Temporal Phosphorus Dynamics in Shallow Eutrophic Lake Suwa, Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Samples
2.2. Chemical Analyses
2.3. Phosphorus Release Test
2.4. Statistical Analyses
3. Results
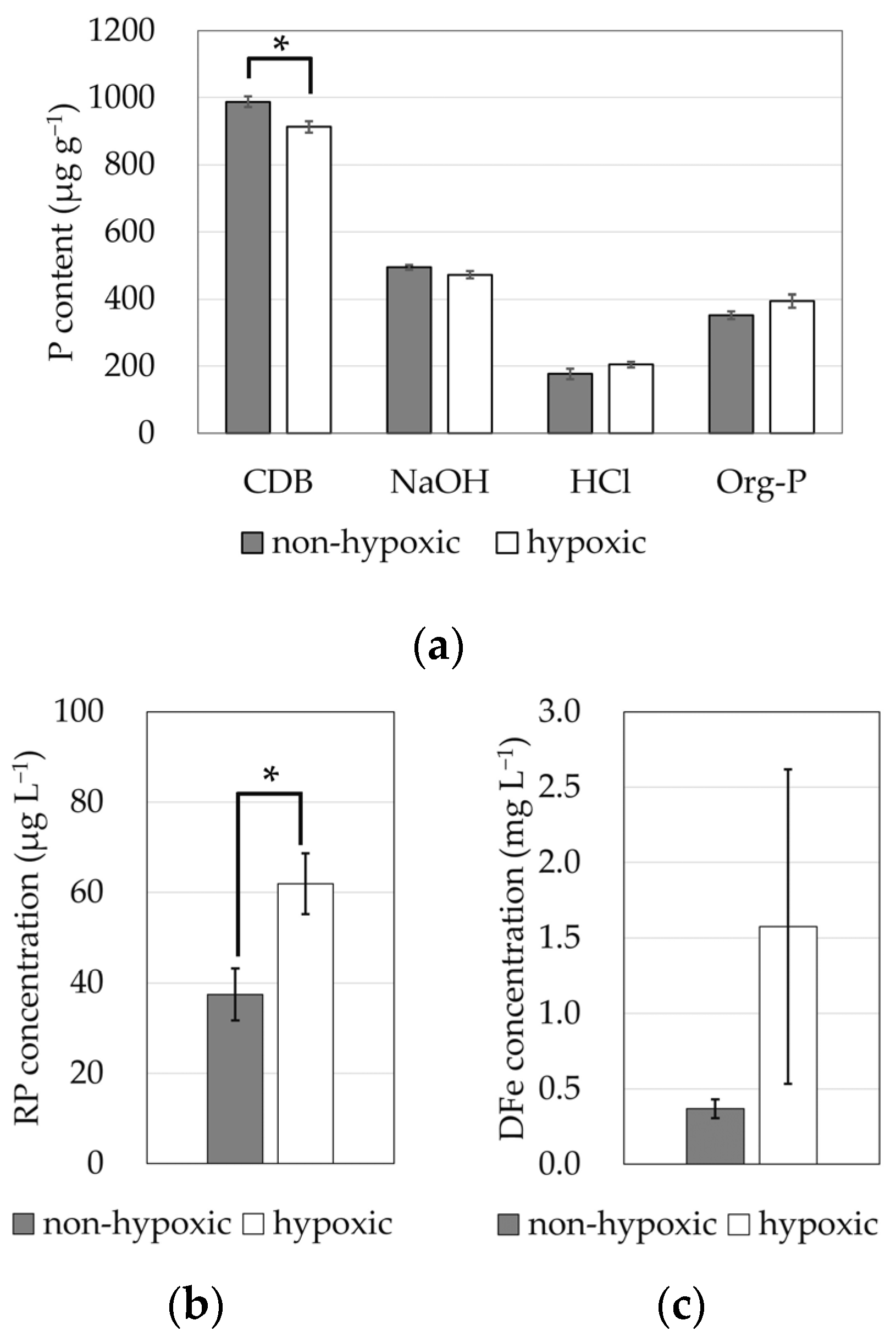
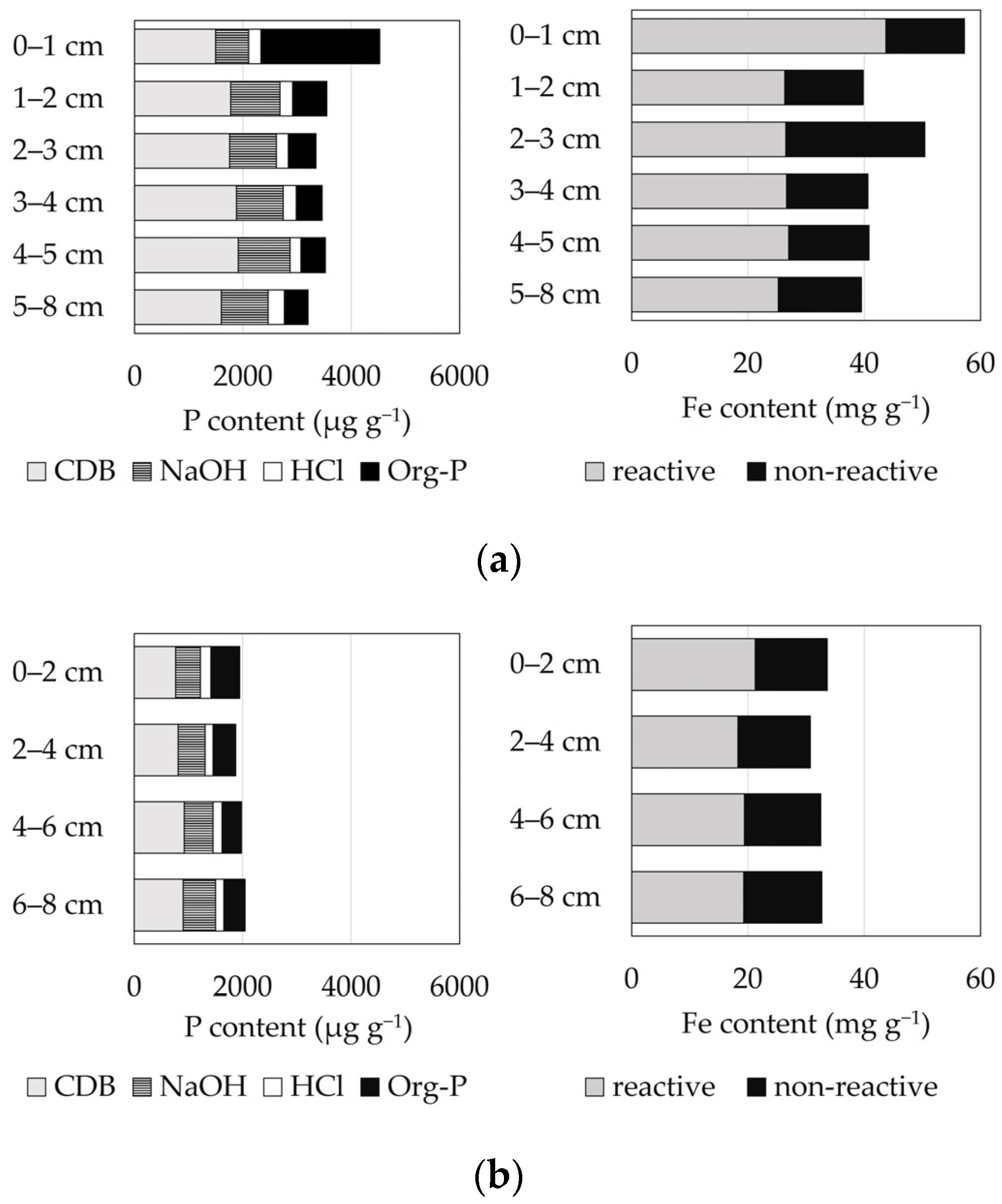
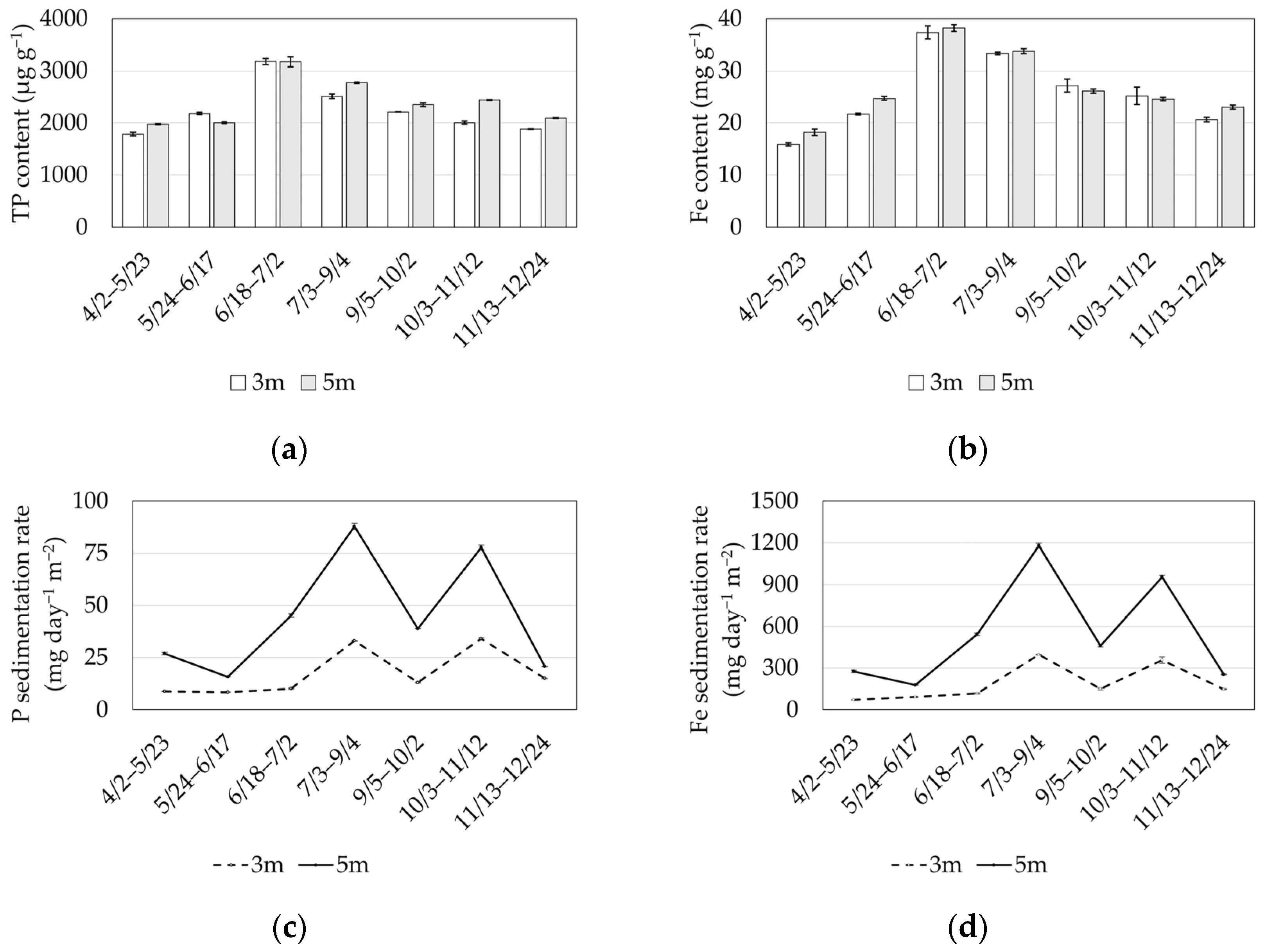
3.1. Field Observation
3.2. Phosphorus Release Test
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Søndergaard, M.; Bjerring, R.; Jeppesen, E. Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 2013, 710, 95–107. [Google Scholar] [CrossRef]
- Horne, A.J.; Goldman, C.R. Limnology, 2nd ed.; McGraw-Hill: New York, NY, USA, 1994; ISBN 978-0071133593. [Google Scholar]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.S.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.M.; Antunes, M.B.; de Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.T.; et al. Liver failure and death after exposure to microcystins at hemodialysis center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Harada, K.I.; Senma, M.; Ito, Y.; Yasuda, N.; Ushida, S.; Kimura, Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: Sudden appearance of toxic cyanobacteria. Nat. Toxins 1999, 7, 81–84. [Google Scholar] [CrossRef]
- Fukushima, T. Have Japanese Lakes Recovered from Polluted State ? Future Directions for Lake Environmental Research. J. Jpn. Soc. Water Environ. 2004, 27, 500–504. (In Japanese) [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake responses to reduced nutrient loading—An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Fukushima, T.; Kawamura, S.; Onda, Y.; Imai, A.; Matsushige, K. Long-term Change in Profile of Sediments and Nutrient Budget in Lakes Kasumigaura and Suwa. J. Jpn. Soc. Water Environ. 2005, 28, 313–319. (In Japanese) [Google Scholar] [CrossRef]
- National Astronomical Observatory of Japan. National Astronomical Observatory of Japan Chronological Science Tables 2020; Maruzen Publishing: Tokyo, Japan, 2020; p. 1047. ISBN 978-4-621-30426-6. [Google Scholar]
- Ibaraki Prefecture; Tochigi Prefecture; Chiba Prefecture. Lake Kasumigaura Water Quality Conservation Plan. 8th Period. 2022. Available online: https://www.pref.ibaraki.jp/seikatsukankyo/kantai/kasumigaura/lake/documents/8ki_honbun.pdf (accessed on 11 April 2024). (In Japanese).
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 1999, 408–409, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Mortensen, E.; Hansen, A.M.; Jørgensen, T. Cascading trophic interactions from fish to bacteria and nutrients after reduced sewage loading: An 18-year study of a shallow hypertrophic lake. Ecosystems 1998, 1, 250–267. [Google Scholar] [CrossRef]
- Watanabe, K.; Park, H.D.; Kumon, F. Historical change of phytoplankton in a eutrophic lake in Japan as determined by analysis of photosynthetic pigments in a lakebed sediment core. Environ. Earth Sci. 2012, 66, 2293–2300. [Google Scholar] [CrossRef]
- Okino, T.; Kato, K. Lake Suwa–Eutrophication and its partial recent recovery. GeoJournal 1987, 14, 373–375. [Google Scholar] [CrossRef]
- Terasawa, J.; Kawamura, M.; Higuchi, S.; Nakazawa, Y. Transition of Water Polluted Load in Lake Suwa. Bull. Nagano Res. Inst. Health Pollut. 1987, 10, 6–11. (In Japanese) [Google Scholar]
- Kurasawa, H. (on behalf of Lake Suwa Researching Group). Watershed Ecosystem Research in Lake Suwa. 1987. Available online: https://soar-ir.repo.nii.ac.jp/records/16456 (accessed on 11 April 2024). (In Japanese).
- Fukuhara, H.; Tanaka, T.; Nakajima, M. The Release of Nutrients from Lake Sediments II. In Lake Suwa Watershed Ecosystem Research Progress Report; Ministry of Education: Tokyo, Japan, 1980; Volume 7, pp. 1–20. (In Japanese) [Google Scholar]
- Okino, T.; Hanazato, T. A Results of the 20-year-monitoring of water quality in Lake Suwa. In Report of the Suwa Hydrobiological Station Shinshu University; Faculty of Science, Shinshu University: Matsumoto, Nagano, Japan, 1997; Volume 10, pp. 7–248. (In Japanese) [Google Scholar]
- Miyabara, Y. A result of the water quality monitoring in Lake Suwa during 2012–2016. In Research Report of the Research and Education Center for Inlandwater Environment, Shinshu University; Department of Atmospheric Environmental & Aquatic Ecosystem, Institute of Mountain Science, Shinshu University: Suwa, Nagano, Japan, 2018; Volume 11, pp. 1–236. (In Japanese) [Google Scholar]
- Nagano Prefecture. Lake Suwa Water Quality Conservation Plan. 8th Period. 2023. Available online: https://www.pref.nagano.lg.jp/mizutaiki/kurashi/shizen/suishitsu/documents/8kikeikaku.pdf (accessed on 11 April 2024). (In Japanese).
- Japan Metrological Agency Past Weather Data Observation. Available online: https://www.data.jma.go.jp/obd/stats/etrn/view/nml_sfc_ym.php?prec_no=48&block_no=47620&year=&month=&day=&elm=normal&view= (accessed on 30 April 2024).
- Urai, A.; Takano, Y.; Matsui, Y.; Iwata, H.; Miyairi, Y.; Yokoyama, Y.; Miyabara, Y.; Ohkouchi, N.; Park, H.D. Origin of Deep Methane from Active Faults along the Itoigawa−Shizuoka Tectonic Line between the Eurasian and North American Plates: 13C/12C and 14C/12C Methane Profiles from a Pull-Apart Basin at Lake Suwa. ACS Earth Space Chem. 2022, 6, 1689–1697. [Google Scholar] [CrossRef]
- Kell, G.S. Effects of isotopic composition, temperature, pressure, and dissolved gases on the density of liquid water. J. Phys. Chem. Ref. Data 1977, 6, 1109–1131. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Menzel, D.W.; Corwin, N. The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulfate oxidation 1. Limnol. Oceanogr. 1965, 10, 280–282. [Google Scholar] [CrossRef]
- Komatsu, N.; Ishii, Y.; Watanabe, K.; Honma, T.; Kitamura, T.; Negishi, M.; Iwasaki, J. Fraction and distribution of phosphorus as evidence of deposition by fish-farming in eutrophic Lake Kasumigaura, Japan. Jpn. J. Limnol. 2009, 69, 193–208. (In Japanese) [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Katsev, S.; Tsandev, I.; L’Heureux, I.; Rancourt, D.G. Factors controlling long-term phosphorus efflux from lake sediments: Exploratory reactive-transport modeling. Chem. Geol. 2006, 234, 127–147. [Google Scholar] [CrossRef]
- Dittrich, M.; Wehrli, B.; Reichert, P. Lake sediments during the transient eutrophication period: Reactive-transport model and identifiability study. Ecol. Modell. 2009, 220, 2751–2769. [Google Scholar] [CrossRef]
- Nagano Prefecture. Lake Suwa Water Quality Conservation Plan; 7th Period; 2018. Available online: https://www.env.go.jp/water/7_suwakokeikaku.pdf (accessed on 11 April 2024). (In Japanese)
- Hanazato, T.; Ogawara, M.; Miyabara, Y. A result of the water quality monitoring in Lake Suwa during 1997–2001. In Research Report of the Research and Education Center for Inlandwater Environment, Shinshu University; Research and Education Center for Inlandwater Environment, Shinshu University: Suwa, Nagano, Japan, 2003; Volume 1, pp. 109–174. (In Japanese) [Google Scholar]
- Miyabara, Y. A result of the water quality monitoring in Lake Suwa during 2002–2006. In Research Report of the Research and Education Center for Inlandwater Environment, Shinshu University; Division of Science for Inland Water Environment, Institute of Mountain Science, Shinshu University: Suwa, Nagano, Japan, 2007; Volume 5, pp. 47–94. (In Japanese) [Google Scholar]
- Miyabara, Y. A result of the water quality monitoring in Lake Suwa during 2007–2011. In Research Report of the Research and Education Center for Inlandwater Environment, Shinshu University; Division of Science for Inland Water Environment, Institute of Mountain Science, Shinshu University: Suwa, Nagano, Japan, 2013; Volume 9, pp. 1–214. (In Japanese) [Google Scholar]
- Wetzel, R.G. Limnology, 2nd ed.; Saunders Publishing: Philadelphia, PA, USA, 1983; ISBN 978-0030330162. [Google Scholar]
- Jane, S.F.; Hansen, G.J.A.; Kraemer, B.M.; Leavitt, P.R.; Mincer, J.L.; North, R.L.; Pilla, R.M.; Stetler, J.T.; Williamson, C.E.; Woolway, R.I.; et al. Widespread deoxygenation of temperate lakes. Nature 2021, 594, 66–70. [Google Scholar] [CrossRef]
- Nagano Environmental Conservation Research Institute. Research on the actual situation and prediction of global warming in Nagano Prefecture. In Research Report on the Development of Global Warming Impact Assessment and Adaptation Planning Methods in Nagano Prefecture; 2015; pp. 2–28. Available online: https://www.pref.nagano.lg.jp/kanken/chosa/kenkyu/coolearth/documents/chap_1.pdf (accessed on 30 October 2023). (In Japanese)
- Caraco, N.F.; Cole, J.J.; Likens, G.E. Sulfate control of phosphorus availability in lakes: A test and re-evaluation of Hasler and Einsele’s model. Hydrobiologia 1993, 253, 275–280. [Google Scholar] [CrossRef]
- Terashima, S.; Inouchi, Y.; Nakao, S.; Yonetani, H. Geochemistry of fourteen elements\ in bottom sediments from Lake Suwa, Central Japan. Bull. Geol. Surv. Jpn. 1990, 41, 147–172. (In Japanese) [Google Scholar]
- Kawano, Y. The Effects of Nutrients in the Lake Sediment on Eutrophication of Lakes—Features in the distribution of nutrients in the sediment of Lake Suwa and their behavior. Jpn. J. Hyg. 1986, 40, 892–902. (In Japanese) [Google Scholar] [CrossRef]
- Futatsugi, Y.; Miyabara, Y.; Saito, Y.; Hanazato, T.; Park, H.D. Long-term Shifts in Dominant Phytoplankton Species and Trophic State Index in Summer in Lake Suwa. J. Jpn. Soc. Water Environ. 2018, 41, 43–54. (In Japanese) [Google Scholar] [CrossRef]
- Gächter, R.; Müller, B. Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol. Oceanogr. 2003, 48, 929–933. [Google Scholar] [CrossRef]
- Schauser, I.; Chorus, I.; Lewandowski, J. Effects of nitrate on phosphorus release: Comparison of two Berlin lakes. Acta Hydrochim. Hydrobiol. 2006, 34, 325–332. [Google Scholar] [CrossRef]
- Kawano, Y.; Terasawa, J.; Harada, T.; Nasu, Y.; Kugimoto, M. Investigation on Release of Nitrogen and Phosphorus from the sediments of Different Depth in Lake Suwa. Bull. Nagano Res. Inst. Health Pollut. 1987, 10, 12–17. (In Japanese) [Google Scholar]
- Terashima, S.; Inouchi, Y.; Miyata, Y.; Katayama, H.; Saito, Y.; Yasuda, A.; Watanabe, K.; Yoshikawa, H.; Inazaki, T. Geochemistry of eleven elements in bottom sediments from Lake Suwa, Japan. Bull. Geol. Surv. Jpn. 1992, 43, 549–564. (In Japanese) [Google Scholar]
- Kanai, Y.; Inouchi, Y.; Katayama, H.; Saito, Y. Estimation of sedimentation rate at the lake Suwa in Nagano Prefecture determined by Pb–210 and Cs–137 radioactivities. Bull. Geol. Surv. Jpn. 1995, 46, 225–238. (In Japanese) [Google Scholar]
- Inouchi, Y.; Saito, Y.; Yokota, S. Sedimentation rate of Lake Kasumigaura Sediments—Sedimentation rate calculation by tephra. J. Geol. Soc. Jpn. 1983, 89, 125–128. (In Japanese) [Google Scholar] [CrossRef]
- Kanai, Y.; Inouchi, Y. Sedimentation rate and sedimentary environment during the past 100 years in Lake Biwa, Shiga Prefecture, central Japan. Bull. Geol. Surv. Jpn. 2016, 67, 67–80. (In Japanese) [Google Scholar] [CrossRef]
- Ikeya, N.; Wada, H.; Ohmori, M. On the Boring Core Sediments from Hamana Lake. Geosci. Rep. Shizuoka Univ. 1987, 13, 67–111. (In Japanese) [Google Scholar] [CrossRef]
- Kumon, F.; Tawara, T.; Yamamoto, M. Sediment age and Sedimentation rate in Lake Kizaki, central Japan. Res. Rep. Res. Educ. Cent. Inlandwater Environ. Shinshu Univ. 2004, 3, 77–84. (In Japanese) [Google Scholar]
- Toyota, M.; Kato, H.; Imai, A.; Miyabara, Y. Influence of Mowing Down Floating-Leaved Plants on Water Body Structure in Lake Suwa. Jpn. J. JSCE 2011, 67, I_1465–I_1470. (In Japanese) [Google Scholar] [CrossRef]
- Akahori, Y.; Takagi, S.; Nishihiro, J.; Kagami, M. Effect of floating-leaved plant, Trapa natans, on the water qualities in Lake Inba. Jpn. J. Limnol. 2016, 77, 155–166. (In Japanese) [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, Y.; Kunito, T.; Miyabara, Y. Temporal Phosphorus Dynamics in Shallow Eutrophic Lake Suwa, Japan. Water 2024, 16, 1340. https://doi.org/10.3390/w16101340
Ichikawa Y, Kunito T, Miyabara Y. Temporal Phosphorus Dynamics in Shallow Eutrophic Lake Suwa, Japan. Water. 2024; 16(10):1340. https://doi.org/10.3390/w16101340
Chicago/Turabian StyleIchikawa, Yutaka, Takashi Kunito, and Yuichi Miyabara. 2024. "Temporal Phosphorus Dynamics in Shallow Eutrophic Lake Suwa, Japan" Water 16, no. 10: 1340. https://doi.org/10.3390/w16101340
APA StyleIchikawa, Y., Kunito, T., & Miyabara, Y. (2024). Temporal Phosphorus Dynamics in Shallow Eutrophic Lake Suwa, Japan. Water, 16(10), 1340. https://doi.org/10.3390/w16101340





