A Critical Review on the Advancement of the Development of Low-Cost Membranes to Be Utilized in Microbial Fuel Cells
Abstract
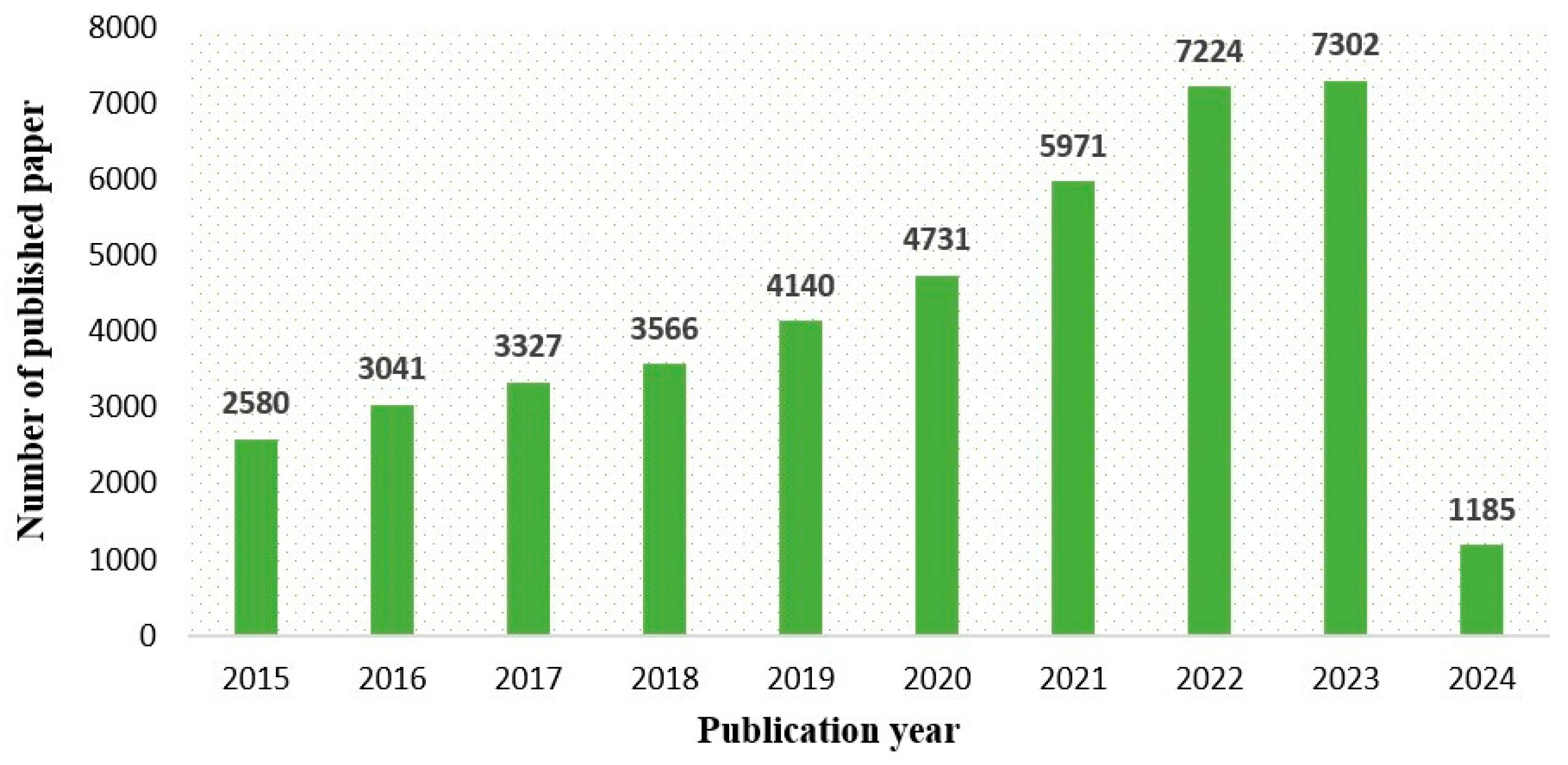
:1. Introduction
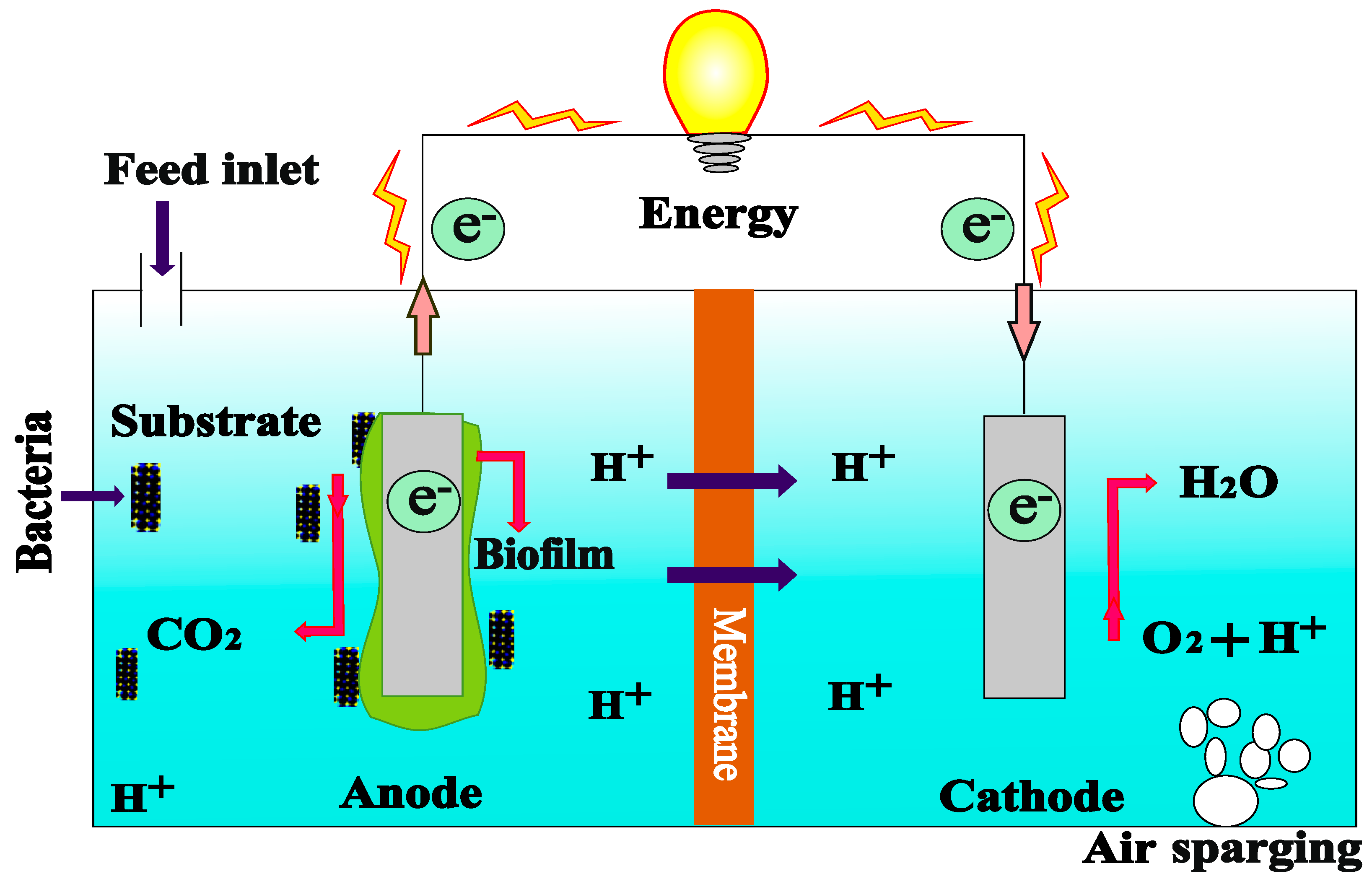
2. Fundamentals of MFCs
2.1. Configuration of MFCs
2.1.1. Dual Compartment of MFC
2.1.2. Single Compartment MFCs
3. Electrodes
3.1. Anode Reaction
3.2. Cathode Reaction
Oxygen Reduction Reaction
4. Membrane
4.1. Types of Earthen Membrane
4.1.1. Earthen Membranes
4.1.2. Clayware Membranes
4.1.3. Ceramic Membranes
5. Ion Transport across Membranes and Its Characterization
5.1. Mass Transport of Oxygen
5.2. Mass Transport of Proton
5.3. Water Uptake
5.4. Ion Exchange Capacity
6. Results and Discussion
6.1. Water Uptake Capacity
6.2. Ion Exchange Capacity
6.3. Power Density
6.4. COD Removal
6.5. Coulombic Efficiency
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, P.; Daverey, A.; Arunachalam, K. Development and characterization of novel low-cost engineered pine needle biochar and montmorillonite clay based proton exchange membrane for microbial fuel cell. J. Water Process. Eng. 2023, 53, 103750. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Dong, C.D.; Chang, J. Microalgae-microbial fuel cell (mMFC): An integrated process for electricity generation, wastewater treatment, CO2 sequestration and biomass production. Int. J. Energy Res. 2020, 19, 44. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.-E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Liang, P.; Liu, P.; Huang, X. Microbial fuel cell sensors for water quality early warning systems: Fundamentals, signal resolution, optimization and future challenges. Renew. Sustain. Energy Rev. 2018, 81, 292–305. [Google Scholar] [CrossRef]
- Kannan, M.; Kumar, G.G. Current status, key challenges and its solutions in the design and development of graphene based ORR catalysts for the microbial fuel cell applications. Biosens. Bioelectron. 2016, 77, 1208–1220. [Google Scholar] [CrossRef]
- Ghasemi, M.; Ismail, M.; Kamarudin, S.K.; Saeedfar, K.; Daud, W.R.W.; Hassan, S.H.; Heng, L.Y.; Alam, J.; Oh, S.-E. Carbon nanotube as an alternative cathode support and catalyst for microbial fuel cells. Appl. Energy 2013, 102, 1050–1056. [Google Scholar] [CrossRef]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Muga, H.E.; Mihelcic, J.R. Sustainability of wastewater treatment technologies. J. Environ. Manag. 2008, 88, 437–447. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Bakeri, G.; Ghasemi, M.; Zirepour, A. A review on the role of proton exchange membrane on the performance of microbial fuel cell. Polym. Adv. Technol. 2014, 25, 1426–1432. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Tiwari, B.; Noori, M.; Ghangrekar, M. A novel low cost polyvinyl alcohol-Nafion-borosilicate membrane separator for microbial fuel cell. Mater. Chem. Phys. 2016, 182, 86–93. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Ben Liew, K.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Theodosiou, P.; Taylor, B.; Greenman, J.; Melhuish, C. Gelatin as a promising printable feedstock for microbial fuel cells (MFC). Int. J. Hydrogen Energy 2017, 42, 1783–1790. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, A.N.; Kumar, G.; Kim, D.-H.; Song, Y.-C.; Kim, S.-H. Electro-fermentation for biofuels and biochemicals production: Current status and future directions. Bioresour. Technol. 2020, 323, 124598. [Google Scholar] [CrossRef]
- Do, M.; Ngo, H.; Guo, W.; Liu, Y.; Chang, S.; Nguyen, D.; Nghiem, L.; Ni, B. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.; Islam, R.; Pervez, N.; Cai, Y.; Naddeo, V.; Islam, S. Microbial Fuel Cell Construction Features and Application for Sustainable Wastewater Treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2008, 1, 417–429. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Flimban, S.G.; Kim, T.; Ismail, I.M.; Oh, S.E. Overview of microbial fuel cell (MFC) recent advancement from fundamentals to applications: MFC designs, major elements, and scalability. Preprints 2018, 2018100763. [Google Scholar] [CrossRef]
- Tamboli, E.; Eswari, J.S. Microbial fuel cell configurations: An overview. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 407–435. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Jain, S.; Mungray, A.A.; Mungray, A.K. SnO2:PANI modified cathode for performance enhancement of air-cathode microbial fuel cell. J. Environ. Chem. Eng. 2020, 8, 103590. [Google Scholar] [CrossRef]
- Das, S.; Mangwani, N. Recent developments in microbial fuel cells: A review. J. Sci. Ind. Res. 2010, 69, 727–731. [Google Scholar]
- Yan, X.; Lee, H.-S.; Li, N.; Wang, X. The micro-niche of exoelectrogens influences bioelectricity generation in bioelectrochemical systems. Renew. Sustain. Energy Rev. 2020, 134, 110184. [Google Scholar] [CrossRef]
- Chang, H.; Zhong, N.; Quan, X.; Qi, X.; Zhang, T.; Hu, R.; Sun, Y.; Wang, C. Membrane technologies for sustainable and eco-friendly microbial energy production. In Membranes for Environmental Applications. Environmental Chemistry for a Sustainable World; Springer: Cham, Switzerland, 2020; pp. 353–381. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Lissens, G.; Siciliano, S.D.; Verstraete, W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol. Lett. 2003, 25, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Hong, L.; Ramanathan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar]
- Logan, B.E.; Regan, J.M. Microbial Fuel Cells—Challenges and Applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef]
- Wang, H.; Park, J.-D.; Ren, Z.J. Practical Energy Harvesting for Microbial Fuel Cells: A Review. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Kaur, R.; Marwaha, A.; Chhabra, V.A.; Kim, K.-H.; Tripathi, S. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sustain. Energy Rev. 2020, 119, 109551. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent insights into microalgae-assisted microbial fuel cells for generating sustainable bioelectricity. Int. J. Hydrogen Energy 2021, 46, 3135–3159. [Google Scholar] [CrossRef]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Ceramic-based microbial fuel cells (MFCs): A review. Int. J. Hydrogen Energy 2017, 42, 1672–1690. [Google Scholar] [CrossRef]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity Production by Geobacter sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef]
- Ben Liew, K.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, H.S.; Kim, H.J.; Kim, G.T.; Chang, I.S.; Lee, J.; Phung, N.T. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 2004, 63, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef]
- Holmes, D.; Bond, D.; O’neil, R.; Reimers, C.; Tender, L.; Lovley, D. Microbial Communities Associated with Electrodes Harvesting Electricity from a Variety of Aquatic Sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Nava, J.; Martínez-Castrejón, M.; García-Mesino, R.L.; López-Díaz, J.A.; Talavera-Mendoza, O.; Sarmiento-Villagrana, A.; Rojano, F.; Hernández-Flores, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.P. Conducting Polymer-Based Microbial Fuel Cells; Materials Research Foundations: Millersville, PA, USA, 2019. [Google Scholar]
- Majidi, M.R.; Farahani, F.S.; Hosseini, M.; Ahadzadeh, I. Low-cost nanowired α-MnO2/C as an ORR catalyst in air-cathode microbial fuel cell. Bioelectrochemistry 2019, 125, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Mou, H.; Wang, L.K.; Matsuura, T.; Wei, Y. Membrane separation: Basics and applications. In Membrane and Desalination Technologies; Humana Press: Totowa, NJ, USA, 2011; pp. 271–332. [Google Scholar]
- Midyurova, B.; Nenov, V. Novel Proton Exchange Membranes and Separators Applied in Microbial Fuel Cells (MFC). J. Balk. Tribol. Assoc. 2016, 22, 2596–2615. [Google Scholar]
- Shabani, M.; Younesi, H.; Pontié, M.; Rahimpour, A.; Rahimnejad, M.; Zinatizadeh, A.A. A critical review on recent proton exchange membranes applied in microbial fuel cells for renewable energy recovery. J. Clean. Prod. 2020, 264, 121446. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Pergamon: Middlesex, UK, 2004; pp. 1166–1175. [Google Scholar]
- Harnisch, F.; Schröder, U. Selectivity versus Mobility: Separation of Anode and Cathode in Microbial Bioelectrochemical Systems. ChemSusChem 2009, 2, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Park, S.-G.; Pandit, S.; Yang, E.; Abdelkareem, M.A.; Jang, J.-K.; Chae, K.-J. Scalability of microbial electrochemical technologies: Applications and challenges. Bioresour. Technol. 2022, 345, 126498. [Google Scholar] [CrossRef]
- Behera, M.; Ghangrekar, M.M. Electricity generation in low cost microbial fuel cell made up of earthenware of different thickness. Water Sci. Technol. 2011, 64, 2468–2473. [Google Scholar] [CrossRef]
- Gryta, M. Resistance of Polypropylene Membrane to Oil Fouling during Membrane Distillation. Membranes 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Dyartanti, E.R.; Purwanto, A.; Widiasa, I.N.; Susanto, H. Ionic Conductivity and Cycling Stability Improvement of PVDF/Nano-Clay Using PVP as Polymer Electrolyte Membranes for LiFePO4 Batteries. Membranes 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Azaman, F.; Nor, M.A.A.M.; Abdullah, W.R.W.; Razali, M.H.; Zulkifli, R.C.; Zaini, M.A.A.; Ali, A. Review on natural clay ceramic membrane: Fabrication and application in water and wastewater treatment. Malays. J. Fundam. Appl. Sci. 2021, 17, 62–78. [Google Scholar] [CrossRef]
- Winfield, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; Ormeno-Cano, N.; Rutkowski, P. Recycled waste polypropylene composite ceramic membranes for extended lifetime of microbial fuel cells. Chem. Eng. J. 2021, 425, 130707. [Google Scholar] [CrossRef]
- Sondhi, R.; Bhave, R.; Jung, G. Applications and benefits of ceramic membranes. Membr. Technol. 2003, 2003, 5–8. [Google Scholar] [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef] [PubMed]
- Eray, E.; Candelario, V.M.; Boffa, V. Ceramic Processing of Silicon Carbide Membranes with the Aid of Aluminum Nitrate Nonahydrate: Preparation, Characterization, and Performance. Membranes 2021, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Merino-Jimenez, I.; Gonzalez-Juarez, F.; Greenman, J.; Ieropoulos, I. Effect of the ceramic membrane properties on the microbial fuel cell power output and catholyte generation. J. Power Sources 2019, 429, 30–37. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Cheraghipoor, M.; Mohebbi-Kalhori, D.; Noroozifar, M.; Maghsoodlou, M.T. Production of greener energy in microbial fuel cell with ceramic separator fabricated using native soils: Effect of lattice and porous SiO2. Fuel 2021, 284, 118938. [Google Scholar] [CrossRef]
- Suransh, J.; Tiwari, A.K.; Mungray, A.K. Modification of clayware ceramic membrane for enhancing the performance of microbial fuel cell. Environ. Prog. Sustain. Energy 2020, 39, e13427. [Google Scholar] [CrossRef]
- Ghadge, A.N.; Jadhav, D.A.; Ghangrekar, M.M. Wastewater treatment in pilot-scale microbial fuel cell using multielectrode assembly with ceramic separator suitable for field applications. Environ. Prog. Sustain. Energy 2016, 35, 1809–1817. [Google Scholar] [CrossRef]
- Freedman, J.C. Cell Membranes; Elsevier eBooks: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chatterjee, P.; Ghangrekar, M.M. Design of Clayware Separator-Electrode Assembly for Treatment of Wastewater in Microbial Fuel Cells. Appl. Biochem. Biotechnol. 2014, 173, 378–390. [Google Scholar] [CrossRef]
- Bakonyi, P.; Koók, L.; Kumar, G.; Tóth, G.; Rózsenberszki, T.; Nguyen, D.D.; Chang, S.W.; Zhen, G.; Bélafi-Bakó, K.; Nemestóthy, N. Architectural engineering of bioelectrochemical systems from the perspective of polymeric membrane separators: A comprehensive update on recent progress and future prospects. J. Membr. Sci. 2018, 564, 508–522. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Foorginezhad, S.; Rezvannasab, G.; Asadnia, M. Natural clay membranes: A sustainable and affordable solution for treating dye solutions, coal mine washery waste, and aquaculture wastewater. J. Water Process. Eng. 2023, 54, 104012. [Google Scholar] [CrossRef]
- Yar’adua, M.M.; Kakale, A.U. Environmental Sustainability: Clay Solution to High Cost of Building Materials in Construction Industry. Br. J. Multidiscip. Adv. Stud. 2018, 2, 82–87. [Google Scholar]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Richerson, D.W.; Lee, W.E. Modern Ceramic Engineering: Properties, Processing, and Use in Design; CRC Press: Boca Raton, FL, USA, 2018; p. 836. [Google Scholar]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Wickramasinghe, S.R.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef] [PubMed]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley & Sons: London, UK, 2007. [Google Scholar]
- Ahmad, H.B. CERAMIC MEMBRANES: NEW TRENDS AND PROSPECTS (SHORT REVIEW). Water and water purification technologies. Sci. Tech. News 2020, 27, 4–31. [Google Scholar]
- Issaoui, M.; Limousy, L. Low-cost ceramic membranes: Synthesis, classifications, and applications. Comptes Rendus Chim. 2019, 22, 175–187. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Milad, M.; Sokri, M.N.M.; Puteh, M.H. Recent progress and technical improvement strategies for mitigating ceramic membrane bottlenecks in water purification processes: A review. Int. J. Appl. Ceram. Technol. 2023, 20, 3327–3356. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Smith, G. Modelling the Proton-Conductive Membrane in Practical Polymer Electrolyte Membrane Fuel Cell (PEMFC) Simulation: A Review. Membranes 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Dharmalingam, S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal. Chim. Acta 2014, 818, 15–22. [Google Scholar] [CrossRef]
- Sabina-Delgado, A.; Kamaraj, S.K.; Hernández-Montoya, V.; Cervantes, F.J. Novel carbon-ceramic composite membranes with high cation exchange properties for use in microbial fuel cell and electricity generation. Int. J. Hydrogen Energy 2023, 48, 25512–25526. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The Effects of Sulfonated Poly(ether ether ketone) Ion Exchange Preparation Conditions on Membrane Properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Start-up investigation of the self-assembled chitosan/montmorillonite nanocomposite over the ceramic support as a low-cost membrane for microbial fuel cell application. Int. J. Hydrogen Energy 2020, 45, 4804–4820. [Google Scholar] [CrossRef]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Application of layer-by-layer assembled chitosan/montmorillonite nanocomposite as oxygen barrier film over the ceramic separator of the microbial fuel cell. Electrochim. Acta 2018, 283, 234–247. [Google Scholar] [CrossRef]
- Sarma, P.J.; Mohanty, K. Epipremnum aureum and Dracaena braunii as indoor plants for enhanced bio-electricity generation in a plant microbial fuel cell with electrochemically modified carbon fiber brush anode. J. Biosci. Bioeng. 2018, 126, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Chhabra, M.; Yadav, P. Performance evaluation of algae assisted microbial fuel cell under outdoor conditions. Bioresour. Technol. 2020, 310, 123418. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Behera, M. Performance evaluation of microbial fuel cells employing ceramic separator of different surface area modified with mineral cation exchanger. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Gunaseelan, K.; Jadhav, D.A.; Gajalakshmi, S.; Pant, D. Blending of microbial inocula: An effective strategy for performance enhancement of clayware Biophotovoltaics microbial fuel cells. Bioresour. Technol. 2021, 323, 124564. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, A.; Sahoo, R.N.; Behera, M. Application of clayware ceramic separator modified with silica in microbial fuel cell for bioelectricity generation during rice mill wastewater treatment. Water Sci. Technol. 2021, 84, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.J.; Mohanty, K. A novel three-chamber modular PMFC with bentonite/fly ash based clay membrane and oxygen reducing biocathode for long term sustainable bioelectricity generation. Bioelectrochemistry 2022, 144, 107996. [Google Scholar]
- Babanova, S.; Jones, J.; Phadke, S.; Lu, M.; Angulo, C.; Garcia, J.; Carpenter, K.; Cortese, R.; Chen, S.; Phan, T.; et al. Continuous flow, large-scale, microbial fuel cell system for the sustained treatment of swine waste. Water Environ. Res. 2020, 92, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipoor, M.; Mohebbi-Kalhori, D.; Noroozifar, M.; Maghsoodlou, M.T. Comparative study of bioelectricity generation in a microbial fuel cell using ceramic membranes made of ceramic powder, Kalporgan’s soil, and acid leached Kalporgan’s soil. Energy 2019, 178, 368–377. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.; Ghangrekar, M. A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem. Eng. J. 2019, 148, 170–177. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Behera, M. Ceramic membrane modified with rice husk ash for application in microbial fuel cells. Electrochim. Acta 2020, 363, 137261. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Sharma, S.; Ghangrekar, M.M. Ameliorated performance of a microbial fuel cell operated with an alkali pre-treated clayware ceramic membrane. Int. J. Hydrogen Energy 2020, 45, 16787–16798. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Dixit, R.; Ghangrekar, M.M. Goethite supplemented natural clay ceramic as an alternative proton exchange membrane and its application in microbial fuel cell. Ionics 2020, 26, 3061–3072. [Google Scholar] [CrossRef]
- Rashid, T.; Sher, F.; Hazafa, A.; Hashmi, R.Q.; Zafar, A.; Rasheed, T.; Hussain, S. Design and feasibility study of novel paraboloid graphite based microbial fuel cell for bioelectrogenesis and pharmaceutical wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104502. [Google Scholar] [CrossRef]
- Das, I.; Ghangrekar, M.M.; Satyakam, R.; Srivastava, P.; Khan, S.; Pandey, H.N. On-Site Sanitary Wastewater Treatment System Using 720-L Stacked Microbial Fuel Cell: Case Study. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020025. [Google Scholar] [CrossRef]
- Obasi, L.A.; Onukwuli, O.D.; Okoye, C.C. Performance of microbial fuel cell operating with clay-manihot starch composite proton exchange membrane using RSM. Curr. Res. Green Sustain. Chem. 2021, 4, 100117. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mais, L.; Campana, R.; Piroddi, L.; Mascia, M.; Gurauskis, J.; Vacca, A.; Palmas, S. Comprehensive characterization of a cost-effective microbial fuel cell with Pt-free catalyst cathode and slip-casted ceramic membrane. Int. J. Hydrogen Energy 2021, 46, 26205–26223. [Google Scholar] [CrossRef]
- Mittal, Y.; Dash, S.; Srivastava, P.; Mishra, P.M.; Aminabhavi, T.M.; Yadav, A.K. Azo dye containing wastewater treatment in earthen membrane based unplanted two chambered constructed wetlands-microbial fuel cells: A new design for enhanced performance. Chem. Eng. J. 2022, 427, 131856. [Google Scholar] [CrossRef]
- Otake, K.; Kitagawa, H. Control of Proton-Conductive Behavior with Nanoenvironment within Metal–Organic Materials. Small 2021, 17, 2006189. [Google Scholar] [CrossRef]
- Gurjar, R.; Behera, M. Exploring necessity to pre-treat organic fraction of waste prior to use in an earthen MFC modified with bentonite. Water Sci. Technol. 2022, 86, 656–671. [Google Scholar] [CrossRef]



| Property | Earthen Membrane | Clayware Membrane | Ceramic Membrane | Reference |
|---|---|---|---|---|
| Composition | Natural materials (soil, sand, clay) | Fired clay | Inorganic materials | [55,56,57] |
| Mechanical Strength | Moderate | Improved | Excellent | [56,57] |
| Chemical Resistance | Limited | Moderate | Excellent | [57,58] |
| Cost | Low | Moderate | High | [57] |
| Availability | Abundant | Widely available | Widely available | [55,57] |
| Eco-friendliness | Yes | Moderate | Moderate | [59,60] |
| Ion Conductivity | Moderate | Good | Excellent | [55,57] |
| Stability | Limited | Moderate | Excellent | [57] |
| Moisture Retention | Moderate | Moderate | Moderate | [57] |
| Lifespan | Short | Moderate | Long | [61] |
| Maintenance | Low | Low | Low | [62] |
| Uniformity | Variable | Moderate | High | [55,63] |
| Pore Size Control | Limited | Limited | Excellent | [55,64] |
| Performance | Variable | Good | Excellent | [65,66] |
| Membrane | Waste-Water Treatment | Modification | Setup | Inoculum | Anode | Cathode | Operation Time (day) | Oxygen Mass Transfer | Proton Mass Transfer | Current Density (mW/m2) | Power Density (mW/m2) | COD Removal (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clayware | Sewage | Bentonite clay | Dual chamber | Anaerobic microbial culture | Carbon fiber | Carbon fiber | 60 | - | - | 38.46 | 15.38 | - | [91] |
| Ceramic | Domestic | CHI/MMT | Dual chamber | Raw wastewater | Carbon cloth | Carbon cloth | - | 0.83 × 10−4 | - | 869.44 ± 27.49 | 119.58 ± 19.16 | 95.67 | [90] |
| Ceramic | Domestic | CHI/MMT | Dual chamber | - | Carbon cloth | Stainless steel | 10 | 9.1 × 10−5 | (222.73 ± 22.7) × 10−3 | 1422.22 ± 41.2 | 229.12 ± 18.5 | 87 | [89] |
| Ceramic | Sanitary sewer | - | Single chamber | Mixed swine waste | Graphite fiber | Graphite fiber | 210 | - | - | 103 ± 7 | 261 | - | [97] |
| Ceramic | Domestic | KS, LKS, and CCP mixed with tap water | Dual chamber | Anaerobic wastewater | Carbon brush | Carbon cloths | - | - | - | 1535.0 ± 29 | 20.18 ± 0.83 | 96.6 | [98] |
| Clayware | Synthetic | Clay (1%, 2%, 5% and 10%) mixed with ACCS | Dual chamber | Anaerobic sludge | Carbon felt | Carbon felt | - | 1.3 × 10−4 | 9 × 10−5 | 779 | - | 81.05 ± 0.08 | [99] |
| Ceramic | Rice mill | Soil with 20% bentonite clay | Dual chamber | Anaerobic sludge | Stainless-steel | Graphite plates | 14 | 1.31 × 10−5 | - | - | 80.15 | 70.7 ± 1.24 | [100] |
| Clayware | Synthetic | Montmorillonite 20% clay | Dual chamber | Anaerobic mixed sludge | Carbon felt | Carbon felt | 3 | (4.02 ± 0.38) × 10−5 | 17.9 × 10−3 | - | 83.5 | 88 | [101] |
| Earthen | Synthetic | Goethite (G-5) | Dual chamber | Anaerobic mixed sludge | Graphite felt | Graphite felt | 3 | 1.95 × 10−5 | 78.71 × 10−3 | - | 112.81 ± 8.74 | 22 | [102] |
| Earthen | Synthetic | Red soil with MMT (20%) + VC (20%) | Single chamber | 1% sludge | Carbon felt | Carbon felt | 30 | (4.01 ± 0.02) ×10−5 | (8.84 ± 0.11) × 10−3 | 168 | 162.74 | 80.48 ± 0 | [67] |
| Earthen | Pharma industry | - | Dual chamber | Municipal solid wastewater | Graphite material | Graphite material | - | - | - | - | - | 80.55 | [103] |
| Ceramic | Sanitary | 20% montmorillonite blended | Single chamber | Sewage | Carbon felt | Carbon felt | 255 | - | - | - | - | 87.29 ± 7.28 | [104] |
| Clayware | Synthetic | Rock phosphate mixed with black soil (5–10%) | Single chamber | Cow manure | Graphite felt | Graphite felt | - | - | 5.34 × 10−6 | - | - | 74.4 ± 4 | [92] |
| Ceramic | Synthetic | Soil mixed with kaolin (10%, 20%, 30%, 40% and 50%) | Dual chamber | Pond sludge | Stainless steel | Graphite plates | 60 | - | 8.18 × 10−6 | - | - | 93.1 | [93] |
| Ceramic | Synthetic | Clay samples | Dual chamber | SUPER-MIX | Carbon felt | Carbon felt | 11 | 2.5 × 10−5 | - | - | 275 | 91 ± 3.96 | [94] |
| Clayware | Sanitary | Starch-kaolinite clay mixture | Dual chamber | Mixed microbial consortium | Graphite rod | Graphite rod | - | - | - | - | 82.4 | - | [105] |
| Ceramic | Rice mill | Soil with 20% w/w bentonite clay and silica 30% | Dual chamber | Anaerobic sludge | Stainless steel | Graphite plate | 40 | 11.35 × 10−4 | 3.64 × 10−5 | - | 71.3 | 76.24 | [95] |
| Ceramic | Activated sludge | (40%) white and (30%) gray ceramic | Single chamber | Activated sludge (75%) and mineral salt medium (25%) | Carbon veil | Carbon veil | 90 | - | - | - | 81 | 98.2 | [60] |
| Clayware | Synthetic | Suspension of clay (20–30%) | Single chamber | Sewage sludge | Carbon felt | Carbon felt | 250 | - | - | 172 | 11.2 | - | [106] |
| Earthen | Domestic | Kalporgan Soil and SiO2 (0–30%) | Dual chamber | Wastewater | Carbon brush | Carbon cloths | - | - | - | 769.23 | - | 85.8 | [66] |
| Earthen | Synthetic | Dual chamber | Graphite rod | Graphite rod | 49 | - | - | 544.6 | - | 94 ± 2.87 | [107] | ||
| Earthen | Kitchen | Red soil with bentonite 20% | Dual chamber | Kitchen waste slurry and leachate | Stainless steel | Graphite plate | 11 | 9.33 × 10−4 | 6.55 × 10−6 | 52 | - | 98.41 | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Yadav, N.; Jadhav, D.A.; Saxena, D.; Anghan, K.; Sandhwar, V.K.; Saxena, S. A Critical Review on the Advancement of the Development of Low-Cost Membranes to Be Utilized in Microbial Fuel Cells. Water 2024, 16, 1597. https://doi.org/10.3390/w16111597
Tiwari A, Yadav N, Jadhav DA, Saxena D, Anghan K, Sandhwar VK, Saxena S. A Critical Review on the Advancement of the Development of Low-Cost Membranes to Be Utilized in Microbial Fuel Cells. Water. 2024; 16(11):1597. https://doi.org/10.3390/w16111597
Chicago/Turabian StyleTiwari, Alok, Niraj Yadav, Dipak A. Jadhav, Diksha Saxena, Kirtan Anghan, Vishal Kumar Sandhwar, and Shivendu Saxena. 2024. "A Critical Review on the Advancement of the Development of Low-Cost Membranes to Be Utilized in Microbial Fuel Cells" Water 16, no. 11: 1597. https://doi.org/10.3390/w16111597
APA StyleTiwari, A., Yadav, N., Jadhav, D. A., Saxena, D., Anghan, K., Sandhwar, V. K., & Saxena, S. (2024). A Critical Review on the Advancement of the Development of Low-Cost Membranes to Be Utilized in Microbial Fuel Cells. Water, 16(11), 1597. https://doi.org/10.3390/w16111597









