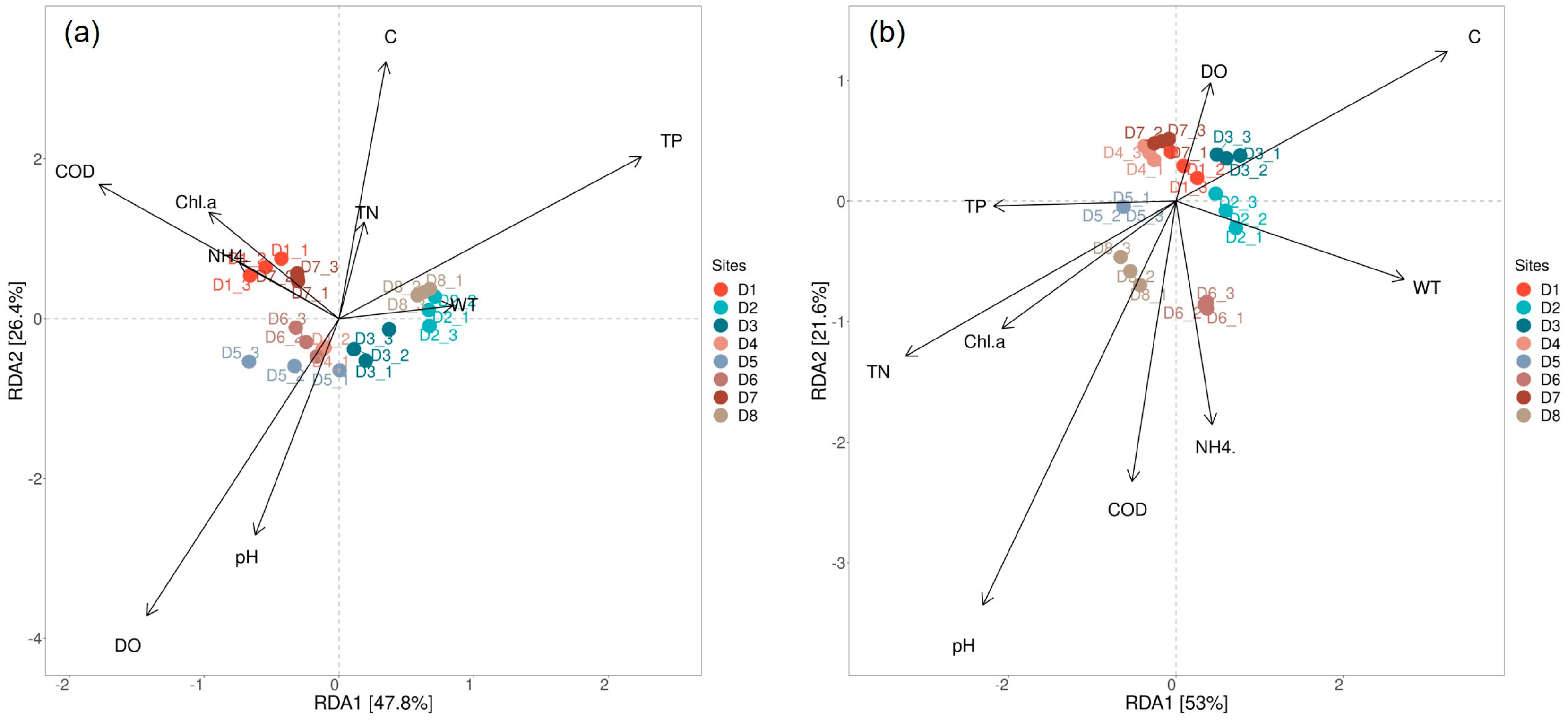
3.4. Relationships between the Phytoplankton Community and Environmental Factors
4. Discussion
4.1. eDNA Metabarcoding Disclosed the Spatial and Temporal Variations in Phytoplankton Diversity in Dianchi Lake
4.2. Phytoplankton Diversity Patterns in Dianchi Lake Were Shaped by Environmental Factors
4.3. eDNA Metabarcoding Is a Useful Tool for Phytoplankton Diversity Monitoring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, C.; Reynolds, C.; Reynolds, C.S.; Reynolds, C.S.; Usher, M.; Saunders, D.; Dobson, A.; Peet, R.; Adam, P.; Birks, H. Ecology of Phytoplankton; Cambridge University: New York, NY, USA, 2006. [Google Scholar]
- De Senerpont Domis, L.N.; Elser, J.J.; Gsell, A.S.; Huszar, V.L.M.; Ibelings, B.W.; Jeppesen, E.; Kosten, S.; Mooij, W.M.; Roland, F.; Sommer, U.; et al. Plankton dynamics under different climatic conditions in space and time. Freshw. Biol. 2013, 58, 463–482. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; Van De Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Phillips, G.; Lyche-Solheim, A.; Skjelbred, B.; Mischke, U.; Drakare, S.; Free, G.; Järvinen, M.; Hoyos, C.; Morabito, G.; Poikane, S. A phytoplankton trophic index to assess the status of lakes for the Water Framework Directive. Hydrobiologia 2013, 704, 75–95. [Google Scholar] [CrossRef]
- Nankabirwa, A.; De Crop, W.; Van der Meeren, T.; Cocquyt, C.; Plisnier, P.-D.; Balirwa, J.; Verschuren, D. Phytoplankton communities in the crater lakes of western Uganda, and their indicator species in relation to lake trophic status. Ecol. Indic. 2019, 107, 105563. [Google Scholar] [CrossRef]
- Carvalho, L.; Poikane, S.; Lyche Solheim, A.; Phillips, G.; Borics, G.; Catalan, J.; De Hoyos, C.; Drakare, S.; Dudley, B.J.; Järvinen, M.; et al. Strength and uncertainty of phytoplankton metrics for assessing eutrophication impacts in lakes. Hydrobiologia 2013, 704, 127–140. [Google Scholar] [CrossRef]
- Ma, T.; Fan, Y.; Li, K.; Hu, Z.; Wu, S. Ecological health assessment of main estuaries of Lake Taihu based on phytoplankton index of biotic integrity. J. Ecol. Rural Environ. 2021, 37, 501–508. [Google Scholar]
- Huo, S.; Zhang, H.; Monchamp, M.E.; Wang, R.; Weng, N.; Zhang, J.; Zhang, H.; Wu, F. Century-long homogenization of algal communities is accelerated by nutrient enrichment and climate warming in lakes and reservoirs of the North Temperate Zone. Environ. Sci. Technol. 2022, 56, 3780–3790. [Google Scholar] [CrossRef]
- Rivera, S.F.; Vasselon, V.; Jacquet, S.; Bouchez, A.; Ariztegui, D.; Rimet, F. Metabarcoding of lake benthic diatoms: From structure assemblages to ecological assessment. Hydrobiologia 2017, 807, 37–51. [Google Scholar] [CrossRef]
- Li, J.L.; Luo, C.L.; Lü, H.; Xu, J.F.; Luo, L.C.; Pan, M.; He, F.; Man, X.M.; Zhang, R.F.; Gong, F.L.; et al. Spatio-temporal variation and driving factors of algal bloom at Dianchi during 2002–2018. Acta Ecol. Sinca 2023, 43, 878–891. [Google Scholar]
- Yang, K.; Yu, Z.; Luo, Y.; Yang, Y.; Zhao, L.; Zhou, X. Spatial and temporal variations in the relationship between lake water surface temperatures and water quality—A case study of Dianchi Lake. Sci. Total Environ. 2018, 624, 859–871. [Google Scholar] [CrossRef]
- Zhang, T.; Zeng, W.H.; Wang, S.R.; Ni, Z.K. Temporal and spatial changes of water quality and management strategies of Dianchi Lake in southwest China. Hydrol. Earth Syst. Sci. 2014, 18, 1493–1502. [Google Scholar] [CrossRef]
- Mu, M.; Wu, C.; Li, Y.; Lyu, H.; Fang, S.; Yan, X.; Liu, G.; Zheng, Z.; Du, C.; Bi, S. Long-term observation of cyanobacteria blooms using multi-source satellite images: A case study on a cloudy and rainy lake. Environ. Sci. Pollut. Res. 2019, 26, 11012–11028. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Yang, C.; He, L.Q.; Dao, G.H.; Du, J.S.; Han, Y.P.; Wu, G.X.; Wu, Q.Y.; Hu, H.Y. Meteorological factors and water quality changes of Plateau Lake Dianchi in China (1990–2015) and their joint influences on cyanobacterial blooms. Sci. Total Environ. 2019, 665, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, S.; Fang, S.; Yu, F.; Feng, W.; Liu, L. Canonical correspondence analysis of relationship between characteristics of phytoplankton community and environmental factors in Dianchi Lake. China Environ. Sci. 2016, 36, 544–552. [Google Scholar]
- Li, L.; Li, G.; Li, G. Winter and spring succession of the phytoplankton community in Dianchi Lake under the influences of environment change. J. Hydroecol. 2020, 41, 57–68. [Google Scholar]
- Wu, L.; Feng, W. Temporal heterogeneity of plankton community in Lake Dianchi and its relation to environmental factors. J. Freshw. Ecol. 2012, 27, 229–241. [Google Scholar] [CrossRef]
- Dai, G.-Y.; Li, J.; Li, L.; Song, L.-R. The Spatio-Temporal Pattern of Phytoplankton in the North Basin of Lake Dianchi and Related Environmental Factors. Acta Hydrobiol. Sin. 2013, 36, 946–956. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, S.; Liu, X.; Liu, Y. Seasonal and spatial variations of phytoplankton communities and correlations with environmental factors in Lake Dianchi. Acta Sci. Nat. Univ. Pekin. 2020, 56, 184–192. [Google Scholar]
- Dong, J.; Li, G.B.; Song, L.R. Historical changes of phytoplankton functional groups in Lake Fuxian, Lake Erhai and Lake Dianchi since 1960s. J. Lake Sci. 2014, 26, 735–742. [Google Scholar]
- Yang, F.; Xu, Q.; Song, Y.; Zhou, X.; Liu, X.; Yan, C.; Huang, L. Evolution trend, governance course and future suggestions of water ecological environment in Dianchi Lake Basin. J. Environ. Eng. Technol. 2022, 12, 633–643. [Google Scholar]
- Mao, J.; Sun, Y.; He, K.; Kong, G.; Yang, Z. Study of water environment improvement effect by Niulan River -Dianchi Lake Water Supplement Project in Waihai area of Dianchi Lake. Water Resour. Prot. 2017, 33, 47–51. [Google Scholar]
- Yang, K.; Pan, M.; Luo, Y.; Chen, K.; Zhao, Y.; Zhou, X. A time-series analysis of urbanization-induced impervious surface area extent in the Dianchi Lake watershed from 1988–2017. Int. J. Remote Sens. 2018, 40, 573–592. [Google Scholar] [CrossRef]
- Li, X.; Janssen, A.B.G.; de Klein, J.J.M.; Kroeze, C.; Strokal, M.; Ma, L.; Zheng, Y. Modeling nutrients in Lake Dianchi (China) and its watershed. Agric. Water Manag. 2019, 212, 48–59. [Google Scholar] [CrossRef]
- Phan, T.; Caillault, E.P.; Bigand, A. Comparative study on supervised learning methods for identifying phytoplankton species. In Proceedings of the 2016 IEEE Sixth International Conference on Communications and Electronics (IEEE ICCE 2016), Ha Long, Vietnam, 27–29 July 2016; pp. 283–288. [Google Scholar]
- Deiner, K.; Bik, H.M.; Machler, E.; Seymour, M.; Lacoursiere-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; de Vere, N.; et al. Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Lin, Y.; Zhong, W.; Zhao, Z.; Shen, L.; Ling, Z.; Zhao, K.; Xu, S. Fish biomonitoring and ecological assessment in the Dianchi Lake Basin based on environmental DNA. Water 2023, 15, 399. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, G.; Yang, J.; Wang, Z.; Xu, Y.; Zhang, X.; Wang, Z. eDNA metabarcoding revealed differential structures of aquatic communities in a dynamic freshwater ecosystem shaped by habitat heterogeneity. Environ. Res. 2021, 201, 111602. [Google Scholar] [CrossRef]
- Diao, C.; Jia, H.; Guo, S.; Hou, G.; Xian, W.; Zhang, H. Biodiversity exploration in autumn using environmental DNA in the South China sea. Environ. Res. 2022, 204, 112357. [Google Scholar] [CrossRef]
- Banerji, A.; Bagley, M.; Elk, M.; Pilgrim, E.; Martinson, J.; Santo Domingo, J. Spatial and temporal dynamics of a freshwater eukaryotic plankton community revealed via 18S rRNA gene metabarcoding. Hydrobiologia 2018, 818, 71–86. [Google Scholar] [CrossRef]
- Ibrahim, A.; Capo, E.; Wessels, M.; Martin, I.; Meyer, A.; Schleheck, D.; Epp, L.S. Anthropogenic impact on the historical phytoplankton community of Lake Constance reconstructed by multimarker analysis of sediment-core environmental DNA. Mol. Ecol. 2021, 30, 3040–3056. [Google Scholar] [CrossRef]
- Yue, Y.; Fu, Z.; Chen, X.; Yang, M.; Wang, B.; Wang, F. Community structure and diversity phytoplankton in the Wujiang River Basin reservoir. J. Shanghai Univ. Nat. Sci. Ed. 2021, 27, 97–105. [Google Scholar]
- Lv, J.; Lin, Y.; Zhao, Z.; Zhou, X. eDNA metabarcoding revealed the seasonal and spatial variation of phytoplankton functional groups in the Chai river and their relationship with environmental factors. J. Freshw. Ecol. 2023, 38, 2176374. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Zhang, Y.; Shi, J.; Yu, H.; Zhang, X. eDNA biomonitoring revealed the ecological effects of water diversion projects between Yangtze River and Tai Lake. Water Res. 2022, 210, 117994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, S.; Zhao, Z.; Zhou, X.; Feng, Q.; Yang, J.; Li, F.; Wang, Z.; Zhang, X. Precision of eDNA metabarcoding technology for biodiversity monitoring of eukaryotic phytoplankton in lakes. Environ. Sci. 2021, 42, 796–807. [Google Scholar]
- Lin, Y.; Zhong, W.; Zhang, X.; Zhou, X.; He, L.; Lv, J.; Zhao, Z. Environmental DNA metabarcoding revealed the impacts of anthropogenic activities on phytoplankton diversity in Dianchi Lake and its three inflow rivers. Ecol. Evol. 2023, 13, e10088. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wang, R.N. Dynamic variation for the species of phytoplankton in Dianchi Lake, China. J. Yunnan Univ. Nat. Sci. Ed. 2006, 28, 73–77. [Google Scholar]
- Zhang, X. The Impacts of Characteristic Pollution Sources on the Water Quality and Plankton Communities of Inflowing Rivers in Dianchi Lake Basin; Shanghai Jiao Tong University: Shanghai, China, 2016. [Google Scholar]
- SL63-94; Quality Standards for Surface Water Resources. Ministry of Water Resources of the People’s Republic of China: Beijing, China, 1994.
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Langsley, G.; Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 2009, 4, e6372. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Hu, H.; Wu, Y. The Freshwater Algae of China, Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2016. [Google Scholar]
- Zhang, C.X.; He, Y.X.; Guo, X.M.; Meng, H.Q.; Wu, L.; Huang, J.; Li, W.G.; Zhao, T.Q. Community structure variations and driving factors of eukaryotes phytoplankton in Danjiangkou Reservoir in summer and winter. J. Henan Polytech. Univ. Nat. Sci. 2022, 41, 110–122. [Google Scholar]
- Cheng, R.; Luo, Y.; Zhang, Y.; Li, Q.; Li, Y.; Shen, Y. eDNA metabarcoding reveals differences in fish diversity and community structure in heterogeneous habitat areas shaped by cascade hydropower. Ecol. Evol. 2023, 13, e10275. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Gan, N.; Zheng, L.; Ma, H.; Shan, K.; Liu, J.; Xiao, B.; Song, L. Seasonal dynamics of water bloom-forming Microcystis morphospecies and the associated extracellular microcystin concentrations in large, shallow, eutrophic Dianchi Lake. J. Environ. Sci. 2014, 26, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.S.; Vera, H.; Carla, K.; Luigi, N.F.; Sergio, M. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Wei, R. Water Quality and Ecological System Monitoring and Treatment Evaluation of the Typical Dianchi Urban Rivers; Shanghai Jiao Tong University: Shanghai, China, 2016. [Google Scholar]
- Hu, Y.; Peng, Y.; Li, R.; Huang, J.; Zhou, Z.; Hu, S.; Wang, Y.; Qiu, G. Plankton diversity and community characteristics in Danjiangkou Reservoir based on environmental DNA metabarcoding. J. Lake Sci. 2021, 33, 1650–1659. [Google Scholar]
- Lin, Y.; Zhao, Z. Application of environmental DNA technologies in monitoring aquatic invasive species. Asian J. Ecotoxicol. 2021, 16, 1–12. [Google Scholar]
- Song, L.; Wu, J.; Song, G.J.; Dong, S.J.; Wang, Z.S. Characteristics of phytoplankton community structure in Liaodong Bay based on environmental DNA technology. Acta Ecol. Sin. 2020, 40, 6243–6257. [Google Scholar]
- Wang, Z.H.; Chen, J.F.; Yang, Y.F. Control and management of harmful algal bloom species introducd by ballastwater. Mar. Environ. Sci. 2010, 29, 920–924. [Google Scholar]
- Lu, Q.Y.; Liu, Y.; Li, C.H.; Wei, X.L.; Liu, Y. Impacts of alien species invasion on the South China Sea ecosystem and related control strategies. Chin. J. Ecol. 2013, 32, 2186–2193. [Google Scholar]
- Li, X.H.; Liu, W.Z.; Zhao, S.; Yang, Y.Q.; Deng, K.P.; Huang, Z.H. Risk assessment of phytoplankton invasion of ballast water. Chin. Front. Health Quar. 2013, 36, 118–123. [Google Scholar]
- Pu, A.M.; Zhang, B.B.; Jia, P.; Wang, Q.G. Analysis of exotic phytoplankton species and their invasion routes in Bohai Bay. J. Agric. Resour. Environ. 2020, 37, 477–483. [Google Scholar]
- Song, L.; Liu, W.; Wu, J.; Song, G.; Song, Y.; Sun, M.; Wang, N. Distribution of the toxic dinoflagellate Stoeckeria algicida in Liaodong Bay. Acta Ecol. Sin. 2017, 37, 1339–1345. [Google Scholar]
- Zhang, S.; Xu, H.; Zhang, Y.; Li, Y.; Wei, J.; Pei, H. Variation of phytoplankton communities and their driving factors along a disturbed temperate river-to-sea ecosystem. Ecol. Indic. 2020, 118, 106776. [Google Scholar] [CrossRef]
- Huang, X.Y.; Ai, S.Y.; He, X.; Wu, X.G.; Chen, X.F. Study on the relationship between phytoplankton community characteristics and environmental factors in River-type reservoirs. J. Hydroecol. 2023. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, D.F.; Yang, Z.J.; Zhang, J.L.; Xu, Y.Q.; Liu, J.G.; Yan, G.H. Vertical stratification characteristics of Dissolved Oxygen and phytoplankton in Thousand-Island Lake and their influencing factors. Environ. Sci. 2017, 38, 1393–1402. [Google Scholar]
- Wang, J.Q.; Wang, S.P.; Zhang, Y.; Lin, J.N.; Gao, X.; Zhang, X.M.; Zhao, Q. Community structure characteristics of eukaryotic planktonic algae in Liaohe River through high-throughput sequencing. Environ. Sci. 2017, 38, 1403–1413. [Google Scholar]
- Chen, J.Q.; Dong, L.; Ma, X.M.; Tian, K.; Bai, J.; Zhao, Y.W. Microbial community monitoring in Baiyangdian Lake based on eDNA technology. J. Agro-Environ. Sci. 2021, 40, 1773–1786. [Google Scholar]
- Kumar, G.; Eble, J.E.; Gaither, M.R. A practical guide to sample preservation and pre-PCR processing of aquatic environmental DNA. Mol. Ecol. Resour. 2020, 20, 29–39. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Giroux, M.S.; Reichman, J.R.; Langknecht, T.; Burgess, R.M.; Ho, K.T. Using eRNA/eDNA metabarcoding to detect community-level impacts of nanoplastic exposure to benthic estuarine ecosystems. Environ. Pollut. 2023, 338, 122650. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Xu, J.; Shen, L.; Zhou, X.; He, L.; Zhao, Z.; Xu, S. Spatial and Temporal Variations in Phytoplankton Community in Dianchi Lake Using eDNA Metabarcoding. Water 2024, 16, 32. https://doi.org/10.3390/w16010032
Lin Y, Xu J, Shen L, Zhou X, He L, Zhao Z, Xu S. Spatial and Temporal Variations in Phytoplankton Community in Dianchi Lake Using eDNA Metabarcoding. Water. 2024; 16(1):32. https://doi.org/10.3390/w16010032
Chicago/Turabian StyleLin, Yuanyuan, Jingge Xu, Liang Shen, Xiaohua Zhou, Liwei He, Zheng Zhao, and Shan Xu. 2024. "Spatial and Temporal Variations in Phytoplankton Community in Dianchi Lake Using eDNA Metabarcoding" Water 16, no. 1: 32. https://doi.org/10.3390/w16010032
APA StyleLin, Y., Xu, J., Shen, L., Zhou, X., He, L., Zhao, Z., & Xu, S. (2024). Spatial and Temporal Variations in Phytoplankton Community in Dianchi Lake Using eDNA Metabarcoding. Water, 16(1), 32. https://doi.org/10.3390/w16010032




