Direct Electrooxidation of Ammonia-Enriched Wastewater Using a Bipolar Membrane-Integrated Electrolytic Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrolytic Cell Configuration
2.2. Wastewater Preparation
2.3. Analytical Method
2.4. Electrochemical Characterization
2.5. Theoretical Calculations
3. Results and Discussion
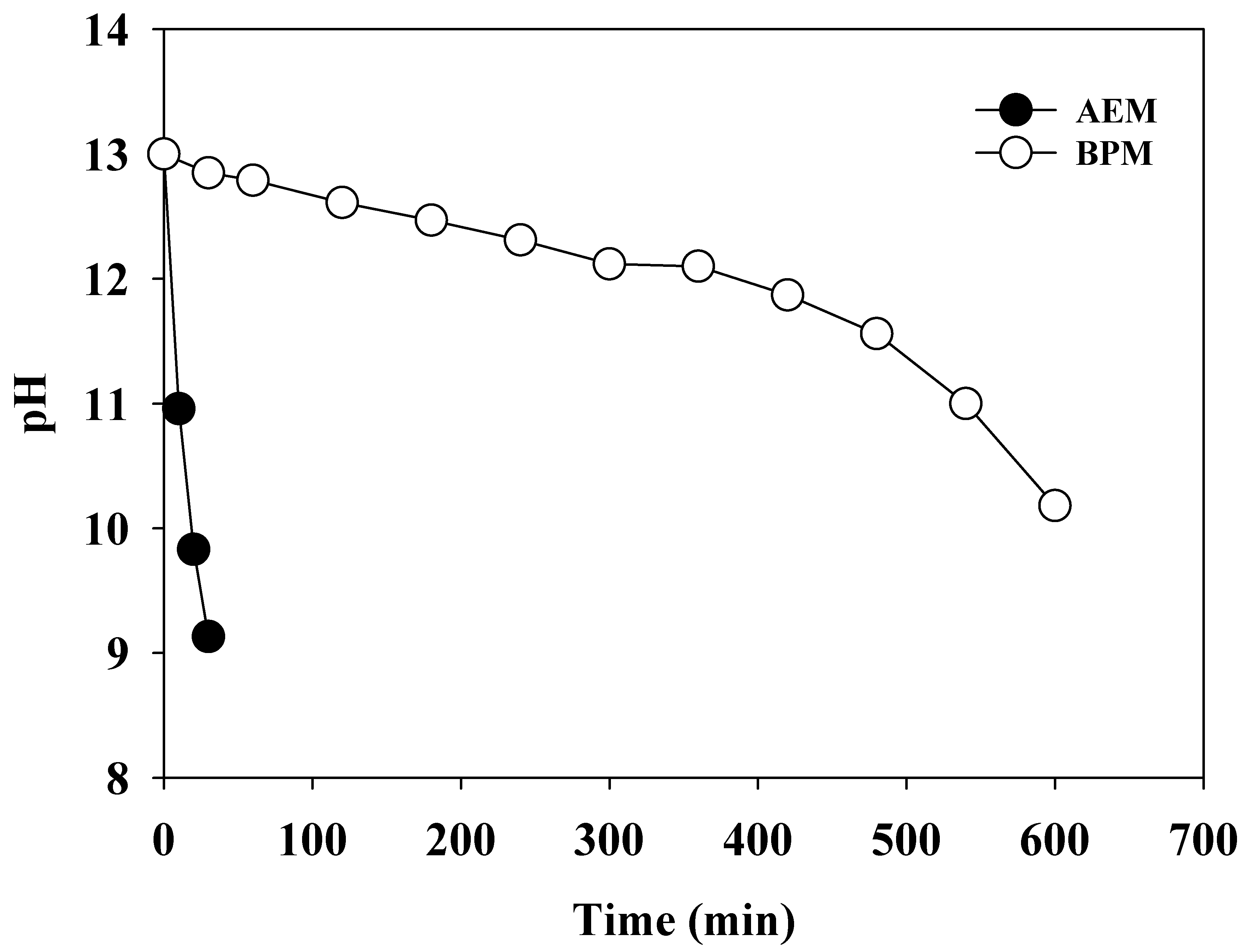
3.1. Role of the BPM
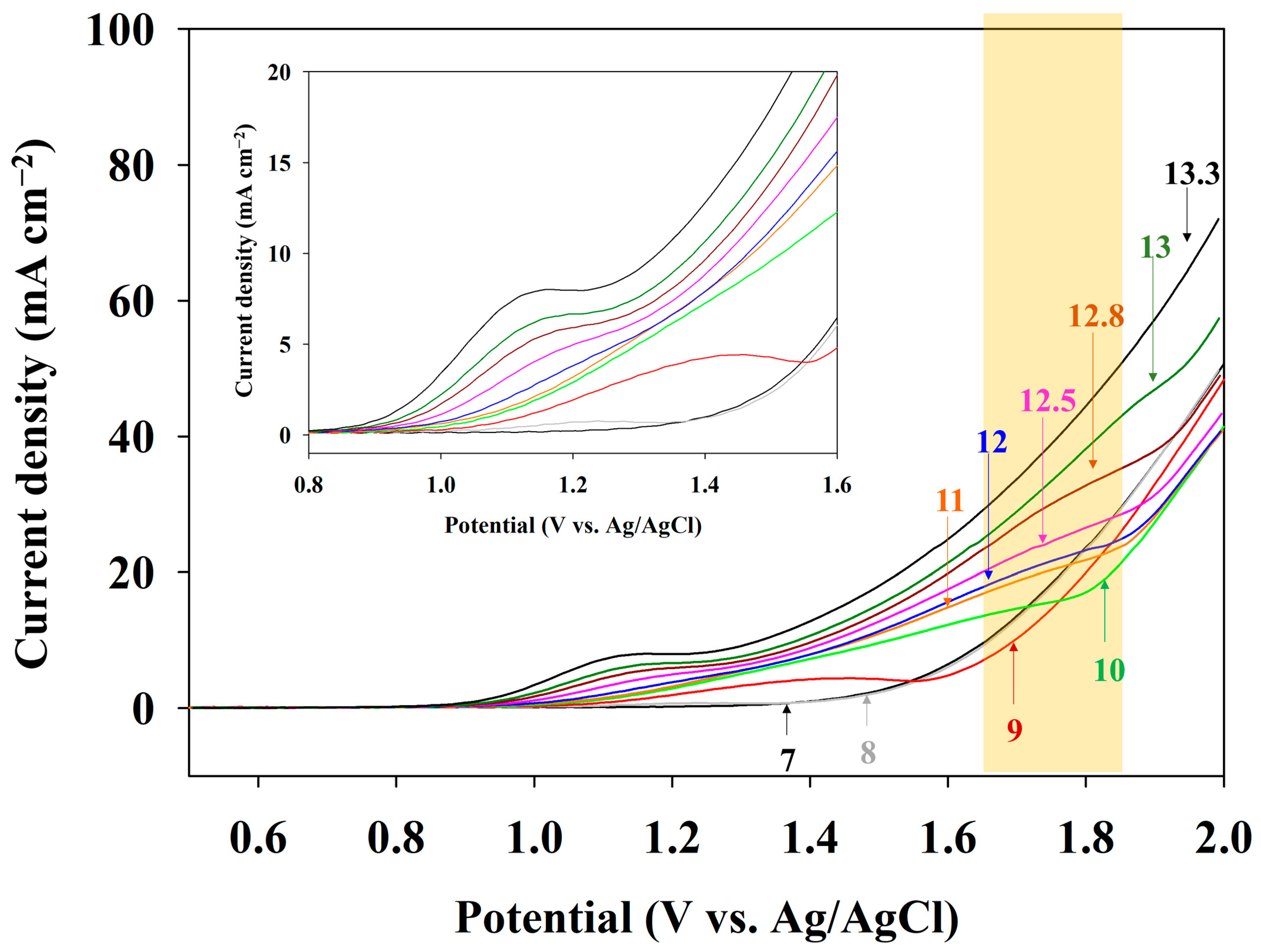
3.2. pH Condition
3.3. Effect of Operation Conditions on Energy Effectiveness
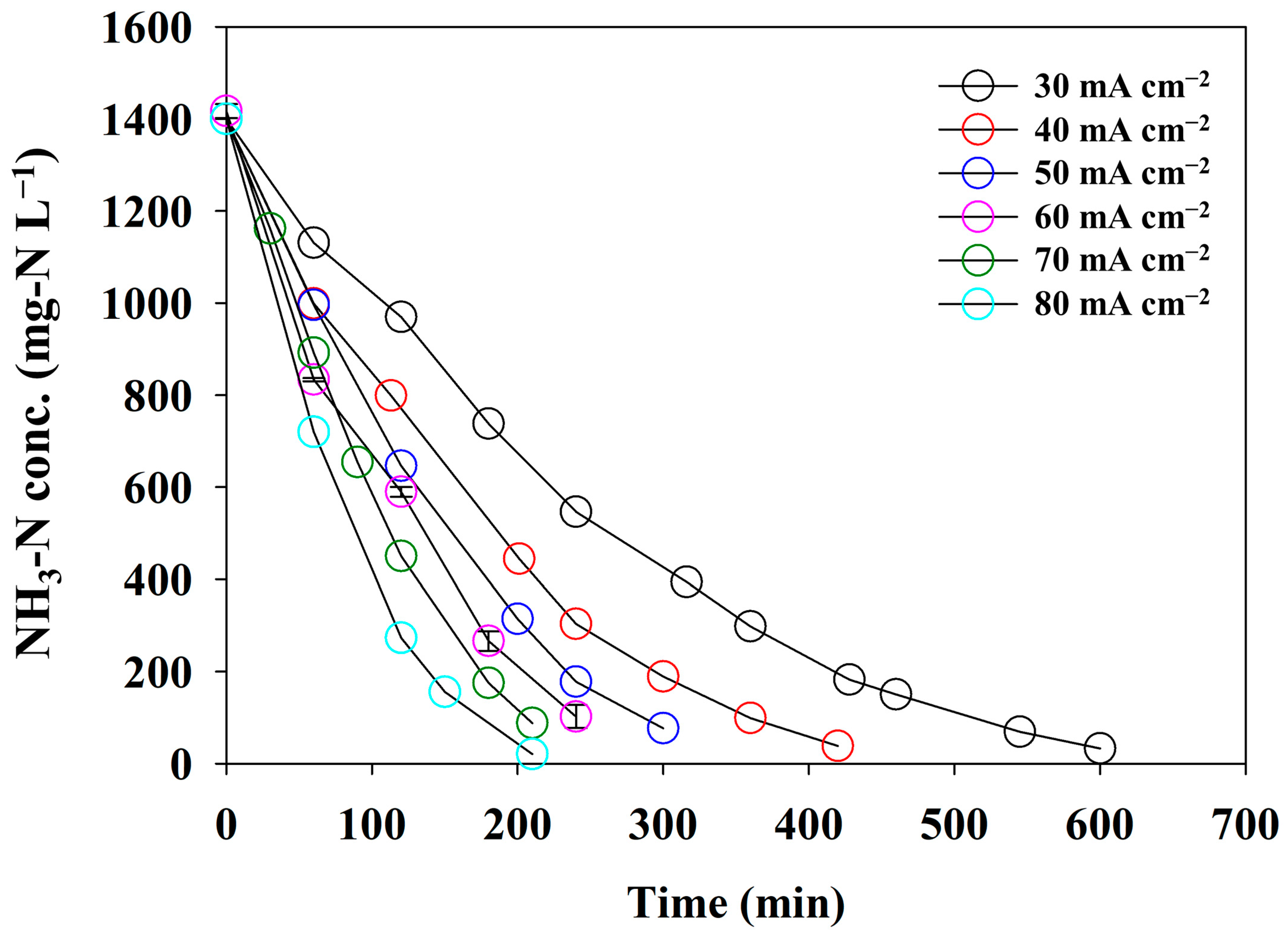
3.3.1. Effect of the Current Density
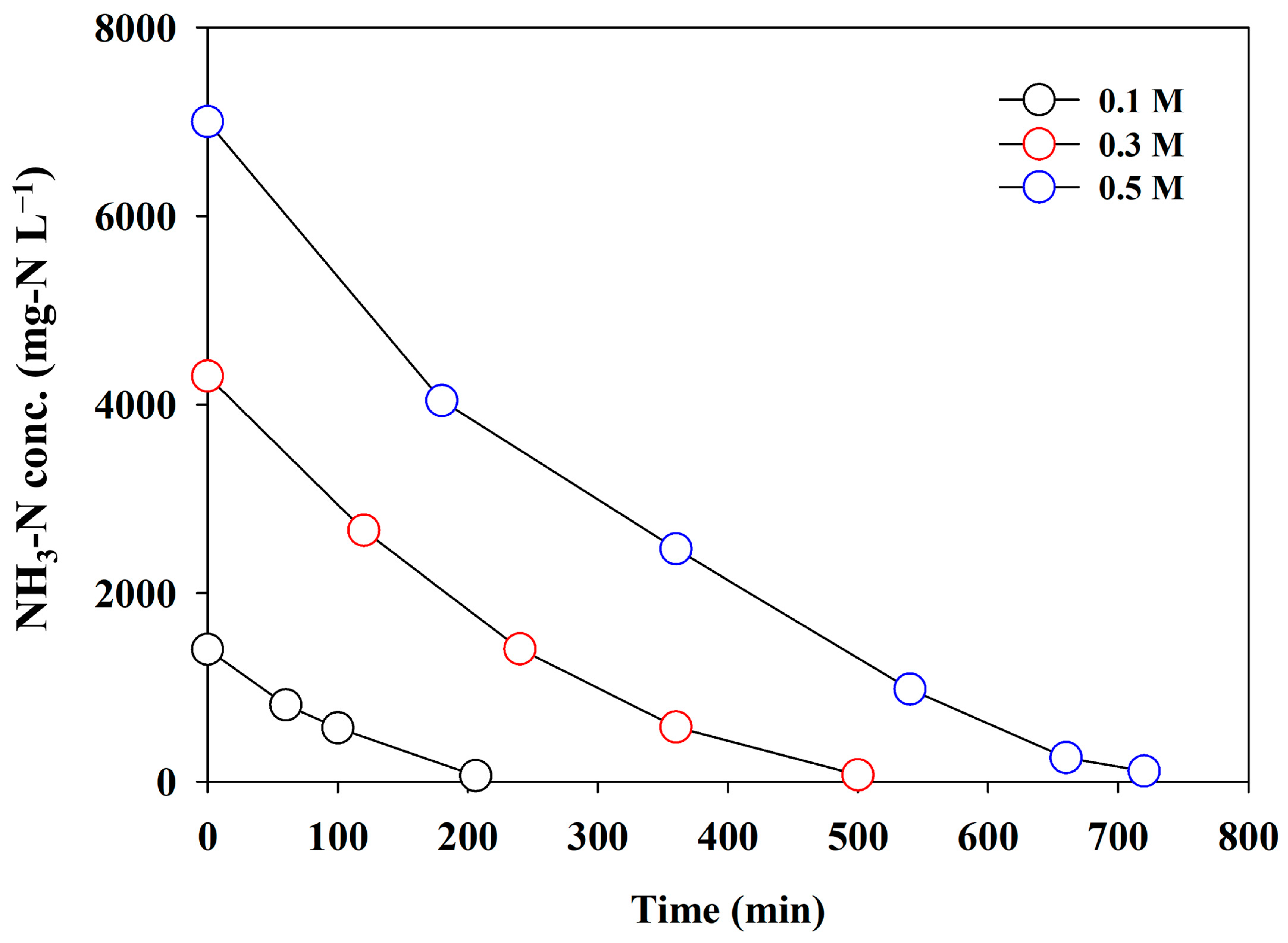
3.3.2. Effect of Initial NH3 Concentration
3.4. Additional Merit of the AmER-AOR: H2 Production
3.5. Practical Application
4. Conclusions and Future Scope
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, B.; Butterly, C.; Zhang, W.; He, J.Z.; Chen, D. Adsorbent materials for ammonium and ammonia removal: A review. J. Clean. Prod. 2021, 283, 124611. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Zilio, M.; Pigoli, A.; Rizzi, B.; Geromel, G.; Meers, E.; Schoumans, O.; Adani, F. Measuring ammonia and odours emissions during full field digestate use in agriculture. Sci. Total Environ. 2021, 782, 146882. [Google Scholar] [CrossRef] [PubMed]
- Riddick, S.N.; Dragosits, U.; Blackall, T.D.; Tomlinson, S.J.; Daunt, F.; Wanless, S.; Sutton, M.A. Global assessment of the effect of climate change on ammonia emissions from seabirds. Atmos. Environ. 2018, 184, 212–223. [Google Scholar] [CrossRef]
- Wen, Q.; Ma, M.; Hou, H.; Yu, W.; Gui, G.; Wu, Q.; Yang, J. Recirculation of reject water in deep-dewatering process to influent of wastewater treatment plant and dewaterability of sludge conditioned with Fe2+/H2O2, Fe2+/Ca(ClO)2, and Fe2+/Na2S2O8: From bench to pilot-scale study. Environ. Res. 2022, 203, 111825. [Google Scholar] [CrossRef] [PubMed]
- Naufal, M.; Wu, J.H. Chemomixoautotrophy and stress adaptation of anammox bacteria: A review. Water Res. 2024, 257, 121663. [Google Scholar] [CrossRef] [PubMed]
- El-Qelish, M.; Mahmoud, M. Overcoming organic matter limitation enables high nutrient recovery from sewage sludge reject water in a self-powered microbial nutrient recovery cell. Sci. Total Environ. 2022, 802, 149851. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Y.; Zhou, M.; Liu, J.; Li, X.; Wang, Y. Metagenomics insight into the long-term effect of ferrous ions on the mainstream anammox system. Environ. Res. 2023, 238, 117243. [Google Scholar] [CrossRef]
- Cruz, H.; Luckman, P.; Seviour, T.; Verstraete, W.; Laycock, B.; Pikaar, I. Rapid removal of ammonium from domestic wastewater using polymer hydrogels. Sci. Rep. 2018, 8, 2912. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Kang, S.; Lee, B.; Ahn, K.H.; Im, S.; Kim, B.; Kim, T.H.; Chae, S. Facile synthesis of copper-substituted Prussian blue analog immobilized ion exchange resins for high-performance ammonium recovery from wastewater: Adsorption kinetics, isotherms, and regeneration. Chem. Eng. J. 2023, 457, 141128. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, L.; Ye, H.; Zhang, Y.; Yan, L.; Li, J.; Kong, H. Removal of ammonia from source-separated urine by electrolytic oxidization using RuO2-IrO2-TiO2/Ti electrodes. Fresenius Environ. Bull. 2010, 19, 991–998. [Google Scholar]
- Zöllig, H.; Remmele, A.; Morgenroth, E.; Udert, K.M. Removal rates and energy demand of the electrochemical oxidation of ammonia and organic substances in real stored urine. Environ. Sci. Water Res. Technol. 2017, 3, 480–491. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Xia, G.; Lu, Y.; Xu, D.; Sun, N.; Chen, J. Process optimization of electrochemical oxidation of ammonia to nitrogen for actual dyeing wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 2931. [Google Scholar] [CrossRef]
- Song, J.; Yang, Y.; Jia, Y.; Wang, T.; Wei, J.; Wang, M.; Yang, B. Improved NH3-N conversion efficiency to N2 activated by BDD substrate on NiCu electrocatalysis process. Sep. Purif. Technol. 2021, 276, 119350. [Google Scholar] [CrossRef]
- Diaz, L.A.; Botte, G.G. Electrochemical deammonification of synthetic swine wastewater. Ind. Eng. Chem. Res. 2012, 51, 12167–12172. [Google Scholar] [CrossRef]
- Kuang, W.; Yan, Z.; Chen, J.; Ling, X.; Zheng, W.; Huang, W.; Feng, C. A Bipolar Membrane-Integrated Electrochlorination Process for Highly Efficient Ammonium Removal in Mature Landfill Leachate: The Importance of ClO• Generation. Environ. Sci. Technol. 2022, 53, 18538–18549. [Google Scholar] [CrossRef]
- Stolov, M.; Freger, V. Degradation of polyamide membranes exposed to chlorine: An impedance spectroscopy study. Environ. Sci. Technol. 2019, 53, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.Z.; Bérubé, P.R. Assessing the effects of sodium hypochlorite exposure on the characteristics of PVDF based membranes. Water Res. 2013, 47, 5392–5399. [Google Scholar] [CrossRef]
- Dutton, K.G.; Lipke, M.C. Correcting Frost Diagram Misconceptions Using Interactive Frost Diagrams. J. Chem. Educ. 2021, 98, 2578–2583. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J. Selective oxidation of ammonium to nitrogen gas by advanced oxidation processes: Reactive species and oxidation mechanisms. J. Environ. Chem. Eng. 2023, 11, 110263. [Google Scholar] [CrossRef]
- Wallace, S.W.; McCrum, I.T.; Janik, M.J. Ammonia electro-oxidation mechanism on the platinum (100) surface. Catal. Today 2021, 371, 50–57. [Google Scholar] [CrossRef]
- Ferrari, F.; Pijuan, M.; Molenaar, S.; Duinslaeger, N.; Sleutels, T.; Kuntke, P.; Radjenovic, J. Ammonia recovery from anaerobic digester centrate using onsite pilot scale bipolar membrane electrodialysis coupled to membrane stripping. Water Res. 2022, 218, 118504. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Juang, Y.; Hu, C.C.; Hua, L.C.; Huang, C. Tuning Cu2O morphologies of Cu2O/Ni foam electrodes for the control of reactivity and nitrogen selectivity in direct ammonia electrooxidation reaction. J. Environ. Chem. Eng. 2024, 12, 112339. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, I.T.; Park, G.I.; Lee, E.H. Electrolytic decomposition of ammonia to nitrogen in a multi-cell-stacked electrolyzer with a self-pH-adjustment function. J. Appl. Electrochem. 2006, 36, 1415–1426. [Google Scholar] [CrossRef]
- Zhang, G.; Ruan, J.; Du, T. Recent advances on photocatalytic and electrochemical oxidation for ammonia treatment from water/wastewater. ACS ES&T Eng. 2020, 1, 310–325. [Google Scholar] [CrossRef]
- Zöllig, H.; Morgenroth, E.; Udert, K.M. Inhibition of direct electrolytic ammonia oxidation due to a change in local pH. Electrochim. Acta 2015, 165, 348–355. [Google Scholar] [CrossRef]
- Katsounaros, I.; Chen, T.; Gewirth, A.A.; Markovic, N.M.; Koper, M.T. Evidence for decoupled electron and proton transfer in the electrochemical oxidation of ammonia on Pt (100). J. Phys. Chem. Lett. 2016, 7, 387–392. [Google Scholar] [CrossRef]
- Marinčić, L.; Leitz, F.B. Electro-oxidation of ammonia in wastewater. J. Appl. Electrochem. 1978, 8, 333–345. [Google Scholar] [CrossRef]
- Shinagawa, T.; Takanabe, K. Towards versatile and sustainable hydrogen production through electrocatalytic water splitting: Electrolyte engineering. ChemSusChem 2017, 10, 1318–1336. [Google Scholar] [CrossRef]
- Zhou, L.; Cheng, Y.F. Catalytic electrolysis of ammonia on platinum in alkaline solution for hydrogen generation. Int. J. Hydrogen Energy 2008, 33, 5897–5904. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, S.Y.; Youn, D.H.; Jang, Y.J. Recent advances in electrocatalysts for ammonia oxidation reaction. Catalysts 2023, 13, 803. [Google Scholar] [CrossRef]
- Nakajima, K.; Toda, H.; Sakata, K.; Nishibayashi, Y. Ruthenium-catalysed oxidative conversion of ammonia into dinitrogen. Nat. Chem. 2019, 11, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Kinetics of electro-oxidation of ammonia-N, nitrites and COD from a recirculating aquaculture saline water system using BDD anodes. Water Res. 2011, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, Y.J.; Kim, I.T.; Park, G.I.; Lee, E.H. Electrochemical conversion characteristics of ammonia to nitrogen. Water Res. 2006, 40, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cheng, Y.; Pan, H.; Kang, P. CuSn Double-Metal Hydroxides for Direct Electrochemical Ammonia Oxidation to Dinitrogen. ChemElectroChem 2022, 9, e202101301. [Google Scholar] [CrossRef]
- Zöllig, H.; Fritzsche, C.; Morgenroth, E.; Udert, K.M. Direct electrochemical oxidation of ammonia on graphite as a treatment option for stored source-separated urine. Water Res. 2015, 69, 284–294. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Molecular hydrogen production from wastewater electrolysis cell with multi-junction BiOx/TiO2 anode and stainless steel cathode: Current and energy efficiency. Appl. Catal. B 2017, 202, 671–682. [Google Scholar] [CrossRef]
- Amstutz, V.; Katsaounis, A.; Kapalka, A.; Comninellis, C.; Udert, K.M. Effects of carbonate on the electrolytic removal of ammonia and urea from urine with thermally prepared IrO2 electrodes. J. Appl. Electrochem. 2012, 42, 787–795. [Google Scholar] [CrossRef]
- Han, J.H.; Jwa, E.; Lee, H.; Kim, E.J.; Nam, J.Y.; Hwang, K.S.; Jeong, N.; Choi, J.; Kim, H.; Jeung, Y.-C.; et al. Direct seawater electrolysis via synergistic acidification by inorganic precipitation and proton flux from bipolar membrane. Chem. Eng. J. 2022, 429, 132383. [Google Scholar] [CrossRef]




| Initial Concentration (mg-N L−1) | Current Efficiency (%) | Specific Energy Demand (kWh kg-N−1) |
|---|---|---|
| 0.1 | 30.37 | 95.29 |
| 0.3 | 45.35 | 65.85 |
| 0.5 | 51.57 | 58.57 |
| Current Density (mA cm−2) | Applied Current (A) | Applied Voltage (volt) | Energy Input (W) | Heat Recovery (W) | Energy Efficiency (%) |
|---|---|---|---|---|---|
| 30 | 0.45 | 4.78 | 2.15 | 0.66 | 30.75 |
| 40 | 0.60 | 4.86 | 2.92 | 0.88 | 30.24 |
| 50 | 0.75 | 4.97 | 3.73 | 1.10 | 29.58 |
| 60 | 0.90 | 5.06 | 4.55 | 1.32 | 29.05 |
| 70 | 1.05 | 5.17 | 5.43 | 1.54 | 28.43 |
| 80 | 1.20 | 5.25 | 6.30 | 1.76 | 28.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-H.; Oh, G.-G.; Lee, B.-J.; Im, S.; Kim, W.; Kang, S.; Han, J.-H. Direct Electrooxidation of Ammonia-Enriched Wastewater Using a Bipolar Membrane-Integrated Electrolytic Cell. Water 2024, 16, 1599. https://doi.org/10.3390/w16111599
Kang J-H, Oh G-G, Lee B-J, Im S, Kim W, Kang S, Han J-H. Direct Electrooxidation of Ammonia-Enriched Wastewater Using a Bipolar Membrane-Integrated Electrolytic Cell. Water. 2024; 16(11):1599. https://doi.org/10.3390/w16111599
Chicago/Turabian StyleKang, Jeong-Hee, Gyung-Geun Oh, Bong-Jae Lee, Seongwon Im, Weonjae Kim, Sungwon Kang, and Ji-Hyung Han. 2024. "Direct Electrooxidation of Ammonia-Enriched Wastewater Using a Bipolar Membrane-Integrated Electrolytic Cell" Water 16, no. 11: 1599. https://doi.org/10.3390/w16111599
APA StyleKang, J.-H., Oh, G.-G., Lee, B.-J., Im, S., Kim, W., Kang, S., & Han, J.-H. (2024). Direct Electrooxidation of Ammonia-Enriched Wastewater Using a Bipolar Membrane-Integrated Electrolytic Cell. Water, 16(11), 1599. https://doi.org/10.3390/w16111599






