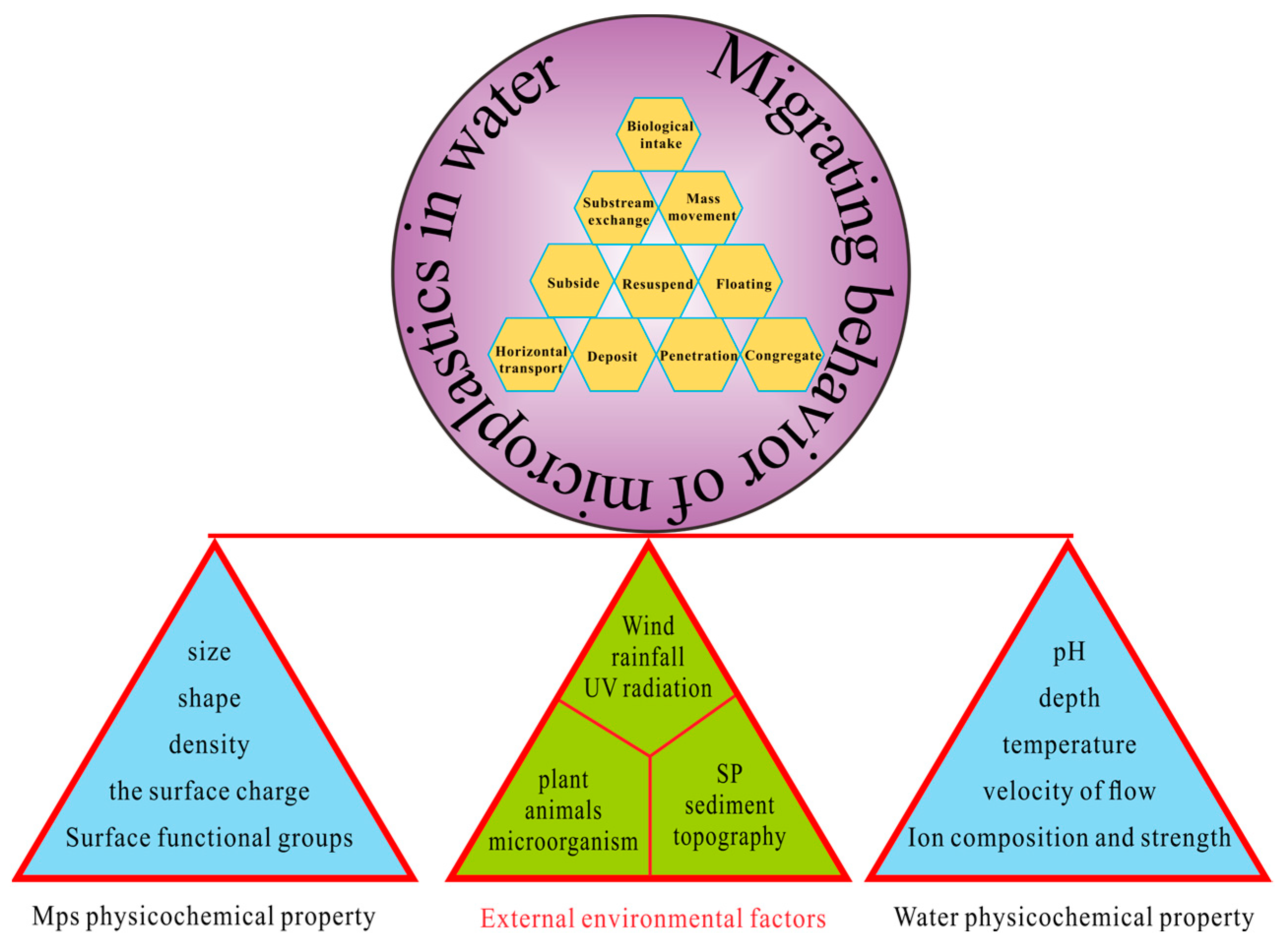
Natural Factors of Microplastics Distribution and Migration in Water: A Review
Abstract
:1. Introduction
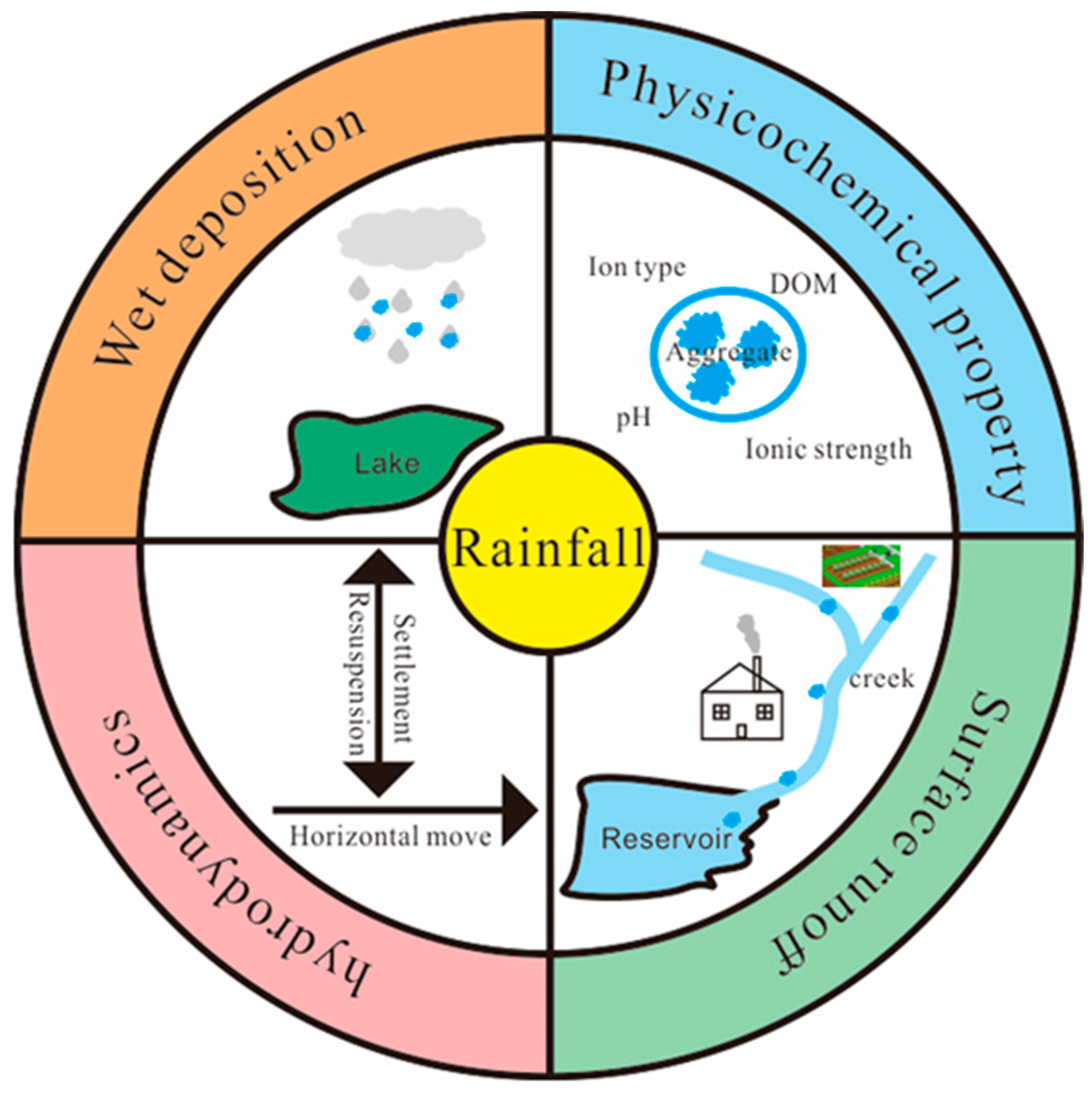
2. Meteorological Factors
2.1. Rainfall
| Country | Event | Sample | Abundance * | Effect ** | Ref. |
|---|---|---|---|---|---|
| China | Rain | Rainwater | 146~8629 items/m2 | Positive | [50] |
| China | Rain | Pearl River | 219.8 ± 160.5 n/L (before); 474 ± 259.7 n/L (after) | Positive | [46] |
| Nigeria | Rain | Oxbow Lake | 3.70 items/L (dry season); 3.08 items/L (rainy season) | Negative | [64] |
| China | Rain | Rainwater | 1.1 × 1013 particle/day (wet); 7.4 × 1012 particles/day (dry) | Positive | [65] |
| Canada | Rain | Catchments | 33.5 pieces/L (rain); 19.1 pieces/L (baseflow) | Positive | [66] |
| Japan | Rain | Surface water | 35 items/L (light rainfalls); 929 items/L (moderate); 331 items/L (heavy) | Positive | [67] |
| Australia | Rain, storm | Cooks River Estuary | 0.4 particles/L (before storm and heavy rain); 17.38 particles/L (after) | Positive | [68] |
| China | Rain | Qing River | 1.16 n/L (before); 1.04 n/L (after) | Negative | [51] |
| Brazil | Rain | Goiana Estuary | 0.56 n/100 m3 (rainy); 0.62 n/100 m3 (dry) | Positive | [69] |
| China | Rain | Urban river | 29.98 n/L (dry season); 90.99 n/L (wet season) | Positive | [70] |
| Sri Lanka | Rain | Beira Lake and Canal | lake: 0.011 (dry), 0.007 (wet); canal: 0.003 (wet), 0.002 (dry) | No | [71] |
| China | Rain | Runoff | 6.0 items/L (beginning); 1.0~4.0 items/L (during); 0.7 items/L (end) | Positive | [72] |
| China | Rain, flood | Dafangying River | 18.62 ± 7.12 items/m3 | Positive | [73] |
| China | Rain | Chaohu Lake | 2133 ± 1534 n/m3 (dry season); 1679 ± 1577 n/m3 (wet season) | Negative | [74] |
| Brazil | Rain | Jurujuba Cove | 14.4~202.8 n/L (rainy season); 91.2~137.4 n/L (dry season) | Negative | [75] |
| Brazil | Rain | Fish farms | 81.12 items/L (dry); 236.96 items/L (rainy) | Positive | [76] |
| South Africa | Rain | Crocodile River | 1058 (cool-dry season); 625 (hot-dry season); 625 particles/m3 (hot-wet season) | Positive | [77] |
| Singapore | Rain | Sea | 164.5 particles/mL | Positive | [78] |
| China | Rain | Lake | 0.59 items/L | Positive | [79] |
| China | Rain | Rainwater | 141 (spring); 140 (winter); 102 (summer); 78 particles/(m2·d) (autumn) | Positive | [80] |
| China | Rain | Maowei Sea | 2.8 particles/L (rainy season); 4.29 particles/L (dry season) | Negative | [81] |
| Mexico | Rain | Runoff | 177.13 particles/L | Positive | [47] |
| USA | Rain | Estuarine rivers | 90,007 pieces/km2 (summer); 95425 pieces/km2 (fall) | Positive | [82] |
| Indian | Rain, Storm | Manipal Lake | 0.423 particles/L (monsoon); 0.117 particles/L (post-monsoon) | Positive | [83] |
| Malaysia | Rain, wind | Sepanggar Bay water | 106.6 ± 23.0 (SWM); 63.0 ± 8.0 (NEM); 31.2 ± 6.7 particles/m3 (INTER) | Positive | [84] |
| China | Rain | Rainwater | 229 n/(m2·d) (wet deposition); 125 n/(m2·d) (dry deposition) | Positive | [85] |
| Colombian | Rain | Estuaries | 0.33 items/m3 (high rain); 0.085 items/L (low rain) | Positive | [86] |
| Canada | Rain | Urban runoff | 186 particles/L | Positive | [87] |
| France | Rain | Liane River | 35.5 (heavy rain); 5.1 (light rain); 12.4 particles/m3 (no rain) | Positive | [88] |
| China | Rain | Karst groundwater | 4.50 items/L | Positive | [89] |
| Brazil | Rain | Paraíba do Sul River | 1~12 particles/m3 (low water season); 1~18.3 particles/m3 (high water season) | Positive | [90] |
| China | Rain | Xincun Lagoon Bay | 60.9 ± 21.5 items/L (rainy season); 72.6 ± 23.7 items/L (dry season) | Negative | [91] |
| Turkey | Flood | Mediterranean Region river | 539,189 MPs/km2 (before flood); 7,699,716 MPs/km2 (afterwards) | Positive | [92] |
| France | Stormwater | Catchment outlet | 29 items/L | Positive | [93] |
| USA | Rain | Tampa Bay surface water | 2.2 particles/L (rain in OTB site); 1.0 particles/L(average) | Positive | [94] |
| Indian | Monsoonal rainfall | Udyavara River | 530.14 ± 352 particles/m3 | Positive | [95] |
| Vietnam | Rain | Saigon River | 53 items/L (rainy season); 75 items/L (dry season) | No | [96] |
| India | Rain | Netravathi River | 36.86 ± 23.12 (2020 monsoon); 70.5 ± 61.22 MP/m3 (after) | Positive | [97] |
| Belgium | Rain | Flanders surface water | 0.48 MPs/L | No | [98] |
| Argentina | Rain | Lake | 100 (spring)~180 MPs/m3 (summer) | Positive | [99] |
| China | Rain, flood | Yangtze Estuary | 300 n/kg (1954 flood at ECS1); 1000 n/kg (1998 flood at CCYY1) | Positive | [100] |
| China | Rain, typhoons | Seawater | 63.6 ± 37.4 items/L (before typhoon); 89.5 ± 20.6 items/L (after typhoon) | Positive | [101] |
| Indonesia | Rain | Jakarta River | 9.80 ± 4.79 (rainy season); 8.01 ± 4.82 particles/m3 (dry season) | Positive | [102] |
| Brazil | Rain | Acaraí Lagoon | 1.4~3.4 n/L | Negative | [103] |
| Australia | Rain | Perth metropolitan waters | 47,164 pieces/km2 (heavy rain in May); 2461 pieces/km2 (March) | Positive | [104] |
| India | Rain | Mandovi-Zuari estuarine | 107 particles/L (wet season); 99 particles/L (dry season) | Positive | [105] |
| Australia | Rain | Storm drains | 139.43 items/effort (before); 132.6 items/effort (during); 294.5 items/effort (after) | Positive | [106] |
| Finland | Rain | Surface flow wetland | 104 MPs/m3 (inflow); 200 (outflow addition deposition) | Positive | [107] |
| Thailand | Rain | Runoff | 1.3 ± 1.3 particles/L (wet season); 2.8 ± 0.9 particles/L (dry season) | Positive | [108] |
| India | Rain | Sharavathi River sediment | 2.5~57.5 pieces/kg (pre-monsoon); 0~15 pieces/kg (post-monsoon) | Positive | [109] |
| China | Rain | Donghu Lake | 5.84 ± 2.95 items/L (equilibrium state); 8.27 ± 5.65 items/L (during rain); 7.60 ± 4.04 items/L (after rainfall) | Positive | [110] |
| China | Rain | WWTP | 36.2~126.2 particles/L(rain); 38.9~75.3 particles/L (no rain) | Positive | [111] |
| China | River | Hanjiang rRiver | 30.9 (base flow); 80.2~114.5 (flood) | Positive | [112] |
| China | Rain, typhoon | Surface seawater in Hong Kong | 0.02 items/L (dry season); 0.10 items/L (wet season) | Positive | [113] |
| China | Rain | Harbor and coastal sediments | 36.5 ± 52.5 items/kg (dry season); 22.6 ± 23.2 items/kg (wet season) | Positive | [114] |
| Italy | Rain, wind | Lake | 0.82~1.24 particles/m3 (before); 2.42~4.41 particles/m3 (after) | Positive | [115] |
| Portugal | Rain | Estuary | 263 items/kg (no rain); 205 items/kg (rain) | No | [116] |
| China | Rain | Jiaozhou Bay sea water | 0.174 pieces/m3 (heavy rain in May); 0.05 piece/m3 (no rain in November) | Positive | [117] |
| USA | Rain | Outfalls | 0.30 ± 0.10~0.80 ± 0.33 MP/L (rain) | Negative | [118] |
| Italy | Rain | Mugnone Creek | 3.5 × 108 items/day (wet season in 2019); 5.2 × 106 (dry season 2020) | Positive | [119] |
| Lithuania | Rain | WWTP | 2982 ± 54 MP/L (wet season); 1964 ± 50 MP/L (dry season) | Positive | [120] |
| Germany | Rain | Weser River | 219.05 items/m3 (no rain day); 14,536.1 items/m3 (rain day) | Positive | [121] |
2.2. Ultraviolet Radiation
2.3. Wind
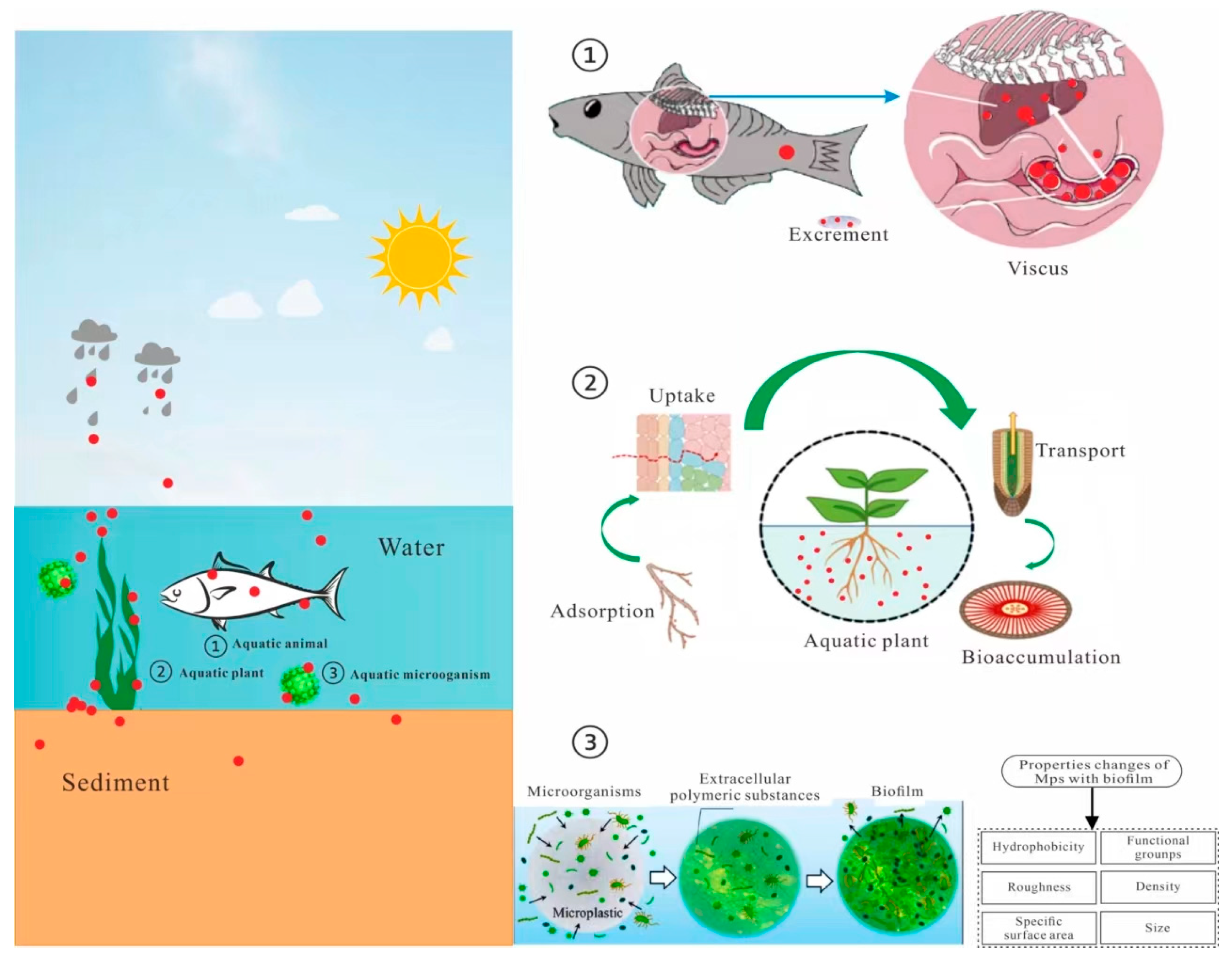
3. Aquatic Life
3.1. Aquatic Plants
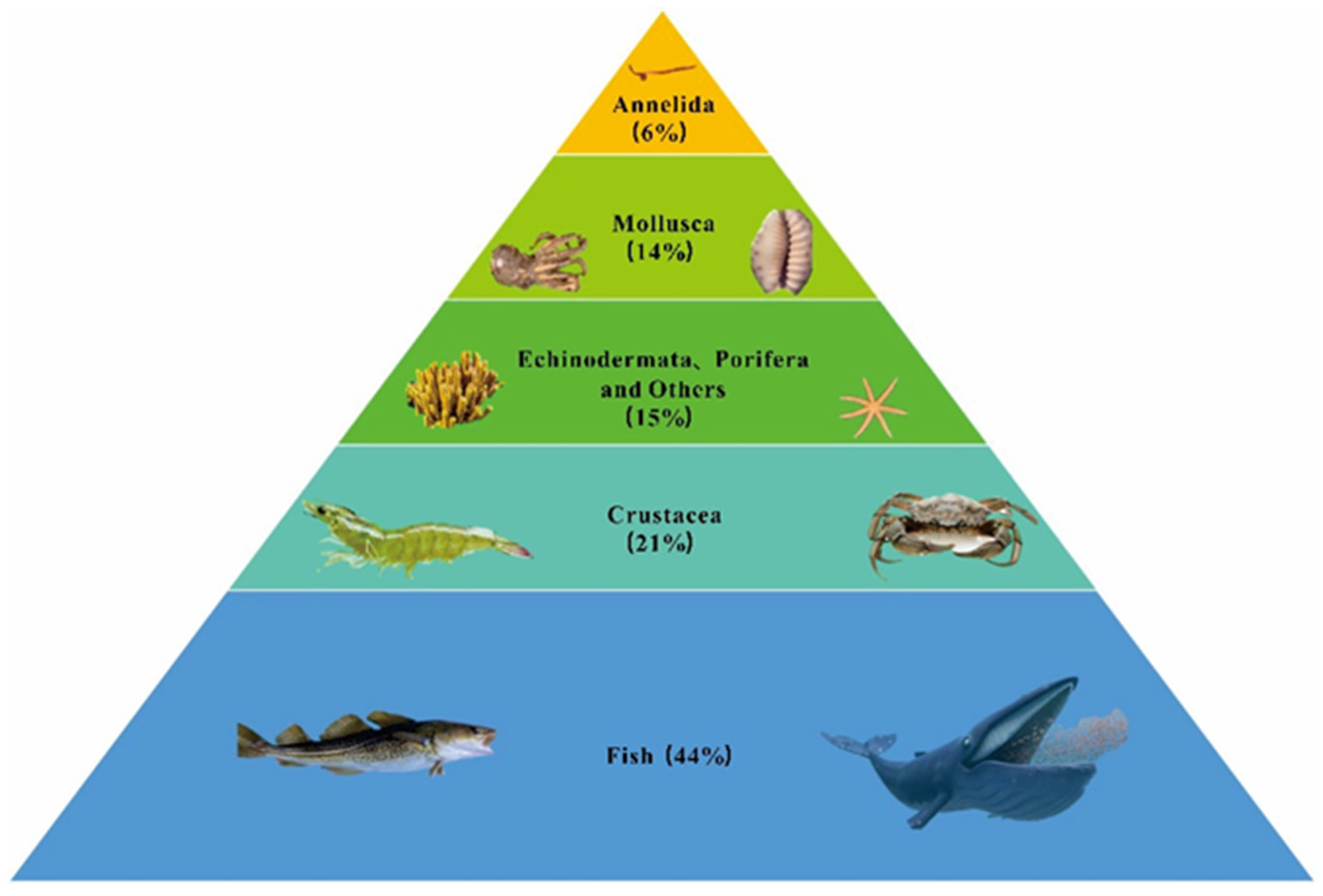
3.2. Aquatic Animals
3.3. Water Microorganisms
4. Water Matrix: Suspended Particulate, Sediment, and Topography
4.1. Suspended Particulate
4.2. Sediments
4.3. Water Topography and Landform
5. Conclusions and Outlook
- Carry out indoor quantitative simulation experiments and outdoor long-term observations on the migration of microplastics under changes in meteorological conditions and understand the migration mechanism and controlling factors of microplastics in the field at a large scale. Attention should be paid to the changes in water environmental conditions in different seasons to better evaluate microplastics’ interannual variation characteristics. Global climate change has led to more frequent extreme weather events, and the transport of microplastics in water bodies and their sediments may be significantly affected by climate change, so it is necessary to strengthen the research on the global distribution and circulation of microplastics under climate change scenarios in the future.
- In different types of water bodies, topography and landforms, as well as the types of particulate matter in water bodies, are very different, and more attention needs to be paid to the movement mechanism of microplastics in different types of lakes and rivers. It is important to clarify the agglomeration behavior and co-migration mechanism between particulate matter and microplastics in clear water to predict microplastics’ environmental behavior and risks accurately. The migration process of microplastics in seawater and surface water has been partially studied, but more attention needs to be paid to the migration of microplastics in groundwater and special ecological regions such as karst water [89].
- The impact of water temperature change on the transport of microplastics and the impact of ocean current processes on the global migration of microplastics were investigated. Currently, most of the research on the migration mechanism of microplastics in water bodies is carried out in the laboratory, which is quite different from the actual environment in which microplastics are located, and the model microplastics used are also significantly different from those in the real environment.
- The interactive migration model of external environmental factors, internal water characteristics, and the physical and chemical properties of microplastics can be constructed. Systematic research can be carried out on multiple factors and scales to have a more comprehensive understanding of the migration process of microplastics in the real environment and better supervise their ecological risks [126,169]. At the same time, large-scale data acquisition needs to be standardized before validating the migration model to increase the applicability of measurements. Strengthening the study of the movement mechanics of microplastics and the spatial mode of pollution, determining the risk location of pollutant accumulation, and better delineating the area with the greatest risk of potential negative effects of microplastic pollution will help to focus on water protection.
- Microplastics in water bodies are important carriers of other pollutants, and the synergistic migration behavior and mechanism of bacteria and viruses of other pollutants, especially newly polluted antibiotics, and microplastics in the actual water environment are still unknown and need to be further studied and improved [183].
- Microplastics are constantly changing in the actual environment and may be affected by various factors in the migration, decomposition, and transformation process. Their original physical and chemical properties will also change.
- Combined with the characteristics of microplastic pollution and the law of migration, an efficient governance system with scientific classification, source treatment, and migration control as the main contents and a sub-regional, sub-type, and sub-time supervision mechanism should be established.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Mennekes, D.; Nowack, B. Predicting microplastic masses in river networks with high spatial resolution at country level. Nat. Water 2023, 1, 523–533. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Luo, X.; Shukla, T.; Gao, T.; Allen, D.; Allen, S.; Bergmann, M. Microplastics and nanoplastics pose risks on the Tibetan Plateau environment. Sci. Bull. 2024, 69, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhagwat, A. Microplastics: A potential threat to groundwater resources. Groundw. Sustain. Dev. 2022, 19, 100852. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Janani, R.; Bhuvana, S.; Geethalakshmi, V.; Jeyachitra, R.; Sathishkumar, K.; Balu, R.; Ayyamperumal, R. Micro and nano plastics in food: A review on the strategies for identification, isolation, and mitigation through photocatalysis, and health risk assessment. Environ. Res. 2023, 241, 117666. [Google Scholar] [CrossRef] [PubMed]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic fibers—Underestimated threat to aquatic organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Fast Facts 2023; Plastics Europe: Brussels, Belgium, 2023. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Jansen, M.; Barnes, P.; Bornman, J.; Rose, K.; Madronich, S.; White, C.; Zepp, R.; Andrady, A. The Montreal Protocol and the fate of environmental plastic debris. Photochem. Photobiol. Sci. 2023, 22, 1203–1211. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef]
- Hollóczki, O.; Gehrke, S. Can nanoplastics alter cell membranes? ChemPhysChem 2020, 21, 9–12. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Ashim, J.; Park, S.; Kim, W.; Ji, S.; Lee, S.-W.; Jung, Y.-R.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C. A preliminary study about the potential risks of the UV-weathered microplastic: The proteome-level changes in the brain in response to polystyrene derived weathered microplastics. Environ. Res. 2023, 233, 116411. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro-and nanoplastics breach the blood–brain barrier (BBB): Biomolecular corona’s role revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Liu, Z.; Sokratian, A.; Duda, A.M.; Xu, E.; Stanhope, C.; Fu, A.; Strader, S.; Li, H.; Yuan, Y.; Bobay, B.G. Anionic nanoplastic contaminants promote Parkinson’s disease–associated α-synuclein aggregation. Sci. Adv. 2023, 9, eadi8716. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C. Microplastics and nanoplastics in atheromas and cardiovascular events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hu, Y.; Yuan, Y.; Hu, B.; Wang, B. Comparative analysis of kinetics and mechanisms for Pb (II) sorption onto three kinds of microplastics. Ecotoxicol. Environ. Saf. 2021, 208, 111451. [Google Scholar] [CrossRef]
- Fajardo, C.; Martín, C.; Costa, G.; Sánchez-Fortún, S.; Rodríguez, C.; de Lucas Burneo, J.J.; Nande, M.; Mengs, G.; Martín, M. Assessing the role of polyethylene microplastics as a vector for organic pollutants in soil: Ecotoxicological and molecular approaches. Chemosphere 2022, 288, 132460. [Google Scholar] [CrossRef]
- Pestana, C.J.; Moura, D.S.; Capelo-Neto, J.; Edwards, C.; Dreisbach, D.; Spengler, B.; Lawton, L.A. Potentially poisonous plastic particles: Microplastics as a vector for cyanobacterial toxins microcystin-LR and microcystin-LF. Environ. Sci. Technol. 2021, 55, 15940–15949. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, G.; Revell, L.E.; Zhang, J.; Zuo, C.; Wang, D.; Le Ru, E.C.; Wu, G.; Mitrano, D.M. Long-range atmospheric transport of microplastics across the southern hemisphere. Nat. Commun. 2023, 14, 7898. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Viaroli, S.; Lancia, M.; Re, V. Microplastics contamination of groundwater: Current evidence and future perspectives. A review. Sci. Total Environ. 2022, 824, 153851. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhou, X.; Su, Y.; Wang, H.; Yu, R.; Zhou, S.; Xu, E.G.; Xing, B. Environmental occurrence, fate, impact, and potential solution of tire microplastics: Similarities and differences with tire wear particles. Sci. Total Environ. 2021, 795, 148902. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Nakaoka, M. Trophic transfer of microplastics from mysids to fish greatly exceeds direct ingestion from the water column. Environ. Pollut. 2021, 273, 116468. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.-F.; Chen, L.-Y.; Lee, H.-J.; Gong, G.-C.; Lee, C. New insights into the role of marine plastic-gels in microplastic transfer from water to the atmosphere via bubble bursting. Water Res. 2022, 222, 118856. [Google Scholar] [CrossRef]
- Cai, Y.; Li, C.; Zhao, Y. A review of the migration and transformation of microplastics in inland water systems. Int. J. Environ. Res. Public Health 2021, 19, 148. [Google Scholar] [CrossRef]
- Gao, S.; Li, Z.; Zhang, S. Trophic transfer and biomagnification of microplastics through food webs in coastal waters: A new perspective from a mass balance model. Mar. Pollut. Bull. 2024, 200, 116082. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L.; Trifuoggi, M. Research progress in transfer, accumulation and effects of microplastics in the oceans. J. Mar. Sci. Eng. 2021, 9, 433. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef]
- Sang, W.; Chen, Z.; Mei, L.; Hao, S.; Zhan, C.; bin Zhang, W.; Li, M.; Liu, J. The abundance and characteristics of microplastics in rainwater pipelines in Wuhan, China. Sci. Total Environ. 2021, 755, 142606. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Li, X.; Zhang, Y.; Gao, W.; Jiang, J.; Mo, A.; He, D. Size/shape-dependent migration of microplastics in agricultural soil under simulative and natural rainfall. Sci. Total Environ. 2022, 815, 152507. [Google Scholar] [CrossRef]
- Yu, H.; Liu, M.; Gang, D.; Peng, J.; Hu, C.; Qu, J. Polyethylene microplastics interfere with the nutrient cycle in water-plant-sediment systems. Water Res. 2022, 214, 118191. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rao, Z.; Niu, S.; Zhan, N.; Wang, X.; Song, X. Microplastics in Sediments of River Yongfeng from Maanshan City, Anhui Province, China. Bull. Environ. Contam. Toxicol. 2020, 104, 166–172. [Google Scholar] [CrossRef]
- Zhang, K.; Xiong, X.; Hu, H.; Wu, C.; Bi, Y.; Wu, Y.; Zhou, B.; Lam, P.K.S.; Liu, J. Occurrence and Characteristics of Microplastic Pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environ. Sci. Technol. 2017, 51, 3794–3801. [Google Scholar] [CrossRef] [PubMed]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; De Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Schmidt, N.; Thibault, D.; Galgani, F.; Paluselli, A.; Sempéré, R. Occurrence of microplastics in surface waters of the Gulf of Lion (NW Mediterranean Sea). Prog. Oceanogr. 2018, 163, 214–220. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Schmidt, N.; Castro-Jiménez, J.; Oursel, B.; Sempéré, R. Phthalates and organophosphate esters in surface water, sediments and zooplankton of the NW Mediterranean Sea: Exploring links with microplastic abundance and accumulation in the marine food web. Environ. Pollut. 2021, 272, 115970. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.F.; Chudyk, W.; Watson, V.; Chapra, S.C.; Suplee, M.W. Influence of biomass and water velocity on light attenuation of Cladophora glomerata L.(Kuetzing) in rivers. Aquat. Bot. 2018, 151, 62–70. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Q.; Sun, L.; Liu, J.; Huang, M.; Wang, T.; Wu, P.; Zhu, N. Impact of persistent rain on microplastics distribution and plastisphere community: A field study in the Pearl River, China. Sci. Total Environ. 2023, 879, 163066. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Piñon-Colin, T.; Rodriguez-Jimenez, R.; Rogel-Hernandez, E.; Alvarez-Andrade, A.; Wakida, F.T. Microplastics in stormwater runoff in a semiarid region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef] [PubMed]
- Rauert, C.; Charlton, N.; Okoffo, E.D.; Stanton, R.S.; Agua, A.R.; Pirrung, M.C.; Thomas, K.V. Concentrations of tire additive chemicals and tire road wear particles in an Australian urban tributary. Environ. Sci. Technol. 2022, 56, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater microplastic concentrations vary through both space and time. Environ. Pollut. 2020, 263, 114481. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Niu, J.; Xu, D.; Zhang, M.; Sun, K.; Gao, B. Wet deposition of globally transportable microplastics (<25 μm) hovering over the megacity of Beijing. Environ. Sci. Technol. 2023, 57, 11152–11162. [Google Scholar]
- Wei, Y.; Dou, P.; Xu, D.; Zhang, Y.; Gao, B. Microplastic reorganization in urban river before and after rainfall. Environ. Pollut. 2022, 314, 120326. [Google Scholar] [CrossRef]
- Ockelford, A.; Cundy, A.; Ebdon, J.E. Storm response of fluvial sedimentary microplastics. Sci. Rep. 2020, 10, 1865. [Google Scholar] [CrossRef]
- Do, T.; Park, Y.; Lim, B.; Kim, S.; Chae, M.-Y.; Chun, C.-H. Effect of the first-flush phenomenon on the quantification of microplastics in rainwater. Mar. Pollut. Bull. 2023, 187, 114559. [Google Scholar] [CrossRef] [PubMed]
- Hajiouni, S.; Mohammadi, A.; Ramavandi, B.; Arfaeinia, H.; De-la-Torre, G.E.; Tekle-Röttering, A.; Dobaradaran, S. Occurrence of microplastics and phthalate esters in urban runoff: A focus on the Persian Gulf coastline. Sci. Total Environ. 2022, 806, 150559. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Hung, P.L.; Fok, L. River microplastic contamination and dynamics upon a rainfall event in Hong Kong, China. Environ. Process. 2019, 6, 253–264. [Google Scholar] [CrossRef]
- Li, Y.; Ke, S.; Xu, D.; Zhuo, H.; Liu, X.; Gao, B. Preferential deposition of buoyant small microplastics in surface sediments of the Three Gorges Reservoir, China: Insights from biomineralization. J. Hazard. Mater. 2024, 468, 133693. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, I.R.; Tufenkji, N. Mobility of functionalized quantum dots and a model polystyrene nanoparticle in saturated quartz sand and loamy sand. Environ. Sci. Technol. 2012, 46, 4449–4457. [Google Scholar] [CrossRef]
- Franchi, A.; O’Melia, C.R. Effects of natural organic matter and solution chemistry on the deposition and reentrainment of colloids in porous media. Environ. Sci. Technol. 2003, 37, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Sadri, B.; Pernitsky, D.; Sadrzadeh, M. Aggregation and deposition of colloidal particles: Effect of surface properties of collector beads. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 46–52. [Google Scholar] [CrossRef]
- Wang, M.; Gao, B.; Tang, D. Review of key factors controlling engineered nanoparticle transport in porous media. J. Hazard. Mater. 2016, 318, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Haapkylä, J.; Unsworth, R.K.; Flavell, M.; Bourne, D.G.; Schaffelke, B.; Willis, B.L. Seasonal rainfall and runoff promote coral disease on an inshore reef. PLoS ONE 2011, 6, e16893. [Google Scholar] [CrossRef]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Zhu, D.; Xia, T.; Qi, Y.; Yao, Y.; Guo, X.; Ji, R.; Chen, W. Polystyrene Nanoplastics-enhanced Contaminant Transport: Role of Irreversible Adsorption in Glassy Polymeric Domain. Environ. Sci. Technol. 2018, 52, 2677–2685. [Google Scholar] [CrossRef]
- Oni, B.A.; Ayeni, A.O.; Agboola, O.; Oguntade, T.; Obanla, O. Comparing microplastics contaminants in (dry and raining) seasons for Ox-Bow Lake in Yenagoa, Nigeria. Ecotoxicol. Environ. Saf. 2020, 198, 110656. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Peng, Z.; Zhu, Z.-R.; Fu, W.; Dai, X.; Ni, B.-J. The atmospheric microplastics deposition contributes to microplastic pollution in urban waters. Water Res. 2022, 225, 119116. [Google Scholar] [CrossRef]
- Ross, M.S.; Loutan, A.; Groeneveld, T.; Molenaar, D.; Kroetch, K.; Bujaczek, T.; Kolter, S.; Moon, S.; Huynh, A.; Khayam, R. Estimated discharge of microplastics via urban stormwater during individual rain events. Front. Environ. Sci. 2023, 11, 1090267. [Google Scholar] [CrossRef]
- Imbulana, S.; Tanaka, S.; Moriya, A.; Oluwoye, I. Inter-event and intra-event dynamics of microplastic emissions in an urban river during rainfall episodes. Environ. Res. 2024, 243, 117882. [Google Scholar] [CrossRef]
- Hitchcock, J.N. Storm events as key moments of microplastic contamination in aquatic ecosystems. Sci. Total Environ. 2020, 734, 139436. [Google Scholar] [CrossRef]
- Lima, A.R.A.; Barletta, M.; Costa, M.F. Seasonal distribution and interactions between plankton and microplastics in a tropical estuary. Estuar. Coast. Shelf Sci. 2015, 165, 213–225. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Wang, F.; Gu, X.; Li, Y.; Liu, Q.; Li, L.; Bai, F. Temporal and spatial variation of microplastics in the urban rivers of Harbin. Sci. Total Environ. 2024, 910, 168373. [Google Scholar] [CrossRef]
- Bandara, R.; Perera, M.; Gomes, P.I.; Yan, X.-F. Profiling Microplastic Pollution in Surface Water Bodies in the Most Urbanized City of Sri Lanka and Its Suburbs to Understand the Underlying Factors. Water Air Soil Pollut. 2023, 234, 157. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, W.; Zou, G.; Wang, X.; Zhao, M.; Guo, S.; Chen, Y. Urban pipeline rainwater runoff is an important pathway for land-based microplastics transport to inland surface water: A case study in Beijing. Sci. Total Environ. 2023, 861, 160619. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Z.; Liu, Y.; Zhao, X.; Liang, Y.; Lu, W.; Song, J. Microplastic contamination assessment in water and economic fishes in different trophic guilds from an urban water supply reservoir after flooding. J. Environ. Manag. 2021, 299, 113667. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, K.; Liu, X.; Yao, R.; Cao, W.; Zhang, L.; Wang, X. Spatial and temporal distributions of microplastics and their macroscopic relationship with algal blooms in Chaohu Lake, China. J. Contam. Hydrol. 2022, 248, 104028. [Google Scholar] [CrossRef]
- Castro, R.O.; Silva, M.L.; Marques, M.R.C.; de Araújo, F.V. Evaluation of microplastics in Jurujuba Cove, Niterói, RJ, Brazil, an area of mussels farming. Mar. Pollut. Bull. 2016, 110, 555–558. [Google Scholar] [CrossRef]
- Dantas Filho, J.V.; Pedroti, V.P.; Santos, B.L.T.; de Lima Pinheiro, M.M.; de Mira, Á.B.; da Silva, F.C.; e Silva, E.C.S.; Cavali, J.; Guedes, E.A.C.; de Vargas Schons, S. First evidence of microplastics in freshwater from fish farms in Rondônia state, Brazil. Heliyon 2023, 9, e15066. [Google Scholar] [CrossRef]
- Nkosi, M.S.; Cuthbert, R.N.; Wu, N.; Shikwambana, P.; Dalu, T. Microplastic abundance, distribution, and diversity in water and sediments along a subtropical river system. Environ. Sci. Pollut. Res. 2023, 30, 91440–91452. [Google Scholar] [CrossRef]
- Curren, E.; Leong, S.C.Y. Spatiotemporal characterisation of microplastics in the coastal regions of Singapore. Heliyon 2023, 9, e12961. [Google Scholar] [CrossRef]
- Dong, H.; Wang, L.; Wang, X.; Xu, L.; Chen, M.; Gong, P.; Wang, C. Microplastics in a remote lake basin of the Tibetan Plateau: Impacts of atmospheric transport and glacial melting. Environ. Sci. Technol. 2021, 55, 12951–12960. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, T.; Yan, M.; Yang, L.; Gong, H.; Wang, W.; Qing, X.; Wang, J. Atmospheric transport and deposition of microplastics in a subtropical urban environment. J. Hazard. Mater. 2021, 416, 126168. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Huang, Y.; Jiang, Y.; Li, J.; Michal, J.J.; Jiang, Z.; Xu, Y.; Lan, W. Long-term trends of microplastics in seawater and farmed oysters in the Maowei Sea, China. Environ. Pollut. 2021, 273, 116450. [Google Scholar] [CrossRef] [PubMed]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, USA. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Warrier, A.K.; Kulkarni, B.; Amrutha, K.; Jayaram, D.; Valsan, G.; Agarwal, P. Seasonal variations in the abundance and distribution of microplastic particles in the surface waters of a Southern Indian Lake. Chemosphere 2022, 300, 134556. [Google Scholar] [CrossRef]
- Tang, C.N.; Kuwahara, V.S.; Leong, S.C.Y.; Moh, P.Y.; Yoshida, T. Effect of monsoon on microplastic bioavailability and ingestion by zooplankton in tropical coastal waters of Sabah. Mar. Pollut. Bull. 2023, 193, 115182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, Y.; Ma, T.; Liu, X.; Wei, H.; Meng, H.; Fu, Y.; Ma, Z.; Zhang, L.; Zhao, J. Distribution and possible sources of atmospheric microplastic deposition in a valley basin city (Lanzhou, China). Ecotoxicol. Environ. Saf. 2022, 233, 113353. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Ordóñez, O.; Castillo-Olaya, V.; Espinosa-Díaz, L.F.; Canals, M. Seasonal variation in plastic litter pollution in mangroves from two remote tropical estuaries of the Colombian Pacific. Mar. Pollut. Bull. 2023, 193, 115210. [Google Scholar] [CrossRef] [PubMed]
- Smyth, K.; Drake, J.; Li, Y.; Rochman, C.; Van Seters, T.; Passeport, E. Bioretention cells remove microplastics from urban stormwater. Water Res. 2021, 191, 116785. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, G.; Doyen, P.; Dehaut, A.; Veillet, G.; Duflos, G.; Amara, R. Vertical distribution of microplastics in a river water column using an innovative sampling method. Environ. Monit. Assess. 2023, 195, 1302. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Li, W.; Lan, J.; Adnan, M. Preliminary study on the distribution, source, and ecological risk of typical microplastics in karst groundwater in Guizhou Province, China. Int. J. Environ. Res. Public Health 2022, 19, 14751. [Google Scholar] [CrossRef] [PubMed]
- da Costa, I.D.; Nunes, N.N.; Costa, L.L.; Zalmon, I.R. Is the Paraíba do Sul River colourful? Prevalence of microplastics in freshwater, south-eastern Brazil. Mar. Freshw. Res. 2022, 73, 1439–1449. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, W.; Xu, Q.; Sun, C.; Wang, X.; Gao, F. Microplastic distribution and influence factor analysis of seawater and surface sediments in a typical bay with diverse functional areas: A case study in Xincun lagoon, China. Front. Environ. Sci. 2022, 10, 829942. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C.; Ayat, B.; Aydoğan, B.; Karaca, S. How microplastics quantities increase with flood events? An example from Mersin Bay NE Levantine coast of Turkey. Environ. Pollut. 2018, 239, 342–350. [Google Scholar] [CrossRef]
- Treilles, R.; Gasperi, J.; Gallard, A.; Saad, M.; Dris, R.; Partibane, C.; Breton, J.; Tassin, B. Microplastics and microfibers in urban runoff from a suburban catchment of Greater Paris. Environ. Pollut. 2021, 287, 117352. [Google Scholar] [CrossRef] [PubMed]
- McEachern, K.; Alegria, H.; Kalagher, A.L.; Hansen, C.; Morrison, S.; Hastings, D. Microplastics in Tampa Bay, Florida: Abundance and variability in estuarine waters and sediments. Mar. Pollut. Bull. 2019, 148, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, V.; Valsan, G.; Amrutha, K.; Sebastian, J.G.; Rangel-Buitrago, N.; Khaleel, R.; Chandran, T.; Reshma, S.; Warrier, A.K. A baseline study of microplastic pollution in a Southern Indian Estuary. Mar. Pollut. Bull. 2023, 186, 114468. [Google Scholar] [CrossRef] [PubMed]
- Strady, E.; Kieu-Le, T.-C.; Gasperi, J.; Tassin, B. Temporal dynamic of anthropogenic fibers in a tropical river-estuarine system. Environ. Pollut. 2020, 259, 113897. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, K.; Warrier, A.K.; Rangel-Buitrago, N. Did the COVID-19 pandemic play a role in the spatial and temporal variations of microplastics? Evidence from a tropical river in southern India. Mar. Pollut. Bull. 2023, 192, 115088. [Google Scholar] [CrossRef] [PubMed]
- Semmouri, I.; Vercauteren, M.; Van Acker, E.; Pequeur, E.; Asselman, J.; Janssen, C. Distribution of microplastics in freshwater systems in an urbanized region: A case study in Flanders (Belgium). Sci. Total Environ. 2023, 872, 162192. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, M.B.; Arias, A.H.; Piccolo, M.C. Microplastics integrating the zooplanktonic fraction in a saline lake of Argentina: Influence of water management. Environ. Monit. Assess. 2020, 192, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, Y.; Wang, Y.; Ding, Y.; Wang, C.; Feng, X.; Fan, Q.; Yuan, F.; Fu, G.; Gao, B. Microplastics: A potential proxy for tracing extreme flood events in estuarine environments. Sci. Total Environ. 2024, 918, 170554. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Wang, M.; Jiang, T.; Liu, X.; Ru, S. Typhoons increase the abundance of microplastics in the marine environment and cultured organisms: A case study in Sanggou Bay, China. Sci. Total Environ. 2019, 667, 1–8. [Google Scholar] [CrossRef]
- Cordova, M.R.; Ulumuddin, Y.I.; Purbonegoro, T.; Puspitasari, R.; Afianti, N.F.; Rositasari, R.; Yogaswara, D.; Hafizt, M.; Iswari, M.Y.; Fitriya, N. Seasonal heterogeneity and a link to precipitation in the release of microplastic during COVID-19 outbreak from the Greater Jakarta area to Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2022, 181, 113926. [Google Scholar] [CrossRef]
- Lorenzi, L.; Reginato, B.C.; Mayer, D.G.; Dantas, D.V. Plastic floating debris along a summer-winter estuarine environmental gradient in a coastal lagoon: How does plastic debris arrive in a conservation unit? Environ. Sci. Pollut. Res. 2020, 27, 8797–8806. [Google Scholar] [CrossRef] [PubMed]
- Hajbane, S.; Pattiaratchi, C. Plastic Pollution Patterns in Offshore, Nearshore and Estuarine Waters: A Case Study from Perth, Western Australia. Front. Mar. Sci. 2017, 4, 63. [Google Scholar] [CrossRef]
- Gupta, P.; Saha, M.; Rathore, C.; Suneel, V.; Ray, D.; Naik, A.; Unnikrishnan, K.; Dhivya, M.; Daga, K. Spatial and seasonal variation of microplastics and possible sources in the estuarine system from central west coast of India. Environ. Pollut. 2021, 288, 117665. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Civiello, A.; Critchell, K.; Hoogenboom, M.; Hamann, M. Input of plastic debris in an urban tropical river system. Mar. Pollut. Bull. 2019, 144, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Büngener, L.; Postila, H.; Löder, M.G.; Laforsch, C.; Ronkanen, A.-K.; Heiderscheidt, E. The fate of microplastics from municipal wastewater in a surface flow treatment wetland. Sci. Total Environ. 2023, 903, 166334. [Google Scholar] [CrossRef]
- Xue, W.; Maung, G.Y.T.; Otiti, J.; Tabucanon, A.S. Land use-based characterization and source apportionment of microplastics in urban storm runoffs in a tropical region. Environ. Pollut. 2023, 329, 121698. [Google Scholar] [CrossRef]
- Amrutha, K.; Shajikumar, S.; Warrier, A.K.; Sebastian, J.G.; Sali, Y.A.; Chandran, T.; Sivadas, S.; Naik, R.; Amrish, V.N.; Kumar, A. Assessment of pollution and risks associated with microplastics in the riverine sediments of the Western Ghats: A heritage site in southern India. Environ. Sci. Pollut. Res. 2023, 30, 32301–32319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, Y.; Zhan, C.; Cheng, B. Impacts of rainfall and lakeshore soil properties on microplastics in inland freshwater: A case study in Donghu Lake, China. Environ. Sci. Process. Impacts 2024, 26, 891–901. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, H.; Xu, H.; Zhou, C.; Wang, P.; Liu, Z.; Yang, Y.; Huang, J.; Wang, C.; Zhao, X. Wastewater treatment plant serves as a potentially controllable source of microplastic: Association of microplastic removal and operational parameters and water quality data. J. Hazard. Mater. 2023, 441, 129974. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Liu, X.; Singh, V.P. Dispersal and transport of microplastic particles under different flow conditions in riverine ecosystem. J. Hazard. Mater. 2023, 442, 130033. [Google Scholar] [CrossRef]
- Cheung, C.K.H.; Not, C. Impacts of extreme weather events on microplastic distribution in coastal environments. Sci. Total Environ. 2023, 904, 166723. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Ju, Y.-R.; Lim, Y.C.; Chen, C.-W.; Dong, C.-D. Seasonal variation of diversity, weathering, and inventory of microplastics in coast and harbor sediments. Sci. Total Environ. 2021, 781, 146610. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.K.; Paglialonga, L.; Czech, E.; Tamminga, M. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Antunes, J.; Pais, J.; Pequeno, J.; Caetano, P.S.; Rocha, F.; Sobral, P.; Costa, M.H. Distribution patterns of microplastics in subtidal sediments from the Sado river estuary and the Arrábida marine park, Portugal. Front. Environ. Sci. 2022, 10, 998513. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Zhu, M.; Liang, J.; Zheng, S.; Sun, X. Seasonal variation of micro-and meso-plastics in the seawater of Jiaozhou Bay, the Yellow Sea. Mar. Pollut. Bull. 2020, 152, 110922. [Google Scholar] [CrossRef] [PubMed]
- Boni, W.; Arbuckle-Keil, G.; Fahrenfeld, N. Inter-storm variation in microplastic concentration and polymer type at stormwater outfalls and a bioretention basin. Sci. Total Environ. 2022, 809, 151104. [Google Scholar] [CrossRef] [PubMed]
- Rimondi, V.; Monnanni, A.; De Beni, E.; Bicocchi, G.; Chelazzi, D.; Cincinelli, A.; Fratini, S.; Martellini, T.; Morelli, G.; Venturi, S. Occurrence and quantification of natural and microplastic items in urban streams: The case of Mugnone Creek (Florence, Italy). Toxics 2022, 10, 159. [Google Scholar] [CrossRef]
- Uogintė, I.; Pleskytė, S.; Pauraitė, J.; Lujanienė, G. Seasonal variation and complex analysis of microplastic distribution in different WWTP treatment stages in Lithuania. Environ. Monit. Assess. 2022, 194, 829. [Google Scholar] [CrossRef]
- Moses, S.R.; Löder, M.G.; Herrmann, F.; Laforsch, C. Seasonal variations of microplastic pollution in the German River Weser. Sci. Total Environ. 2023, 902, 166463. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Yee Leung, K.M.; Wu, F. Color: An important but overlooked factor for plastic photoaging and microplastic formation. Environ. Sci. Technol. 2022, 56, 9161–9163. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Yadav, B.P.; Siddiqui, N.A.; Chaturvedi, S.K. Mathematical modelling and analysis of plastic waste pollution and its impact on the ocean surface. J. Ocean Eng. Sci. 2020, 5, 136–163. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, J.-S.; Kim, S.-Y.; Song, N.-S.; La, H.S.; Yang, E.J. Arctic Ocean sediments as important current and future sinks for marine microplastics missing in the global microplastic budget. Sci. Adv. 2023, 9, eadd2348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef] [PubMed]
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar. Pollut. Bull. 2016, 110, 383–395. [Google Scholar] [CrossRef]
- Lattin, G.L.; Moore, C.J.; Zellers, A.F.; Moore, S.L.; Weisberg, S.B. A comparison of neustonic plastic and zooplankton at different depths near the southern California shore. Mar. Pollut. Bull. 2004, 49, 291–294. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; Van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, R.; Shi, X.; Zhang, S.; Sun, B.; Zhao, S.; Huotari, J. Application of a microplastic trap to the determination of the factors controlling the lakebed deposition of microplastics. Sci. Total Environ. 2022, 843, 156883. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.; Hecq, J.-H.; Glagani, F.; Voisin, P.; Collard, F.; Goffart, A. Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Xu, E.G.; Li, L.; Zhou, A.; Peijnenburg, W.J.; Grossart, H.-P.; Liu, W.; Yang, Y. Tracing and trapping micro-and nanoplastics: Untapped mitigation potential of aquatic plants? Water Res. 2023, 242, 120249. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, G.; Scalici, M. Riparian vegetation as a trap for plastic litter. Environ. Pollut. 2022, 292, 118410. [Google Scholar] [CrossRef] [PubMed]
- Helcoski, R.; Yonkos, L.T.; Sanchez, A.; Baldwin, A.H. Wetland soil microplastics are negatively related to vegetation cover and stem density. Environ. Pollut. 2020, 256, 113391. [Google Scholar] [CrossRef] [PubMed]
- Sundbæk, K.B.; Koch, I.D.W.; Villaro, C.G.; Rasmussen, N.S.; Holdt, S.L.; Hartmann, N.B. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J. Appl. Phycol. 2018, 30, 2923–2927. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; He, S.; Chi, H.-Y.; Li, Z.-C.; Zhou, X.-X.; Yan, B. Quantification of nanoplastic uptake in cucumber plants by pyrolysis gas chromatography/mass spectrometry. Environ. Sci. Technol. Lett. 2021, 8, 633–638. [Google Scholar] [CrossRef]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef]
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef]
- Gan, Q.; Cui, J.; Jin, B. Environmental microplastics: Classification, sources, fates, and effects on plants. Chemosphere 2023, 313, 137559. [Google Scholar] [CrossRef]
- da Costa, J.P.; Chamkha, M.; Ksibi, M.; Sayadi, S. Effects of microplastics’ physical and chemical properties on aquatic organisms: State-of-the-art and future research trends. TrAC Trends Anal. Chem. 2023, 166, 117192. [Google Scholar]
- Azevedo-Santos, V.M.; Goncalves, G.R.; Manoel, P.S.; Andrade, M.C.; Lima, F.P.; Pelicice, F.M. Plastic ingestion by fish: A global assessment. Environ. Pollut. 2019, 255 Pt 1, 112994. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Olden, J.D. Global meta-analysis reveals diverse effects of microplastics on freshwater and marine fishes. Fish Fish. 2022, 23, 1439–1454. [Google Scholar] [CrossRef]
- Miranda, D.d.A.; de Carvalho-Souza, G.F. Are we eating plastic-ingesting fish? Mar. Pollut. Bull. 2016, 103, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Mchugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Vroom, R.J.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Godbold, J.A.; Lewis, C.N.; Savage, G.; Solan, M.; Galloway, T.S. Microplastic burden in marine benthic invertebrates depends on species traits and feeding ecology within biogeographical provinces. Nat. Commun. 2023, 14, 8023. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Zhao, J.; Lan, R.; Wang, Z.; Su, W.; Song, D.; Xue, R.; Liu, Z.; Liu, X.; Dai, Y.; Yue, T. Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution. Nat. Nanotechnol. 2024, 19, 406–414. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef]
- Chen, C.-S.; Shiu, R.-F.; Hsieh, Y.-Y.; Xu, C.; Vazquez, C.I.; Cui, Y.; Hsu, I.C.; Quigg, A.; Santschi, P.H.; Chin, W.-C. Stickiness of extracellular polymeric substances on different surfaces via magnetic tweezers. Sci. Total Environ. 2021, 757, 143766. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Xue, J. Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 2021, 55, 12780–12790. [Google Scholar] [CrossRef]
- He, S.; Jia, M.; Xiang, Y.; Song, B.; Xiong, W.; Cao, J.; Peng, H.; Yang, Y.; Wang, W.; Yang, Z. Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. J. Hazard. Mater. 2022, 424, 127286. [Google Scholar] [CrossRef]
- Tu, C.; Chen, T.; Zhou, Q.; Liu, Y.; Wei, J.; Waniek, J.J.; Luo, Y. Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci. Total Environ. 2020, 734, 139237. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Wu, G.-M.; Neu, T.R.; Wendt-Potthoff, K. Biofouling, metal sorption and aggregation are related to sinking of microplastics in a stratified reservoir. Water Res. 2020, 176, 115748. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Qi, K.; Wang, J.; Wang, W.; Wang, Z.; Lu, N.; Qu, J. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res. 2020, 184, 116205. [Google Scholar] [CrossRef]
- Meng, D.; Li, Y. Assessing the Settling Velocity of Biofilm-Encrusted Microplastics: Accounting for Biofilms as an Equivalent to Surface Roughness. Environ. Sci. Technol. 2024, 58, 1329–1337. [Google Scholar] [CrossRef]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Nauendorf, A.; Krause, S.; Bigalke, N.K.; Gorb, E.V.; Gorb, S.N.; Haeckel, M.; Wahl, M.; Treude, T. Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar. Pollut. Bull. 2016, 103, 168–178. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Ha, S.Y.; Han, G.M.; Jang, M.; Hong, S.H. Microplastic emission characteristics of stormwater runoff in an urban area: Intra-event variability and influencing factors. Sci. Total Environ. 2023, 866, 161318. [Google Scholar] [CrossRef]
- Singh, N.; Tiwari, E.; Khandelwal, N.; Darbha, G.K. Understanding the stability of nanoplastics in aqueous environments: Effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals. Environ. Sci. Nano 2019, 6, 2968–2976. [Google Scholar] [CrossRef]
- Andersen, T.J.; Rominikan, S.; Olsen, I.S.; Skinnebach, K.H.; Fruergaard, M. Flocculation of PVC microplastic and fine-grained cohesive sediment at environmentally realistic concentrations. Biol. Bull. 2021, 240, 42–51. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, W.; Xia, X.; Liu, C.; Min, J.; Zhang, W.; Crittenden, J.C. Interactions between nano/micro plastics and suspended sediment in water: Implications on aggregation and settling. Water Res. 2019, 161, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Paul-Pont, I.; Hegaret, H.; Moriceau, B.; Lambert, C.; Huvet, A.; Soudant, P. Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ. Pollut. 2017, 228, 454–463. [Google Scholar] [CrossRef]
- Oriekhova, O.; Stoll, S. Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ. Sci. Nano 2018, 5, 792–799. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Quik, J.T.; Sun, M.; Koelmans, A.A. Fate of nano-and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef]
- Xie, M.; Alsina, M.A.; Yuen, J.; Packman, A.I.; Gaillard, J.-F. Effects of resuspension on the mobility and chemical speciation of zinc in contaminated sediments. J. Hazard. Mater. 2019, 364, 300–308. [Google Scholar] [CrossRef]
- Corcoran, P.L.; Belontz, S.L.; Ryan, K.; Walzak, M.J. Factors controlling the distribution of microplastic particles in benthic sediment of the Thames River, Canada. Environ. Sci. Technol. 2019, 54, 818–825. [Google Scholar] [CrossRef]
- Dong, Z.; Qiu, Y.; Zhang, W.; Yang, Z.; Wei, L. Size-dependent transport and retention of micron-sized plastic spheres in natural sand saturated with seawater. Water Res. 2018, 143, 518–526. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, C.; Zhang, F.; Wei, K.; Shan, S.; Zhao, Y.; Man, Y.B.; Wong, M.H.; Zhang, J. Microplastics removal mechanisms in constructed wetlands and their impacts on nutrient (nitrogen, phosphorus and carbon) removal: A critical review. Sci. Total Environ. 2024, 918, 170654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hernández-Crespo, C.; Du, B.; Van Hulle, S.W.; Rousseau, D.P. Fate and removal of microplastics in unplanted lab-scale vertical flow constructed wetlands. Sci. Total Environ. 2021, 778, 146152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T.; Hu, H.; Ao, H.; Xiong, X.; Shi, H.; Wu, C. Transport and fate of microplastics in constructed wetlands: A microcosm study. J. Hazard. Mater. 2021, 415, 125615. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wu, T.; Sun, H.-J.; Ding, J.; Pang, J.-W.; Zhang, L.; Ren, N.-Q.; Yang, S.-S. Recent advances towards micro (nano) plastics research in wetland ecosystems: A systematic review on sources, removal, and ecological impacts. J. Hazard. Mater. 2023, 452, 131341. [Google Scholar] [CrossRef] [PubMed]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.; McGrattan, S.; Sullivan, P.J.; Walter, M.T. The effect of dams on river transport of microplastic pollution. Sci. Total Environ. 2019, 664, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Cai, C.; He, Y.; Chen, L.; Xiong, X.; Huang, H.; Tao, S.; Liu, W. Occurrence and characteristics of microplastics in the Haihe River: An investigation of a seagoing river flowing through a megacity in northern China. Environ. Pollut. 2020, 262, 114261. [Google Scholar] [CrossRef] [PubMed]
- Kan, A.T.; Tomson, M.B. Ground water transport of hydrophobic organic compounds in the presence of dissolved organic matter. Environ. Toxicol. Chem. Int. J. 1990, 9, 253–263. [Google Scholar] [CrossRef]
- Packman, A.I.; Salehin, M.; Zaramella, M. Hyporheic exchange with gravel beds: Basic hydrodynamic interactions and bedform-induced advective flows. J. Hydraul. Eng. 2004, 130, 647–656. [Google Scholar] [CrossRef]
- Elliott, A.H.; Brooks, N.H. Transfer of nonsorbing solutes to a streambed with bed forms: Theory. Water Resour. Res. 1997, 33, 123–136. [Google Scholar] [CrossRef]
- Ren, S.; Xia, Y.; Jin, X.; Sun, D.; Luo, D.; Wei, W.; Yang, Q.; Ding, J.; Lv, M.; Chen, L. Influence of microplastics on the availability of antibiotics in soils. Sci. Total Environ. 2024, 924, 171514. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Wang, Y.; Adnan, M.; Li, W.; Zhang, Y. Natural Factors of Microplastics Distribution and Migration in Water: A Review. Water 2024, 16, 1595. https://doi.org/10.3390/w16111595
An X, Wang Y, Adnan M, Li W, Zhang Y. Natural Factors of Microplastics Distribution and Migration in Water: A Review. Water. 2024; 16(11):1595. https://doi.org/10.3390/w16111595
Chicago/Turabian StyleAn, Xianjin, Yanling Wang, Muhammad Adnan, Wei Li, and Yaqin Zhang. 2024. "Natural Factors of Microplastics Distribution and Migration in Water: A Review" Water 16, no. 11: 1595. https://doi.org/10.3390/w16111595
APA StyleAn, X., Wang, Y., Adnan, M., Li, W., & Zhang, Y. (2024). Natural Factors of Microplastics Distribution and Migration in Water: A Review. Water, 16(11), 1595. https://doi.org/10.3390/w16111595







