Nutrient Removal from Aqueous Solutions Using Biosorbents Derived from Rice and Corn Husk Residues: A Systematic Review from the Environmental Management Perspective
Abstract
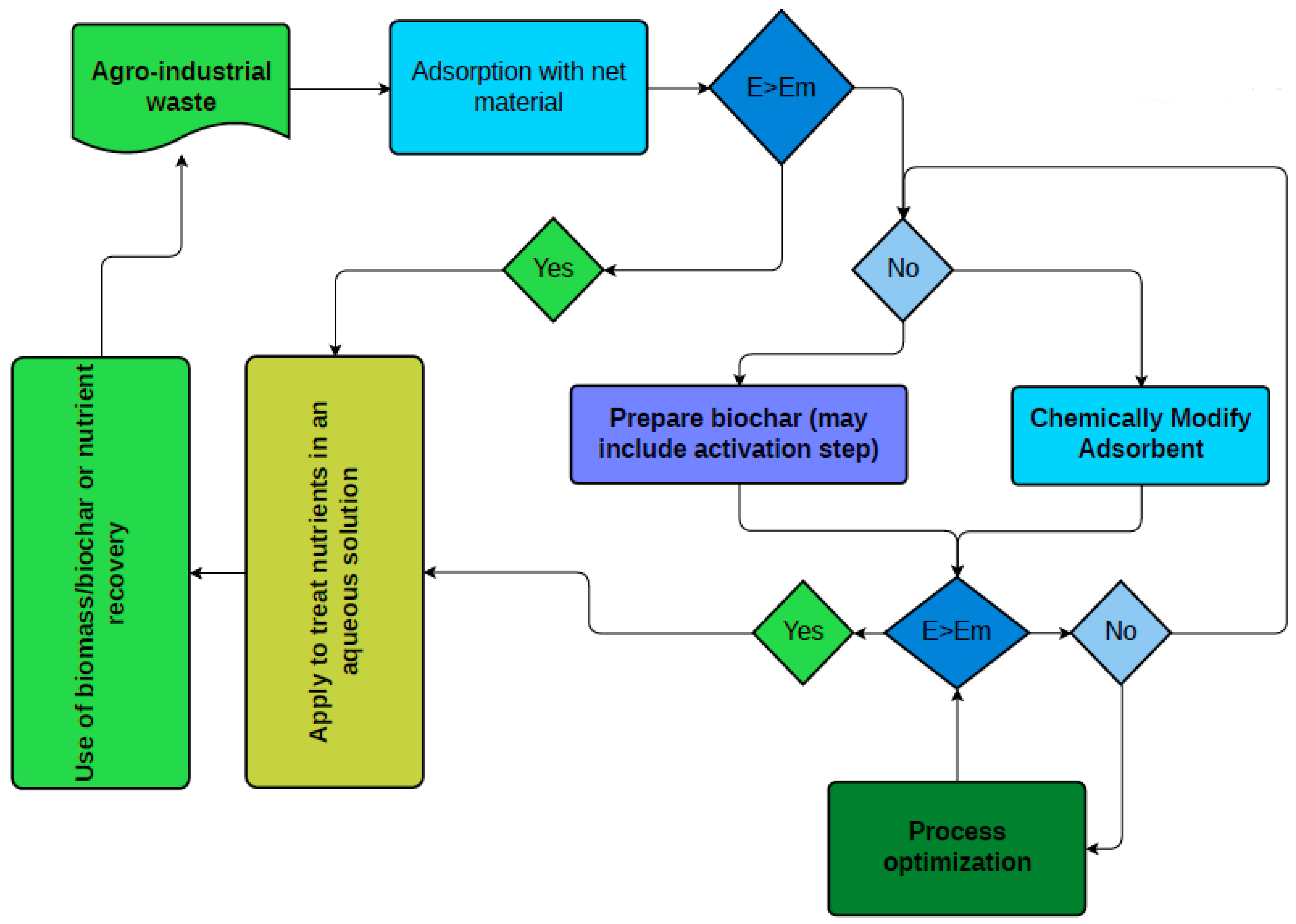
:1. Introduction
2. Effects of Nutrients Dissolved in Water on Human Health
| Nutrient or Contaminant | Effect on Health (Disease) | Reference | Permissible Limit |
|---|---|---|---|
| Phosphate | Urine damage | [34,41] | 0.5 mg/L [42] |
| Osteoporosis | [34] | ||
| Nitrate | Infantile cyanosis syndrome (blue baby syndrome) | [38,43,44] | 10 mg/L [45,46] |
| Cancer | [43,44] | ||
| Hepatic damage | [39] | ||
| Vomiting | [38] | ||
| Hypertension | [38] | ||
| Diarrhea | [38] | ||
| Respiratory tract disease | [38] | ||
| Spontaneous abortions (miscarriages) | [38] | ||
| Ammonium | Hyperammonemia (affects developing central nervous system) | [35] | 1.5 mg/L [47] |
| Liver failure | [48,49] | ||
| Nitrite | Cancer | [40] | 0.2 mg/L [50] |
| Methemoglobinemia | [40] | ||
| Effect on the thyroid gland | [40] | ||
| Urinary tract tumors | [40] | ||
| Algae cyanotoxin | Paralytic effects | [33,51] | 1-µg/L Microcystin [52,53] |
| Diarrhea | [33,51] | ||
| Neurotoxic affectation | [33,51] | ||
| Hypoxia | [54] |
3. Importance of Biosorption in Removing Phosphorus and Nitrogen from Wastewater
4. Agro-Industrial Residues and Biosorbents
| Biosorbent/Classification | Operation Mode | Geographic Location | Type of Water Loaded with Nutrients | Pollutant | Removal Efficiency (%) | Data for Biosorption Capacity | Reference |
|---|---|---|---|---|---|---|---|
| Rice husk biochar/No activation used | Batch | Fukuoka (Japan). | Synthetic water solutions | Nitrates and phosphates | 5–25 | Co = 5–20 mg/L. | [85] |
| Da = 5 g/L q = 2.1–5 mg/g | |||||||
| Wheat straw chemically modified with Epichlorohydrin/Biosorbent used after chemical activation | Batch | China | Synthetic water solutions | Nitrates | 50 | Co = 50 mg/L. | [93] |
| Da = 4 g/L q = 2.1 mmol/g | |||||||
| Corn straw biochar chemically modified with Fe3O4/Biosorbent used after chemical activation | Batch | China | Synthetic water solutions | Phosphates | 55–95 | Co = 20 mg/L. | [94] |
| Da = 4 g/L q = 2–20 mg/g | |||||||
| Eggshell and rice straw biochar chemically modified with CaO/Biosorbent used after chemical activation | Batch | China | Synthetic water solutions | Phosphates | 45 | Co = 5–200 mg/L. | [95] |
| Da = 0,2 g/L q = 231 mg/g | |||||||
| Date palm wastes (surface fibers)/No activation used | Batch | Iraq | Synthetic water solutions | Phosphates | 85 | Co = 50 mg/L. | [96] |
| Da = 5 g/L q = 8–9 mg/g | |||||||
| Date palm wastes (date stones)/No activation used | Batch | Iraq | Synthetic water solutions | Phosphates | 87 | Co = 50 mg/L. | [96] |
| Da = 5 g/L q = 8–9 mg/g | |||||||
| Pine cone (raw and sodium hydroxide-modified)/Biosorbent used after chemical activation | Batch | Turkey | Synthetic water solutions | Ammonium | 19–99 | Co= 50 mg/L. | [97] |
| Da = 0.5–10 g/L q = 35–55 mg/g | |||||||
| Pomegranate peel (raw)/No activation used | Batch | Hungary | Synthetic water solutions | Ammonium | 27–97 | Co = 5–90 mg/L. | [98] |
| Da = 0.5–10 g/L q = 4–5 mg/g |
5. Rice Husks in Nutrient Removal from Aqueous Solutions
6. Corn Residues for Treating the Nutrients in Aqueous Solutions
| Biosorbent/Classification | Biosorption Method (Operation Mode) | Geographic Location | Type of Water Loaded with Nutrients | Pollutant | Removal Efficiency (%) | Data for Biosorption Capacity | Reference |
|---|---|---|---|---|---|---|---|
| Rice husk biochar/No activation used | Batch | Khordha (India) | Synthetic water solutions | Nitrates and phosphates | 65–75 | Co = 0.5–10 mg/L. Da = 200 g/L. q = 0.07–0.5 mg/g | [16] |
| Rice husk: 1) ground, 2) chemically modified with (3-chloro-2-hydroxypropyl)-trimethylammonium/Biosorbent used after chemical activation | Batch | Chonburi (Thailand) | Wastewater from swine processes | Nitrates and phosphates | 65–84 | Co = 0.5–10 mg/L. Da = 5–30 g/L. q = 2–12 mg/g | [117] |
| Rice husk/No activation used | Fixed bed | Iraq | Synthetic water | Total phosphorus | 95 | Co = 1 mg/L. bh = 1 m | [78] |
| A layer mix of recycled concrete aggregate (RCA), crushed glass, and rice husk/No activation used | Fixed bed | Rio Grande Valley of Texas (United States) | Synthetic waters to simulate storm runoff | Nitrates and phosphates | 88–99 | Co = 6–75 mg/L. bh = 0.5 m | [125] |
| NaOH-modified rice husk biochar/Biosorbent used after chemical activation | Batch | Besut, Terengganu (Malaysia) | Synthetic water | Phosphates | 97 | Co = 2–10 mg/L. Da = 0.2–1 g/L. q = 1.8 mg/g | [26] |
| Rice husk biochar/No activation used | Batch | Pakistan | Synthetic water | Nitrates and phosphates | 40–95 | Co = 50–100 mg/L. Da = 1 g/L. q = 47–95 mg/g | [16] |
| Rice husk biochar/No activation used | Batch | Beijing (China) | Residual water from a pig-manure digester plant | Nitrates and nitrites | 50–80 | Co = 35–60 mg/L. Da = 1–50 g/L. q = 40–45 mg/g | [17] |
| HCl-modified rice husk biochar/Biosorbent used after chemical activation | Batch | Panipat (India) | Synthetic water | Phosphates | 89 | Co = 10 mg/L. Da= 0.4–4 g/L. q= 0.8–1.4 mg/g | [10] |
| Rice husk/No activation used | Fixed bed | Iraq | Synthetic water | Total nitrogen | 97 | Co = 1 mg/L. bh = 0.4 m | [5] |
| Rice husk biochar/No activation used | Batch | Huzhou (China) | Synthetic water | Ammonium Ion | 10–50 | Co = 40 mg/L. Da = 0.8–8 g/L. q = 0.6–2 mg/g | [18] |
| Rice husk biochar treated with chemical solutions, such as NaOH and H2SO4/Biosorbent used after chemical activation | Batch | Benarés (India) | Synthetic water | Phosphates | 60–97 | Co = 10–30 mg/L. Da = 2–7 g/L. q = 9–13 mg/g | [82] |
| Rice husk biochar/No activation used | Batch | Juja (Kenya) | Wastewater from an animal slaughterhouse | Nitrates and nitrites | 35–65 | Co = 13–130 mg/L. Da = 0.8–8 g/L. q = 0.3–13 mg/g | [39] |
| Silica compound MCM-41 synthesized from rice husk/Biosorbent used after chemical activation | Batch | Egypt | Synthetic water | Phosphates | 36–76 | Co = 0.5–2.5 mM of Na2HPO4·2H2O. Da = 2–8 g/L. q = 11–16 mg/g | [142] |
| Rice husk biochar mixed with calcite/Biosorbent used after chemical activation | Batch | Jiaxing (China) | Synthetic water | Phosphates | 54–87 | Co = 25–125 mg/L. Da = 0.25–0,35 g/L. q = 11 mg/g | [123] |
| Rice husk biochar activated with MgO/Biosorbent used after chemical activation | Batch | Thai Nguyen (Vietnam) | Synthetic water | Ammonium ion and phosphates | 44–71 | Co = 100 mg/L. Da = 1 g/L. q = 17–118 mg/g | [74] |
| Rice husk biochar with and without a mixture of sludge/No activation used | Batch | Ho Chi Minh City (Vietnam) | Synthetic water | Phosphate and ammonium | 46–74 | Co = 50–100 mg/L. Da = 1–1.2 g/L. q = 61–67 mg/g | [143] |
| Mixture of rice husk biochar and oyster shell/No activation used | Batch | Jiaxing (China) | Synthetic water and domestic sewage | Total phosphorus | 93–99 | Co = 3–100 mg/L. Da = 0.2 g/L. q = 150–200 mg/g | [144] |
| Rice husk biochar activated with Ca/Biosorbent used after chemical activation | Batch | Taiwan | Synthetic water | Nitrates | 20–55 | Co = 100 mg/L. Da = 0.1 g/L. q = 2.4–32 mg/g | [145] |
| Corn straw biochar chemically modified with ferrous sulfate/Biosorbent used after chemical activation | Fixed bed | Henan (China) | Synthetic water to simulate runoff | Total phosphorus | 80–99 | Co = 1.9–2.5 mg/L. bh = 0.5 m. q = 0.7 mg/g | [99] |
| Corn cob biochar/No activation used | Batch | Beijing (China) | Synthetic water | Ammonium Ion | 7–15 | Co = 100 mg/L. Da = 10 g/L. q = 0.6–1.1 mg/g | [76] |
| Corn stalk biochar chemically modified with Mg/Biosorbent used after chemical activation | Batch | Beijing (China) | Swine plant wastewater | Total phosphorus | 83–95 | Co = 84–2600 mg/L. Da = 10 g/L. q = 7–18 mg/g | [134] |
| Cellulose extracted from corn stalks chemically modified with dimethylformamide, pyridine, and diethylamine/Biosorbent used after chemical activation | Batch | Shaanxi (China) | Synthetic water | Nitrates and phosphates | 10–60 | Co = 0.5–100 mg/L. Da = 2 g/L. q = 14–23 mg/g | [19] |
| Corn stover biochar/No activation used | Batch | New York (United States) | Synthetic water | Nitrates and phosphates | 98–99 | Co = 0.1–10 mg/L. Da = 10 g/L | [20] |
| (1) Corn cobs modified with graft amines. (2) Unmodified corn cob./Biosorbent used after chemical activation | Batch | Sydney (Australia) | Synthetic water | Nitrates | 10–50 | Co = 20 mg/L. Da = 0.1–1 g/L. q = 50 mg/g | [75] |
| Raw granular corn cob (GCC)/No activation used | Batch | Baghdad (Iraq) | Domestic wastewater | Ammonium Ion | 56 | Co = 5–100 mg/L. Da = 3 g/L. q = 2–10 mg/g | [146] |
| Corn cob biochar/No activation used | Batch | Bogor (Indonesia) | Synthetic water | Ammonium ion, nitrate, and phosphate | 90 | Co = 0.1–50 mg/L. Da = 8 g/L. q = 0.18 mg/g | [107] |
| Corn cob biochar/No activation used | Batch | Bhubaneswar (India) | Synthetic gray water | Phosphates | 39–63 | Co = 16–22 mg/L. Da = 0.1–0.55 g/L. q = 4–13 mg/g | [135] |
| Corn straw biochar/No activation used | Batch | China | Synthetic water | Ammoniacal nitrogen | 45–89 | Co = 30 mg/L. Da = 33 g/L. q = 0.8–1 mg/g | [147] |
| Corn straw biochar modified with magnesium chloride (MgCl2) /Biosorbent used after chemical activation | Batch | Guilin (China) | Synthetic water | Ammonium ion and total phosphorus | 30–80 | Co = 20–350 mg/L. Da = 5 g/L. q = 10–25 mg/g | [128] |
| Corn stalk biochar assembled with double-layer hydroxyls (Ni–Fe, Mg–Al, and Zn–Al)/Biosorbent used after chemical activation | Batch | Harbin (China) | Synthetic water | Phosphates | 30–93 | Co = 50 mg/L. Da = 0.25 g/L. q = 152 mg/g | [52] |
| Corn stalk biochar, previously modifying the stalk with FeCl3/Biosorbent used after chemical activation | Batch | China | Real eutrophic water | Total nitrogen and phosphorus | 35–85 | Co = 2.5–50 mg/L. Da = 1.25 g/L. q = 14–90 mg/g | [104] |
| Corn cob biochar/No activation used | Fixed bed | Chiang Mai (Thailand) | Wastewater from a pig farm | Ammonium ion and phosphates | 72–76 | Co = 0.7–15 mg/L. bh = 1 m | [136] |
| Corn stover biochar modified with calcium/Biosorbent used after chemical activation | Batch | China | Synthetic water | Phosphates | 30–86 | Co = 30 mg/L. Da = 1 g/L. q = 34 mg/g | [133] |
| Corn straw biochar, modified with (1) potassium hydroxide (KOH) and (2) ferric chloride (FeCl3)/Biosorbent used after chemical activation | Batch | China | Synthetic water | Ammonium ion | 5–38 | Co = 100 mg/L. Da = 2 g/L. q = 5–22 mg/g | [139] |
7. Selection Criteria and Application of Biosorbents for Treating Wastewater Nutrients
- Low cost. Agro-industrial residues are low-cost when their biosorption is simple or when they require low energy content to obtain them, such as when only applying washing and drying to the material or biomass (raw material without additional treatment), such as husks of rice, cob, stalk, and corn straw. However, physical or chemical modifications, such as pyrolysis (to obtain biochar) or chemical activation (through impregnation of precursor substances to increase the biosorption capacity of biosorbents), on agro-industrial waste increases their cost because of the energy required for heating or the use of substances as activating agents, among other aspects.
- Innocuous (nontoxic for the environment).
- Abundant (such as agro-industrial waste), and the use of nonrenewable materials should be avoided.
- Readily available in various territories.
- Generate less negative environmental impacts. For example, prioritizing biodegradable materials or materials that have an affinity with the environment for reuse or exploitation.
- Potential for reuse or recovery of materials, promoting the circular economy. For example, biosorption residue can be used in the production chain, as is the case for improving soil properties and power generation.
- Easy to prepare, operate, and maintain.
- Should not inhibit or reduce the efficiency of another wastewater treatment process (such as a biological process for wastewater treatment) if it is required to be coupled with other wastewater treatment technologies. In case of negatively affecting another process, the characteristics or variables related to it should be studied to guarantee the effectiveness or proper functioning of the sequential set of operations and unitary processes for eliminating contaminants from residual water according to the water quality requirement of the treated effluent.
- Does not require expensive equipment or supplies to obtain an activating agent or biosorbent to be applied in biosorption.
8. Current Challenges in Studying Biosorbents with Regard to the Treatment of Wastewater Nutrients
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, N.; Wang, L.; Lin, L.; Li, Y.; Zhang, W.; Niu, L.; Zhang, H.; Wang, L. Pelagic-benthic coupling of the microbial food web modifies nutrient cycles along a cascade-dammed river. Front. Environ. Sci. Eng. 2022, 16, 50. [Google Scholar] [CrossRef]
- Lugo, J.L.; Lugo, E.R.; Puente, M. A systematic review of microorganisms as indicators of recreational water quality in natural and drinking water systems. J. Water Health 2021, 19, 20–28. [Google Scholar] [CrossRef]
- Lugo-Arias, J.; Burgos-Vergara, J.; Lugo-Arias, E.; Gould, A.; Ovallos-Gazabon, D. Evaluation of low-cost alternatives for water purification in the stilt house villages of Santa Marta’s Ciénaga Grande. Heliyon 2020, 6, e03062. [Google Scholar] [CrossRef]
- Fageria, N.; Baligar, V. Nutrient availability. Encycl. Soils Environ. 2005, 3, 63–71. [Google Scholar] [CrossRef]
- Maleh, G. Utilization of rice husk in the sorption of eutrophication nitrogen and producing a useful organic fertilizer for plant production. J. Eng. Sustain. Dev. 2018, 22, 65–76. Available online: https://jeasd.uomustansiriyah.edu.iq/index.php/jeasd/article/view/324 (accessed on 12 September 2023). [CrossRef]
- Wang, X.; Zhang, S.; Liu, S.; Chen, J. A two-dimensional numerical model for eutrophication in Baiyangdian Lake. Front. Environ. Sci. Eng. 2012, 6, 815–824. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Pinzon, J.; Oppenheimer, J.; Jacangelo, J. Sources of nutrients impacting surface waters in Florida: A review. J. Environ. Manag. 2012, 109, 80–92. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, B.; Wester, A.E.; Chen, J.; He, F.; Chen, H.; Gao, B. Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chem. Eng. J. 2019, 362, 460–468. [Google Scholar] [CrossRef]
- Shukla, N.; Sahoo, D.; Remya, N. Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J. Clean. Prod. 2019, 235, 1073–1079. [Google Scholar] [CrossRef]
- Mor, S.; Chhoden, K.; Ravindra, K. Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J. Clean. Prod. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B.; Khan, M. Recent advances on activated carbon-based materials for nitrate adsorption: A review. J. Anal. Appl. Pyrolysis. 2023, 169, 105856. [Google Scholar] [CrossRef]
- Almanassra, I.; Kochkodan, V.; Mckay, G.; Atieh, M.; Al-Ansari, T. Review of phosphate removal from water by carbonaceous sorbents. J. Environ. Manag. 2021, 287, 112245. [Google Scholar] [CrossRef]
- Bar, N.; Mitra, T.; Das, S. Biosorption of Cu (II) ions from industrial effluents by rice husk: Experiment, statistical, and ANN modeling. J. Environ. Eng. Landsc. Manag. 2021, 29, 441–448. [Google Scholar] [CrossRef]
- Sarkar, S.; Bar, N.; Das, S. Cr (VI) and Cu (II) removal from aqueous solution in fixed bed column using rice bran; experimental, statistical and GA modelling. J. Indian Chem. Soc. 2021, 98, 100216. [Google Scholar] [CrossRef]
- Mandal, A.; Bar, N.; Das, S. Phenol Adsorption by Biological and Industrial Wastes and ANN Modeling. In Effective Waste Management and Circular Economy: Legislative Framework and Strategies; 2022; p. 141. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003231608-16/phenol-adsorption-biological-industrial-wastes-ann-modeling-ashanendu-mandal-nirjhar-bar-sudip-kumar-das (accessed on 15 December 2023).
- Fatima, I.; Ahmad, M.; Vithanage, M.; Iqbal, S. Abstraction of nitrates and phosphates from water by sawdust- and rice husk-derived biochars: Their potential as N- and P-loaded fertilizer for plant productivity in nutrient deficient soil. J. Anal. Appl. Pyrolysis 2021, 155, 105073. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Zhu, K.; Fu, H.; Zhang, J.; Lv, X.; Tang, J.; Xu, X. Studies on removal of NH4+-N from aqueous solution by using the activated carbons derived from rice husk. Biomass Bioenergy 2012, 43, 18–25. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, Y. Adsorption isotherms, kinetics and thermodynamics of nitrate and phosphate in binary systems on a novel adsorbent derived from corn stalks. J. Geochem. Explor. 2018, 188, 95–100. [Google Scholar] [CrossRef]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, nitrate, and phosphate sorption to and solute leaching from biochars prepared from corn stover (Zea mays L.) and oak wood (Quercus spp.). J. Environ. Qual. 2013, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Montenegro Orozco, K.T.; Rojas Carpio, A.S.; Cabeza Rojas, I.; Hernández Pardo, M.A. Potencial de biogás de los residuos agroindustriales generados en el departamento de Cundinamarca. Rev. ION 2017, 29, 23–36. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Orozco Colonia, B.S. Producción y procesamiento del maíz en Colombia. Rev. Guillermo Ockham 2013, 11, 97–110. Available online: https://www.redalyc.org/pdf/1053/105327548008.pdf (accessed on 20 September 2023). [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent advances on pollutants removal by rice husk as a bio-based adsorbent: A critical review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Danish, M.; Anastopoulos, I.; Iwuozor, K.O. Recent progress on corn (Zea mays L.)-based materials as raw, chemically modified, carbonaceous, and composite adsorbents for aquatic pollutants: A review. J. Anal. Appl. Pyrolysis 2023, 172, 106004. [Google Scholar] [CrossRef]
- Karam, D.S.; Nagabovanalli, P.; Rajoo, K.S.; Ishak, C.F.; Abdu, A.; Rosli, Z.; Muharam, F.M.; Zulperi, D. An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J. Saudi Soc. Agric. Sci. 2022, 21, 149–159. [Google Scholar] [CrossRef]
- Hamzah, S.; Razali, N.A.; Yatim, N.I.; Alias, M.; Ali, A.; Zaini, N.S.; Abuhabib, A.A.M. Characterisation and performance of thermally treated rice husk as efficient adsorbent for phosphate removal. J. Water Supply Res. Technol. AQUA 2018, 67, 766–778. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Zhao, H.; Dang, J.; Jin, R.; Zhao, W.; Li, Y. Wheat straws and corn straws as adsorbents for the removal of Cr(VI) and Cr(III) from aqueous solution: Kinetics, isotherm, and mechanism. ACS Omega 2020, 5, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Abaide, E.R.; Dotto, G.L.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Adsorption of 2–nitrophenol using rice straw and rice husks hydrolyzed by subcritical water. Bioresour. Technol. 2019, 284, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhang, Q.; Owens, G.; Chen, Z. A cellulose degrading bacterial strain used to modify rice straw can enhance Cu(II) removal from aqueous solution. Chemosphere 2020, 256, 127142. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X. Removal of Cr (VI) from aqueous solution using modified corn stalks: Characteristic, equilibrium, kinetic and thermodynamic study. Chem. Eng. J. 2011, 168, 909–917. [Google Scholar] [CrossRef]
- Mejbel, H.; Irwin, C.; Dodsworth, W.; Higgins, S.; Paterson, M.; Pick, F. Long-term cyanobacterial dynamics from lake sediment DNA in relation to experimental eutrophication, acidification and climate change. Freshw. Biol. 2023, 68, 1875–1893. [Google Scholar] [CrossRef]
- Bates, S.S.; Freitas, A.; Milley, J.E.; Pocklington, R.; Quilliam, M.A.; Smith, J.C.; Worms, J. Controls on domoic acid production by the diatom Nitzschia pungens f. Multiseries in culture: Nutrients and irradiance. Can. J. Fish. Aquat. Sci. 1991, 48, 1136–1144. [Google Scholar] [CrossRef]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef]
- Braissant, O.; McLin, V.; Cudalbu, C. Ammonia toxicity to the brain. J. Inherit. Metab. Dis. 2013, 36, 595–612. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.; Reichl, F.; Nieder, R.; Benbi, D.; Reichl, F. Reactive water-soluble forms of nitrogen and phosphorus and their impacts on environment and human health. Soil Compon. Hum. Health 2018, 223–255. Available online: https://link.springer.com/chapter/10.1007/978-94-024-1222-2_5 (accessed on 18 September 2023).
- EPA. Drinking Water from Household Wells; EPA: Washington, DC, USA, 2002; EPA 816-K-02–003. [Google Scholar]
- Fewtrell, L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: A discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef]
- Konneh, M.; Wandera, S.M.; Murunga, S.I.; Raude, J.M. Adsorption and desorption of nutrients from abattoir wastewater: Modelling and comparison of rice, coconut and coffee husk biochar. Heliyon 2021, 7, e08458. [Google Scholar] [CrossRef] [PubMed]
- Parvizishad, M.; Dalvand, A.; Mahvi, A.; Goodarzi, F. A review of adverse effects and benefits of nitrate and nitrite in drinking water and food on human health. Health Scope 2017, 6, e14164. [Google Scholar] [CrossRef]
- Oliveira, M.; Machado, A.V.; Nogueira, R. Phosphorus removal from eutrophic waters with an aluminium hybrid nanocomposite. Water Air Soil Pollut. 2012, 223, 4831–4840. [Google Scholar] [CrossRef]
- Romero, J. Calidad del Agua, 3rd ed.; Escuela Colombiana de Ingeniería Julio Garavito: Bogotá, Colombia, 2009. [Google Scholar]
- EPA. Estimated National Occurrence and Exposure to Nitrate/Nitrite in Public Drinking Water Supplies; US Environmental Protection Agency: Washington, DC, USA, 1990. [Google Scholar]
- Moradzadeh, M.; Moazed, H.; Sayyad, G.; Khaledian, M. Transport of nitrate and ammonium ions in a sandy loam soil treated with potassium zeolite—Evaluating equilibrium and non-equilibrium equations. Acta Ecol. Sin. 2014, 34, 342–350. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations; United States Environmental Protection Agency: Washington, DC, USA, 2009; EPA 816-F-09-004. [Google Scholar]
- Zhang, Y.; Zhang, C.; Qiu, Y.; Li, B.; Pang, H.; Xue, Y.; Liu, Y.; Yuan, Z.; Huang, X. Wastewater treatment technology selection under various influent conditions and effluent standards based on life cycle assessment. Resour. Conserv. Recycl. 2020, 154, 104562. [Google Scholar] [CrossRef]
- Lewoyehu, M. Evaluation of drinking water quality in rural area of Amhara region, Ethiopia: The case of Mecha district. J. Chem. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Adeva, M.; Souto, G.; Blanco, N.; Donapetry, C. Ammonium metabolism in humans. Metabolism 2012, 61, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Warren, K. The differential toxicity of ammonium salts. J. Clin. Investig. 1958, 37, 497–501. [Google Scholar] [CrossRef]
- Moazeni, M.; Ebrahimi, A.; Atefi, M.; Mahaki, B.; Rastegari, A. Determination of nitrate and nitrite exposure and their health risk assessment in 21 brands of bottled waters in Isfahan’s market in 2013. Int. J. Environ. Health Eng. 2014, 3, 71–75. Available online: https://www.researchgate.net/publication/265604514_Determination_of_nitrate_and_nitrite_exposure_and_their_health_risk_assessment_in_21_brands_of_bottled_waters_in_Isfahan%27s_market_in_2013 (accessed on 15 December 2023).
- Richardson, K. Harmful or exceptional phytoplankton blooms in the marine ecosystem. Adv. Mar. Biol. 1997, 31, 301–385. [Google Scholar] [CrossRef]
- Nguyen, T.A.D.; Nguyen, L.T.; Enright, A.; Pham, L.T.; Tran, H.Y.T.; Tran, T.T.; Nguyen, V.H.T.; Tran, D.N. Health risk assessment related to cyanotoxins exposure of a community living near Tri an reservoir, Vietnam. Environ. Sci. Pollut. Res. Int. 2021, 28, 56079–56091. [Google Scholar] [CrossRef]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Sun, Y.; Tsang, D.C.W.; Cheng, K.; Ok, Y.S. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery. J. Hazard. Mater. 2019, 365, 665–673. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Enhanced removal of nitrate from water using surface modification of adsorbents—A review. J. Environ. Manag. 2013, 131, 363–374. [Google Scholar] [CrossRef]
- Rout, P.R.; Shahid, M.K.; Dash, R.R.; Bhunia, P.; Liu, D.; Varjani, S.; Zhang, T.C.; Surampalli, R.Y. Nutrient removal from domestic wastewater: A comprehensive review on conventional and advanced technologies. J. Environ. Manag. 2021, 296, 113246. [Google Scholar] [CrossRef]
- Znad, H.; Al Ketife, A.M.D.; Judd, S.; AlMomani, F.; Vuthaluru, H.B. Bioremediation and nutrient removal from wastewater by Chlorella vulgaris. Ecol. Eng. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef] [PubMed]
- Milmile, S.N.; Pande, J.V.; Karmakar, S.; Bansiwal, A.; Chakrabarti, T.; Biniwale, R.B. Equilibrium isotherm and kinetic modeling of the adsorption of nitrates by anion exchange Indion NSSR resin. Desalination 2011, 276, 38–44. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Yüzak, Y.; Thomas, K.M. Adsorption and desorption kinetics for hydrophilic and hydrophobic vapors on activated carbon. Carbon 2006, 44, 989–1004. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Días, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Worch, E.E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modelling; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2021. [Google Scholar]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-Bedoya, M.; Villamizar, L. Modelamiento de la cinética de bioadsorción de Cr(III) usando cáscara de naranja. DYNA 2009, 76, 95–106. Available online: https://revistas.unal.edu.co/index.php/dyna/article/view/13588 (accessed on 17 December 2023).
- Ho, Y.S.; McKay, G. The sorption of lead(II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Lima, E.C.; Sher, F.; Guleria, A.; Saeb, M.R.; Anastopoulos, I.; Tran, H.N.; Hosseini-Bandegharaei, A. Is one performing the treatment data of adsorption kinetics correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
- Quadra, G.; Brovini, E. Nutrient Pollution. In The Palgrave Handbook of Global Sustainability; Palgrave Macmillan: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.; Fetzer, I.; Bennett, E.; Reinette, B.; Sörlin, S. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. Available online: https://www.science.org/doi/10.1126/science.1259855 (accessed on 16 December 2023). [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. Nat. Resour. Forum 2020, 44, 40–51. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/1477-8947.12187 (accessed on 10 October 2023). [CrossRef]
- Bunce, J.; Ndam, E.; Ofiteru, I.; Moore, A.; Graham, D. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Tran, D.; Pham, T.; Dang, V.; Pham, T.; Nguyen, M.; Dang, N.; Ha, M.; Nguyen, V.; Nghiem, L.D. A facile technique to prepare MgO-biochar nanocomposites for cationic and anionic nutrient removal. J. Water Process Eng. 2022, 47, 102702. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Shim, W.G.; Kandasamy, J.; Ngo, H.H.; Vigneswaran, S. Enhanced removal of nitrate from water using amine-grafted agricultural wastes. Sci. Total Environ. 2016, 565, 503–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Mahmood, I.B. Recovery of NH 4 + by corn cob produced biochars and its potential application as soil conditioner. Front. Environ. Sci. Eng. 2014, 8, 825–834. [Google Scholar] [CrossRef]
- Devi, M.K.; Manikandan, S.; Oviyapriya, M.; Selvaraj, M.; Assiri, M.A.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; et al. Recent advances in biogas production using agro-industrial waste: A comprehensive review outlook of techno-economic analysis. Bioresour. Technol. 2022, 363, 127871. [Google Scholar] [CrossRef]
- Abbas, M.N. Phosphorus removal from wastewater using rice husk and subsequent utilization of the waste residue. Desalination Water Treat. 2015, 55, 970–977. [Google Scholar] [CrossRef]
- Huang, C.; Tan, C. A review: CO2 utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Ghezzehei, T.A.; Sarkhot, D.V.; Berhe, A.A. Biochar can be used to capture essential nutrients from dairy wastewater and improve soil physico-chemical properties. Solid Earth 2014, 5, 953–962. [Google Scholar] [CrossRef]
- Girmay, G.; Singh, B.R.; Mitiku, H.; Borresen, T.; Lal, R. Carbon stocks in Ethiopian soils in relation to land use and soil management. Land Degrad. Dev. 2008, 19, 351–367. [Google Scholar] [CrossRef]
- Yadav, D.; Kapur, M.; Kumar, P.; Mondal, M.K. Adsorptive removal of phosphate from aqueous solution using rice husk and fruit juice residue. Process Saf. Environ. Prot. 2015, 94, 402–409. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, J.; Zhang, R.; Li, K.; Hu, R.; Liu, Y.; Zhang, L.; Li, J. Enhanced nitrate, fluoride, and phenol removal using polyurethane sponges loaded with rice husk biochar in immobilized bioreactor. Bioresour. Technol. 2022, 364, 128098. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, E.P.A.; Hillary, A.K.; Fukuda, T.; Shinogi, Y. The effects of rice husk char on ammonium, nitrate and phosphate retention and leaching in loamy soil. Geoderma 2016, 277, 61–68. [Google Scholar] [CrossRef]
- Manyatshe, A.; Cele, Z.E.D.; Balogun, M.O.; Nkambule, T.T.I.; Msagati, T.A.M. Chitosan modified sugarcane bagasse biochar for the adsorption of inorganic phosphate ions from aqueous solution. J. Environ. Chem. Eng. 2022, 10, 108243. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Hameed, B.H.; Ok, Y.S.; Omirou, M. A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J. Mol. Liq. 2017, 240, 179–188. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F. Preparation and application of Mg–Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 2021, 126, 293–304. [Google Scholar] [CrossRef]
- Wang, X.; Lü, S.; Gao, C.; Feng, C.; Xu, X.; Bai, X.; Gao, N.; Yang, J.; Liu, M.; Wu, L. Recovery of ammonium and phosphate from wastewater by wheat straw-based amphoteric adsorbent and reusing as a multifunctional slow-release compound fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 2068–2079. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Li, W.; Xu, X.; Zhong, Q. Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw. Chem. Eng. J. 2011, 171, 1209–1217. [Google Scholar] [CrossRef]
- Micháleková-Richveisová, B.; Frišták, V.; Pipíška, M.; Ďuriška, L.; Moreno-Jimenez, E.; Soja, G. Iron-impregnated biochars as effective phosphate sorption materials. Environ. Sci. Pollut. Res. Int. 2017, 24, 463–475. [Google Scholar] [CrossRef]
- Zheng, L.; Peng, D.; Meng, P. Promotion effects of nitrogenous and oxygenic functional groups on cadmium (II) removal by carboxylated corn stalk. J. Clean. Prod. 2018, 201, 609–623. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.; Yue, W.; Yue, Q. Preparation and utilization of wheat straw anionic sorbent for the removal of nitrate from aqueous solution. J. Environ. Sci. 2007, 19, 1305–1310. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Ren, Z.; Gao, B. Removal of phosphate and chromium (VI) from liquids by an amine-crosslinked nano-Fe3O4 biosorbent derived from corn straw. RSC Adv. 2016, 6, 47237–47248. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef]
- Ismail, Z.Z. Kinetic study for phosphate removal from water by recycled date-palm wastes as agricultural by-products. Int. J. Environ. Stud. 2012, 69, 135–149. [Google Scholar] [CrossRef]
- Demirak, A.; Keskin, F.; Şahin, Y.; Kalemci, V. Removal of ammonium from water by pine cone powder as biosorbent, Mugla. J. Sci. Technol. 2015, 1, 5–12. [Google Scholar] [CrossRef]
- Bellahsen, N.; Varga, G.; Halyag, N.; Kertész, S.; Tombácz, E.; Hodúr, C. Pomegranate peel as a new low-cost adsorbent for ammonium removal. Int. J. Environ. Sci. Technol. 2021, 18, 711–722. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, J.; Chi, T.; Wang, P.; Yang, B. Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 2015, 9, 1066–1075. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of biochar for the removal of nitrogen and phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A.R. Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci. Total Environ. 2014, 487, 26–36. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lin, F.; Dou, X.; Zheng, M.; Tan, W.; Wang, C. Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides. J. Clean. Prod. 2018, 187, 205–214. [Google Scholar] [CrossRef]
- Min, L.; Zhongsheng, Z.; Zhe, L.; Haitao, W. Removal of nitrogen and phosphorus pollutants from water by FeCl3− impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef]
- Kilpimaa, S.; Runtti, H.; Kangas, T.; Lassi, U.; Kuokkanen, T. Physical activation of carbon residue from biomass gasification: Novel sorbent for the removal of phosphates and nitrates from aqueous solution. J. Ind. Eng. Chem. 2015, 21, 1354–1364. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Das, P. Compost preparation technique standardization for raw material mixture of rice straw and rice husk in bulk amount. Indian For. 2015, 141, 1117–1123. Available online: https://indianforester.co.in/index.php/indianforester/article/view/84347 (accessed on 16 December 2023).
- Nathen, A.; Ravi, R. Indian rice husk ash–improving the mechanical properties of concrete: A review. Int. J. Eng. Res. Appl. 2017, 07, 76–79. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, Q.; Du, D. Adsorptive removal of Cd(II) from aqueous solution using natural and modified rice husk. Bioresour. Technol. 2010, 101, 5175–5179. [Google Scholar] [CrossRef]
- Pérez, S.; Rodríguez, C. Metodología para la formación del precio del arroz en Cuba. Rev. Cuba. Finanz. Precios 2019, 3, 91–101. Available online: https://core.ac.uk/download/pdf/304203407.pdf (accessed on 16 December 2023).
- Chica, J.; Tirado, Y.C.; Barreto, J.M. Indicadores de competitividad del cultivo del arroz en Colombia y Estados Unidos. Rev. De Cienc. Agrícolas 2016, 33, 16–31. [Google Scholar] [CrossRef]
- Siddique, R. Waste Materials and by-Products in Concrete; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Available online: https://link.springer.com/book/10.1007/978-3-540-74294-4 (accessed on 16 December 2023).
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.Y.T. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Li, A.; Xie, H.; Qiu, Y.; Liu, L.; Lu, T.; Wang, W.; Qiu, G. Resource utilization of rice husk biomass: Preparation of MgO flake-modified biochar for simultaneous removal of heavy metals from aqueous solution and polluted soil. Environ. Pollut. 2022, 310, 119869. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef] [PubMed]
- Sooksawat, N.; Santibenchakul, S.; Kruatrachue, M.; Inthorn, D. Recycling rice husk for removal of phosphate and nitrate from synthetic and swine wastewater: Adsorption study and nutrient analysis of modified rice husk. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2021, 56, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chi, L.; Wang, X.; Sui, Y.; Wang, Y.; Arandiyan, H. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water. J. Environ. Chem. Eng. 2018, 6, 5269–5286. [Google Scholar] [CrossRef]
- Delaney, P.; McManamon, C.; Hanrahan, J.P.; Copley, M.P.; Holmes, J.D.; Morris, M.A. Development of chemically engineered porous metal oxides for phosphate removal. J. Hazard. Mater. 2011, 185, 382–391. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef]
- Das, S.; Mishra, S.; Sahu, H. A review of activated carbon to counteract the effect of iron toxicity on the environment. Environ. Chem. Ecotoxicol. 2023, 5, 86–94. [Google Scholar] [CrossRef]
- Manivannan, Y.; Bhagyashree, M.; Beach, T.; Halden, R. Role of environmental contaminants in the etiology of Alzheimer’s disease: A review. Curr. Alzheimer Res. 2015, 12, 116–146. [Google Scholar] [CrossRef]
- Ramola, S.; Belwal, T.; Li, C.J.; Liu, Y.X.; Wang, Y.Y.; Yang, S.M.; Zhou, C.H. Preparation and application of novel rice husk biochar–calcite composites for phosphate removal from aqueous medium. J. Clean. Prod. 2021, 299, 126802. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Alam, T.; Bezares-Cruz, J.C.; Mahmoud, A.; Jones, K.D. Nutrients and solids removal in bioretention columns using recycled materials under intermittent and frequent flow operations. J. Environ. Manag. 2021, 297, 113321. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF); World Health Organization. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017. Special Focus on Inequalities. New York. 2019. Available online: https://www.who.int/publications-detail-redirect/9789240030848 (accessed on 16 December 2023).
- Lei, C.; Bian, Y.; Zhi, F.; Hou, X.; Lv, C.; Hu, Q. Enhanced adsorption capacity of cellulose hydrogel based on corn stalk for pollutants removal and mechanism exploration. J. Clean. Prod. 2022, 375, 134130. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X. Research on corn production efficiency and influencing factors of typical farms: Based on data from 12 corn-producing countries from 2012 to 2019. PLoS ONE 2021, 16, e0254423. [Google Scholar] [CrossRef]
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Usman, M.; Kizito, S.; Lu, J.; Dong, R.; Wu, S. Phosphate removal from aqueous solution using iron oxides: Adsorption, desorption and regeneration characteristics. J. Colloid Interface Sci. 2018, 528, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, P.; Kumar, P.; Korving, L.; Witkamp, G.; Van Loosdrecht, M. The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: A review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Zhuo, S.N.; Dai, T.C.; Ren, H.Y.; Liu, B.F. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism. Bioresour. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.F.; Wang, Y.C. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, K.; Behera, M.; Neelancherry, R. Graywater treatment in sequencing batch reactor using simultaneous nitrification, denitrification, and phosphorus removal, with kinetic studies of phosphate adsorption onto corncob. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020017. [Google Scholar] [CrossRef]
- Gotore, O.; Rameshprabu, R.; Itayama, T. Adsorption performances of corn cob-derived biochar in saturated and semi-saturated vertical-flow constructed wetlands for nutrient removal under erratic oxygen supply. Environ. Chem. Ecotoxicol. 2022, 4, 155–163. [Google Scholar] [CrossRef]
- Sui, Q.; Liu, C.; Dong, H.; Zhu, Z. Effect of ammonium nitrogen concentration on the ammonia-oxidizing bacteria community in a membrane bioreactor for the treatment of anaerobically digested swine wastewater. J. Biosci. Bioeng. 2014, 118, 277–283. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, Y.; Zhao, Y.; Kumar, L. Achieving an extraordinary high organic and hydraulic loadings with good performance via an alternative operation strategy in a multi-stage constructed wetland system. Environ. Sci. Pollut. Res. Int. 2018, 25, 11841–11853. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, H.; Liu, J.; Wang, X.; Li, J.; Shi, E.; Wang, C.; Yang, J.; Zhang, Z. A study on and adsorption mechanism of ammonium nitrogen by modified corn straw biochar. R. Soc. Open Sci. 2023, 10, 221535. [Google Scholar] [CrossRef]
- Faraj, H.; Jamrah, A.; Al-Omari, S.; Al-Zghoul, T. Optimization of an electrocoagulation-assisted adsorption treatment system for dairy wastewater. Case Stud. Chem. Environ. Eng. 2024, 9, 100574. [Google Scholar] [CrossRef]
- Othmani, A.; Kesraoui, A.; Elaissaoui, I.; Seffen, M. Coupling anodic oxidation, biosorption and alternating current as alternative for wastewater purification. Chemosphere 2020, 249, 126480. [Google Scholar] [CrossRef] [PubMed]
- Seliem, M.K.; Komarneni, S.; Khadra, M.R.A. Phosphate removal from solution by composite of MCM-41 silica with rice husk: Kinetic and equilibrium studies. Microporous Mesoporous Mater. 2016, 224, 51–57. [Google Scholar] [CrossRef]
- Thanh, N.; Hang, V.; Thuy, N.; Hien, L.; Huy, N. Nutrient recovery from poultry wastewater by modified biochar: An optimization study, current. Appl. Sci. Technol. 2023, 23, 1–16. [Google Scholar] [CrossRef]
- Xu, C.; Liu, R.; Chen, L. Removal of phosphorus from domestic sewage in rural areas using oyster shell-modified agricultural waste–rice husk biochar. Processes 2023, 11, 2577. [Google Scholar] [CrossRef]
- Chang, J.; Sivasubramanian, P.; Dong, C.; Kumar, M. Study on adsorption of ammonium and nitrate in wastewater by modified biochar. Bioresour. Technol. Rep. 2023, 21, 101346. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Hameed, B.B. Recycling of raw corn cob residues as an agricultural waste material for ammonium removal: Kinetics, isotherms, and mechanisms. Int. J. Waste Manag. 2014, 13, 217–230. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Z.T.; Wu, Z.C.; Zhan, X.M.; Zhang, K.; Zhao, E.F.; Han, X.R. Adsorption characteristics of ammonium nitrogen by biochar from diverse origins in water. Adv. Mater. Res. 2013, 664, 305–312. [Google Scholar] [CrossRef]
- Das, A.; Bar, N.; Das, S. Adsorptive removal of Pb (II) ion on Arachis hypogaea’s shell: Batch experiments, statistical, and GA modeling. Int. J. Environ. Sci. Technol. 2023, 20, 537–550. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bar, N.; Rajbansi, B.; Das, S. Synthesis of Chitosan-nTiO2 Nanocomposite, Application in Adsorptive Removal of Cu (II)-Adsorption and Desorption study, Mechanism, Scale-up Design, Statistical and Genetic Algorithm Modeling. Appl. Organomet. Chem. 2023, 37, e7094. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B. Insight into the co-pyrolysis of different blended feedstocks to biochar for the adsorption of organic and inorganic pollutants: A review. J. Clean. Prod. 2020, 265, 121762. [Google Scholar] [CrossRef]
- Kwikima, M.; Mateso, S.; Chebude, Y. Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review. Sci. Afr. 2021, 13, e00934. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugo-Arias, J.; Vargas, S.B.; Maturana, A.; González-Álvarez, J.; Lugo-Arias, E.; Rico, H. Nutrient Removal from Aqueous Solutions Using Biosorbents Derived from Rice and Corn Husk Residues: A Systematic Review from the Environmental Management Perspective. Water 2024, 16, 1543. https://doi.org/10.3390/w16111543
Lugo-Arias J, Vargas SB, Maturana A, González-Álvarez J, Lugo-Arias E, Rico H. Nutrient Removal from Aqueous Solutions Using Biosorbents Derived from Rice and Corn Husk Residues: A Systematic Review from the Environmental Management Perspective. Water. 2024; 16(11):1543. https://doi.org/10.3390/w16111543
Chicago/Turabian StyleLugo-Arias, José, Sandra Bibiana Vargas, Aymer Maturana, Julia González-Álvarez, Elkyn Lugo-Arias, and Heidy Rico. 2024. "Nutrient Removal from Aqueous Solutions Using Biosorbents Derived from Rice and Corn Husk Residues: A Systematic Review from the Environmental Management Perspective" Water 16, no. 11: 1543. https://doi.org/10.3390/w16111543
APA StyleLugo-Arias, J., Vargas, S. B., Maturana, A., González-Álvarez, J., Lugo-Arias, E., & Rico, H. (2024). Nutrient Removal from Aqueous Solutions Using Biosorbents Derived from Rice and Corn Husk Residues: A Systematic Review from the Environmental Management Perspective. Water, 16(11), 1543. https://doi.org/10.3390/w16111543







