Critical Review on Two-Stage Anaerobic Digestion with H2 and CH4 Production from Various Wastes
Abstract
1. Introduction
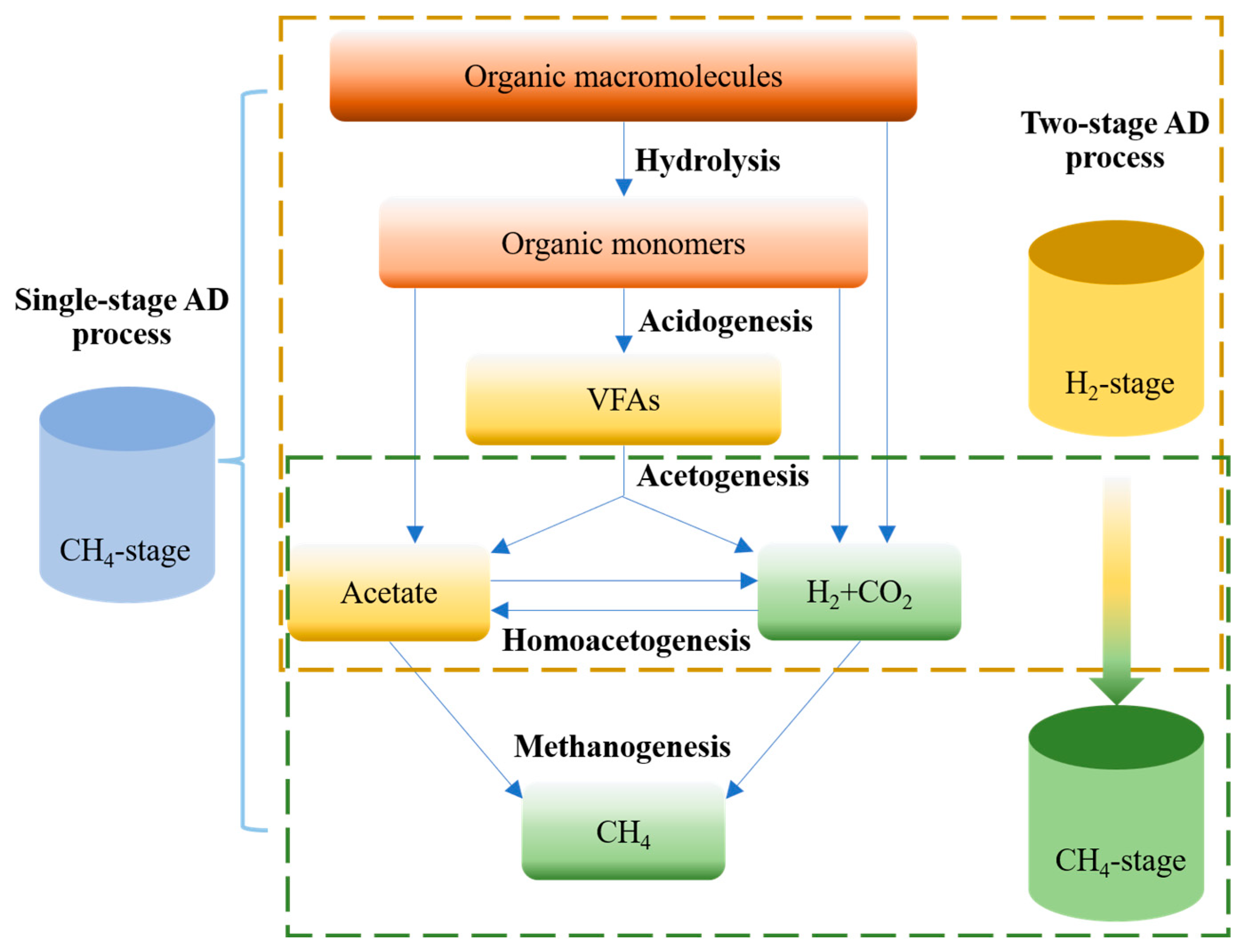
2. Comparison of Single- and Two-Stage AD Processes
- Hydrolysis, in which organic macromolecules, such as carbohydrates, proteins, lipids, and other large polymers, are degraded into small monomers by hydrolytic and fermentative bacterial consortia;
- Acidogenesis, in which the monomers are further degraded into various metabolic products, mainly consisting of volatile fatty acids (VFAs), alcohols, lactate, NH4+, H2, and CO2 by acidogenic bacteria;
- Acetogenesis, in which products from acidogenesis are converted to H2, CO2, and acetate by acetogenic bacteria;
- Methanogenesis, in which CH4 is produced from CO2, acetate, or methyl compounds by methanogens.
2.1. Biogas Production
2.2. Degradation of Organics
2.3. Energy Recovery
2.4. Operation Stability
3. Influence Factors of the Two-Stage AD Process
3.1. Substrate
3.1.1. Sole Substrates
3.1.2. Co-Substrates
3.2. Inoculum
3.2.1. Inoculum of the H2 Stage
3.2.2. Inoculum of the CH4 Stage
3.3. pH
3.4. Temperature
3.5. OLR
3.6. HRT
3.7. Nutrients
3.8. Inhibitors
4. Upgrading Technology
4.1. Type of Reactors
4.2. Integrating Technology
4.3. Digestate Recirculation
4.4. Biogas Recirculation
5. Microbial Communities
5.1. Bacterial Communities
5.2. Archaeal Communities
5.3. Influence Factors on Microbial System
6. Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, T.G.; Bian, S.W.; Ko, J.H.; Wu, H.N.; Xu, Q.Y. Enhancement of hydrogen production using untreated inoculum in two-stage food waste digestion. Bioresour. Technol. 2019, 282, 189–196. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane production in two-stage anaerobic digestion system. Int. J. Hydrogen Energy 2019, 44, 17363–17380. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Murphy, J.D. Innovation in biological production and upgrading of methane and hydrogen for use as gaseous transport biofuel. Biotechnol. Adv. 2016, 34, 451–472. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent developments in biohythane production from household food wastes: A review. Bioresour. Technol. 2018, 257, 311–319. [Google Scholar] [CrossRef]
- Sen, B.; Aravind, J.; Kanmani, P.; Lay, C.H. State of the art and future concept of food waste fermentation to bioenergy. Renew. Sustain. Energy Rev. 2016, 53, 547–557. [Google Scholar] [CrossRef]
- Liu, Z.D.; Si, B.C.; Li, J.M.; He, J.W.; Zhang, C.; Lu, Y.; Zhang, Y.H.; Xing, X.H. Bioprocess engineering for biohythane production from low-grade waste biomass: Technical challenges towards scale up. Curr. Opin. Biotechnol. 2018, 50, 25–31. [Google Scholar] [CrossRef]
- Lavagnolo, M.C.; Girotto, F.; Rafieenia, R.; Danieli, L.; Alibardi, L. Two-stage anaerobic digestion of the organic fraction of municipal solid waste—Effects of process conditions during batch tests. Renew. Energy 2018, 126, 14–20. [Google Scholar] [CrossRef]
- Odedina, M.J.; Charnnok, B.; Saritpongteeraka, K.; Chaiprapat, S. Effects of size and thermophilic pre-hydrolysis of banana peel during anaerobic digestion, and biomethanation potential of key tropical fruit wastes. Waste Manag. 2017, 68, 128–138. [Google Scholar] [CrossRef]
- Si, B.C.; Liu, Z.D.; Zhang, Y.H.; Li, J.M.; Shen, R.X.; Zhu, Z.B.; Xing, X.H. Towards biohythane production from biomass: Influence of operational stage on anaerobic fermentation and microbial community. Int. J. Hydrogen Energy 2016, 41, 4429–4438. [Google Scholar] [CrossRef]
- Vaez, E.; Zilouei, H. Towards the development of biofuel production from paper mill effluent. Renew. Energy 2020, 146, 1408–1415. [Google Scholar] [CrossRef]
- Mamimin, C.; Singkhala, A.; Kongjan, P.; Suraraksa, B.; Prasertsan, P.; Imai, T.; O-Thong, S. Two-stage thermophilic fermentation and mesophilic methanogen process for biohythane production from palm oil mill effluent. Int. J. Hydrogen Energy 2015, 40, 6319–6328. [Google Scholar] [CrossRef]
- Fernández, C.; Cuetos, M.J.; Martínez, E.J.; Gómez, X. Thermophilic anaerobic digestion of cheese whey: Coupling H2 and CH4 production. Biomass Bioenergy 2015, 81, 55–62. [Google Scholar] [CrossRef]
- Weide, T.; Brügging, E.; Wetter, C.; Ierardi, A.; Wichern, M. Use of organic waste for biohydrogen production and volatile fatty acids via dark fermentation and further processing to methane. Int. J. Hydrogen Energy 2019, 44, 24110–24125. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, L.K.; Lin, R.C.; Liu, M.; Zhou, J.H.; Cen, K.F. Physicochemical characterization of typical municipal solid wastes for fermentative hydrogen and methane co-production. Energy Convers. Manag. 2016, 117, 297–304. [Google Scholar] [CrossRef]
- Wan, J.J.; Jing, Y.H.; Rao, Y.; Zhang, S.C.; Luo, G. Thermophilic Alkaline Fermentation Followed by Mesophilic Anaerobic Digestion for Efficient Hydrogen and Methane Production from Waste-Activated Sludge: Dynamics of Bacterial Pathogens as Revealed by the Combination of Metagenomic and Quantitative PCR Analyses. Appl. Environ. Microbiol. 2018, 84, 14. [Google Scholar]
- Baldi, F.; Pecorini, I.; Lannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Bolzonella, D.; Micolucci, F.; Battista, F.; Cavinato, C.; Gottardo, M.; Piovesan, S.; Pavan, P. Producing Biohythane from Urban Organic Wastes. Waste Biomass Valorization 2020, 11, 2367–2374. [Google Scholar] [CrossRef]
- Tena, M.; Perez, M.; Solera, R. Benefits in the valorization of sewage sludge and wine vinasse via a two-stage acidogenic-thermophilic and methanogenic-mesophilic system based on the circular economy concept. Fuel 2021, 296, 120654. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, R.Y.; Ji, M. Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge. Energies 2019, 12, 2748. [Google Scholar] [CrossRef]
- Kim, M.; Liu, C.G.; Noh, J.W.; Yang, Y.N.; Oh, S.; Shimizu, K.; Lee, D.Y.; Zhang, Z.Y. Hydrogen and methane production from untreated rice straw and raw sewage sludge under thermophilic anaerobic conditions. Int. J. Hydrogen Energy 2013, 38, 8648–8656. [Google Scholar] [CrossRef]
- Farhat, A.; Miladi, B.; Hamdi, M.; Bouallagui, H. Fermentative hydrogen and methane co-production from anaerobic co-digestion of organic wastes at high loading rate coupling continuously and sequencing batch digesters. Environ. Sci. Pollut. Res. 2018, 25, 27945–27958. [Google Scholar] [CrossRef]
- Voelklein, M.A.; Jacob, A.; Shea, R.O.; Murphy, J.D. Assessment of increasing loading rate on two-stage digestion of food waste. Bioresour. Technol. 2016, 202, 172–180. [Google Scholar] [CrossRef]
- Das, D.; Roy, S. Biohythane: Fuel for the Future; Jenny Stanford Publishing: Singapore, 2016. [Google Scholar]
- Rózsenberszki, T.; Koók, L.; Bakonyi, P.; Nemestóthy, N.; Logroño, W.; Pérez, M.; Urquizo, G.; Recalde, C.; Kurdi, R.; Sarkady, A. Municipal waste liquor treatment via bioelectrochemical and fermentation (H2 + CH4) processes: Assessment of various technological sequences. Chemosphere 2017, 171, 692–701. [Google Scholar] [CrossRef]
- Batista, L.P.P.; Paulinetti, A.P.; Ferraz, A.D.N.; Albanez, R.; Ratusznei, S.M.; Etchebehere, C.; Lovato, G.; Rodrigues, J.A.D. Two-stage thermophilic anaerobic digestion of cheese whey: Process optimization, comparison with single-stage, and full-scale estimation. Chem. Eng. Process. Process Intensif. 2023, 183, 109260. [Google Scholar] [CrossRef]
- Lateef, S.A.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Influence of cefazolin contamination on performance of two-stage and single stage anaerobic batch digesters. Energy Sustain. Dev. 2018, 44, 117–124. [Google Scholar] [CrossRef]
- Rafieenia, R.; Girotto, F.; Peng, W.; Cossu, R.; Pivato, A.; Raga, R.; Lavagnolo, M.C. Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manag. 2017, 59, 194–199. [Google Scholar] [CrossRef]
- Yue, L.C.; Cheng, J.; Hua, J.J.; Dong, H.Q.; Zhou, J.H.; Li, Y.Y. Improving fermentative methane production of glycerol trioleate and food waste pretreated with ozone through two-stage dark hydrogen fermentation and anaerobic digestion. Energy Convers. Manag. 2020, 203, 13. [Google Scholar] [CrossRef]
- O-Thong, S.; Suksong, W.; Promnuan, K.; Thipmunee, M.; Mamimin, C.; Prasertsan, P. Two-stage thermophilic fermentation and mesophilic methanogenic process for biohythane production from palm oil mill effluent with methanogenic effluent recirculation for pH control. Int. J. Hydrogen Energy 2016, 41, 21702–21712. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Ghrayeb, O.A. Role of organic loading rate in bioenergy generation from palm oil mill effluent in a two-stage up-flow anaerobic sludge blanket continuous-stirred tank reactor. J. Clean. Prod. 2017, 142, 3044–3049. [Google Scholar] [CrossRef]
- Mamimin, C.; Probst, M.; Gómez-Brandón, M.; Podmirseg, S.M.; Insam, H.; Reungsang, A.; O-Thong, S. Trace metals supplementation enhanced microbiota and biohythane production by two-stage thermophilic fermentation. Int. J. Hydrogen Energy 2019, 44, 3325–3338. [Google Scholar] [CrossRef]
- Salem, A.H.; Mietzel, T.; Brunstermann, R.; Widmann, R. Two-stage anaerobic fermentation process for bio-hydrogen and bio-methane production from pre-treated organic wastes. Bioresour. Technol. 2018, 265, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Balachandar, G.; Das, D. Improvement in biohythane production using organic solid waste and distillery effluent. Waste Manag. 2017, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mamimin, C.; Kongjan, P.; O-Thong, S.; Prasertsan, P. Enhancement of biohythane production from solid waste by co-digestion with palm oil mill effluent in two-stage thermophilic fermentation. Int. J. Hydrogen Energy 2019, 44, 17224–17237. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Kopsahelis, A.; Zagklis, D.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew. Energy 2020, 147, 610–619. [Google Scholar]
- Intanoo, P.; Rangsunvigit, P.; Namprohm, W.; Thamprajamchit, B.; Chavadej, J.; Chavadej, S. Hydrogen production from alcohol wastewater by an anaerobic sequencing batch reactor under thermophilic operation: Nitrogen and phosphorous uptakes and transformation. Int. J. Hydrogen Energy 2012, 37, 11104–11112. [Google Scholar] [CrossRef]
- Liu, D.; Li, R.Y.; Ji, M.; Cai, Y.M. Enhanced Hydrogen and Methane Production from Sewage Sludge by Addition of Cornstalk in Two-Stage Fermentation Process. Asian J. Chem. 2013, 25, 6535–6539. [Google Scholar] [CrossRef]
- Jehlee, A.; Rodjaroen, S.; Waewsak, J.; Reungsang, A.; O-Thong, S. Improvement of biohythane production from Chlorella sp. TISTR 8411 biomass by co-digestion with organic wastes in a two-stage fermentation. Int. J. Hydrogen Energy 2019, 44, 17238–17247. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, L.K.; Lin, R.C.; Yue, L.C.; Liu, J.Z.; Zhou, J.H.; Cen, K.F. Fermentative biohydrogen and biomethane co-production from mixture of food waste and sewage sludge: Effects of physiochemical properties and mix ratios on fermentation performance. Appl. Energy 2016, 184, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Wu, S.B.; Wang, D.H. Hydrogen-methane production from pulp & paper sludge and food waste by mesophilic-thermophilic anaerobic co-digestion. Int. J. Hydrogen Energy 2013, 38, 15055–15062. [Google Scholar]
- Paudel, S.; Kang, Y.; Yoo, Y.S.; Seo, G.T. Effect of volumetric organic loading rate (OLR) on H2 and CH4 production by two-stage anaerobic co-digestion of food waste and brown water. Waste Manag. 2017, 61, 484–493. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. Biogas yield using single and two stage anaerobic digestion: An experimental approach. Energy Sustain. Dev. 2023, 74, 6–19. [Google Scholar] [CrossRef]
- Yun, Y.M.; Sung, S.; Choi, J.S.; Kim, D.H. Two-stage co-fermentation of lipid-extracted microalgae waste with food waste leachate: A viable way to reduce the inhibitory effect of leftover organic solvent and recover additional energy. Int. J. Hydrogen Energy 2016, 41, 21721–21727. [Google Scholar] [CrossRef]
- Chen, Y.G.; Liu, H.; Zheng, X.; Wang, X.; Wu, J. New method for enhancement of bioenergy production from municipal organic wastes via regulation of anaerobic fermentation process. Appl. Energy 2017, 196, 190–198. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Wu, J.; Xiao, B.Y.; Hojo, T.; Kubota, K.; Cheng, J.; Li, Y.Y. Co-production of biohydrogen and biomethane from food waste and paper waste via recirculated two-phase anaerobic digestion process: Bioenergy yields and metabolic distribution. Bioresour. Technol. 2019, 276, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.G.; Parker, W.; Conidi, D.; Basnar, R.; Seto, P. Eliminating methanogenic activity in hydrogen reactor to improve biogas production in a two-stage anaerobic digestion process co-digesting municipal food waste and sewage sludge. Bioresour. Technol. 2011, 102, 7086–7092. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Q.; Liang, J.J.; Wu, S.B.; Wang, B.H. Was pretreatment beneficial for more biogas in any process? Chemical pretreatment effect on hydrogen-methane co-production in a two-stage process. J. Ind. Eng. Chem. 2013, 19, 316–321. [Google Scholar] [CrossRef]
- Shah, A.T.; Favaro, L.; Alibardi, L.; Cagnin, L.; Sandon, A.; Cossu, R.; Casella, S.; Basaglia, M. Bacillus sp. strains to produce bio-hydrogen from the organic fraction of municipal solid waste. Appl. Energy 2016, 176, 116–124. [Google Scholar] [CrossRef]
- Nualsri, C.; Reungsang, A.; Plangklang, P. Biochemical hydrogen and methane potential of sugarcane syrup using a two-stage anaerobic fermentation process. Ind. Crops Prod. 2016, 82, 88–99. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Wirasembada, Y.C.; Shin, B.; Shin, J.; Kurniawan, A.; Cho, J. Effects of sudden shock load on simultaneous biohythane production in two-stage anerobic digestion of high-strength organic wastewater. Bioresour. Technol. 2024, 394, 130186. [Google Scholar] [CrossRef]
- Rafieenia, R.; Pivato, A.; Lavagnolo, M.C. Effect of inoculum pre-treatment on mesophilic hydrogen and methane production from food waste using two-stage anaerobic digestion. Int. J. Hydrogen Energy 2018, 43, 12013–12022. [Google Scholar] [CrossRef]
- Liu, Z.D.; Li, Q.; Zhang, C.; Wang, L.J.; Han, B.; Li, B.M.; Zhang, Y.H.; Chen, H.Z.; Xing, X.H. Effects of operating parameters on hydrogen production from raw wet steam-exploded cornstalk and two-stage fermentation potential for biohythane production. Biochem. Eng. J. 2014, 90, 234–238. [Google Scholar] [CrossRef]
- Wang, S.J.; Ma, Z.H.; Su, H.J. Two-step continuous hydrogen production by immobilized mixed culture on corn stalk. Renew. Energy 2018, 121, 230–235. [Google Scholar] [CrossRef]
- Ta, D.T.; Lin, C.Y.; Chu, C.Y.; Ta, T.M.N. Performance characteristics of single-stage biohythane production by immobilized anaerobic bacteria. Energetika 2018, 64, 2. [Google Scholar] [CrossRef]
- Lay, C.H.; Vo, T.P.; Lin, P.Y.; Abdul, P.M.; Liu, C.M.; Lin, C.Y. Anaerobic hydrogen and methane production from low-strength beverage wastewater. Int. J. Hydrogen Energy 2019, 44, 14351–14361. [Google Scholar] [CrossRef]
- Lindner, J.; Zielonka, S.; Oechsner, H.; Lemmer, A. Effect of different pH-values on process parameters in two-phase anaerobic digestion of high-solid substrates. Environ. Technol. 2015, 36, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.C.B.; Schlienz, M.; Greger, M. Production of bio-hydrogen and methane during semi-continuous digestion of maize silage in a two-stage system. Int. J. Hydrogen Energy 2017, 42, 5768–5779. [Google Scholar] [CrossRef]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Notodarmojo, P.A.; Fujiwara, T.; Habuer; Van, D.P. Effectiveness of oyster shell as alkali additive for two-stage anaerobic co-digestion: Carbon flow analysis. Energy 2022, 239, 122177. [Google Scholar]
- Thungklin, P.; Sittijunda, S.; Reungsang, A. Sequential fermentation of hydrogen and methane from steam-exploded sugarcane bagasse hydrolysate. Int. J. Hydrogen Energy 2018, 43, 9924–9934. [Google Scholar] [CrossRef]
- Xu, R.Z.; Fang, S.Y.; Zhang, L.; Huang, W.X.; Shao, Q.Q.; Fang, F.; Feng, Q.; Cao, J.S.; Luo, J.Y. Distribution patterns of functional microbial community in anaerobic digesters under different operational circumstances: A review. Bioresour. Technol. 2021, 341, 125823. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Ríos-Leal, E.; Ponce-Noyola, M.T.; Poggi-Varaldo, H.M. Gas biofuels from solid substrate hydrogenogenic-methanogenic fermentation of the organic fraction of municipal solid waste. Process Biochem. 2012, 47, 1572–1587. [Google Scholar] [CrossRef]
- Zainal, B.S.; Danaee, M.; Mohd, N.S.; Ibrahim, S. Effects of temperature and dark fermentation effluent on biomethane production in a two-stage up-flow anaerobic sludge fixed-film (UASFF) bioreactor. Fuel 2020, 263, 116729. [Google Scholar] [CrossRef]
- Li, Q.Y.; Li, Y.F. Coproduction of hydrogen and methane in a CSTR-IC two-stage anaerobic digestion system from molasses wastewater. Water Sci. Technol. 2019, 79, 270–277. [Google Scholar] [CrossRef]
- Yeshanew, M.M.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Production of biohythane from food waste via an integrated system of continuously stirred tank and anaerobic fixed bed reactors. Bioresour. Technol. 2016, 220, 312–322. [Google Scholar] [CrossRef]
- Nualsri, C.; Kongjan, P.; Reungsang, A. Direct integration of CSTR-UASB reactors for two-stage hydrogen and methane production from sugarcane syrup. Int. J. Hydrogen Energy 2016, 41, 17884–17895. [Google Scholar] [CrossRef]
- Guneratnam, A.J.; Xia, A.; Murphy, J.D. Comparative study of single- and two-stage fermentation of the brown seaweed Laminaria digitata. Energy Convers. Manag. 2017, 148, 405–412. [Google Scholar] [CrossRef]
- Sun, C.H.; Xia, A.; Fu, Q.; Huang, Y.; Lin, R.C.; Murphy, J.D. Effects of pre-treatment and biological acidification on fermentative hydrogen and methane co-production. Energy Convers. Manag. 2019, 185, 431–441. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Biohythane production from sugarcane bagasse and water hyacinth: A way towards promising green energy production. J. Clean. Prod. 2019, 207, 689–701. [Google Scholar] [CrossRef]
- Guo, Y.C.; Dai, Y.; Bai, Y.X.; Li, Y.H.; Fan, Y.T.; Hou, H.W. Co-producing hydrogen and methane from higher-concentration of corn stalk by combining hydrogen fermentation and anaerobic digestion. Int. J. Hydrogen Energy 2014, 39, 14204–14211. [Google Scholar] [CrossRef]
- Reungsang, A.; Sittijunda, S.; Sreela, C. Methane production from acidic effluent discharged after the hydrogen fermentation of sugarcane juice using batch fermentation and UASB reactor. Renew. Energy 2016, 86, 1224–1231. [Google Scholar] [CrossRef]
- Macêdo, W.V.; Oliveira, G.H.D.; Zaiat, M. Tetrabromobisphenol A (TBBPA) anaerobic biodegradation occurs during acidogenesis. Chemosphere 2021, 282, 130995. [Google Scholar] [CrossRef]
- Forouzanmehr, F.; Solon, K.; Maisonnave, V.; Daniel, O.; Volcke, E.I.P.; Gillot, S.; Buffiere, P. Sulfur transformations during two-stage anaerobic digestion and intermediate thermal hydrolysis. Sci. Total Environ. 2022, 810, 151247. [Google Scholar] [CrossRef]
- Promnuan, K.; Higuchi, T.; Imai, T.; Kongjan, P.; Reungsang, A.; O-Thong, S. Simultaneous biohythane production and sulfate removal from rubber sheet wastewater by two-stage anaerobic digestion. Int. J. Hydrogen Energy 2020, 45, 263–274. [Google Scholar] [CrossRef]
- Yang, S.M.; Luo, F.; Yan, J.; Zhang, T.L.; Xian, Z.Y.; Huang, W.Y.; Zhang, H.G.; Cao, Y.J.; Huang, L. Biogas production of food waste with in-situ sulfide control under high organic loading in two-stage anaerobic digestion process: Strategy and response of microbial community. Bioresour. Technol. 2023, 373, 128712. [Google Scholar] [CrossRef]
- Sani, K.; Jariyaboon, R.; O-Thong, S.; Cheirsilp, B.; Kaparaju, P.; Wang, Y.; Kongjan, P. Performance of pilot scale two-stage anaerobic co-digestion of waste activated sludge and greasy sludge under uncontrolled mesophilic temperature. Water Res. 2022, 221, 118736. [Google Scholar] [CrossRef]
- Seengenyoung, J.; Mamimin, C.; Prasertsan, P.; O-Thong, S. Pilot-scale of biohythane production from palm oil mill effluent by two-stage thermophilic anaerobic fermentation. Int. J. Hydrogen Energy 2019, 44, 3347–3355. [Google Scholar] [CrossRef]
- Shah, T.A.; Ali, S.; Afzal, A.; Tabassum, R. Simultaneous Pretreatment and Biohydrogen Production from Wheat Straw by Newly Isolated Ligninolytic Bacillus Sp Strains with Two-Stage Batch Fermentation System. Bioenergy Res. 2018, 11, 835–849. [Google Scholar] [CrossRef]
- Jung, K.W.; Moon, C.; Cho, S.K.; Kim, S.H.; Shin, H.S.; Kim, D.H. Conversion of organic solid waste to hydrogen and methane by two-stage fermentation system with reuse of methane fermenter effluent as diluting water in hydrogen fermentation. Bioresour. Technol. 2013, 139, 120–127. [Google Scholar] [CrossRef]
- Lin, R.C.; Cheng, J.; Yang, Z.B.; Ding, L.K.; Zhang, J.B.; Zhou, J.H.; Cen, K.F. Enhanced energy recovery from cassava ethanol wastewater through sequential dark hydrogen, photo hydrogen and methane fermentation combined with ammonium removal. Bioresour. Technol. 2016, 214, 686–691. [Google Scholar] [CrossRef]
- Yun, J.; Lee, Y.Y.; Choi, H.; Cho, K.S. Process contribution evaluation for COD removal and energy production from molasses wastewater in a BioH2-BioCH4-MFC-integrated system. Bioprocess Biosyst. Eng. 2017, 40, 55–62. [Google Scholar] [CrossRef]
- Schievano, A.; Sciarria, T.P.; Gao, Y.C.; Scaglia, B.; Salati, S.; Zanardo, M.; Quiao, W.; Dong, R.J.; Adani, F. Dark fermentation, anaerobic digestion and microbial fuel cells: An integrated system to valorize swine manure and rice bran. Waste Manag. 2016, 56, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, J.L.; Kumari, S.; Das, D. Improvement of energy recovery from water hyacinth by using integrated system. Int. J. Hydrogen Energy 2018, 43, 1303–1318. [Google Scholar] [CrossRef]
- Clark, J.H. Green biorefinery technologies based on waste biomass. Green Chem. 2019, 21, 1168–1170. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. An efficient multiphase bioprocess for enhancing the renewable energy production from almond shells. Energy Convers. Manag. 2020, 203, 112235. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Ricci, M.; Bianchi, D.; Wandera, S.M.; Adani, F.; Dong, R.J. Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renew. Energy 2019, 130, 1108–1115. [Google Scholar] [CrossRef]
- Kobayashi, T.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Effect of sludge recirculation on characteristics of hydrogen production in a two-stage hydrogen-methane fermentation process treating food wastes. Int. J. Hydrogen Energy 2012, 37, 5602–5611. [Google Scholar] [CrossRef]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Shah, A.A.; Adnan, F.; Ijaz, U.Z.; Ahmed, S.; Badshah, M. Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, O.; Dinsdale, R.; Hawkes, F.R.; Hawkes, D.L.; Noike, T. Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresour. Technol. 2000, 73, 59–65. [Google Scholar] [CrossRef]
- Liu, D.W.; Liu, D.P.; Zeng, R.J.; Angelidaki, I. Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res. 2006, 40, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Nualsri, C.; Kongjan, P.; Reungsang, A.; Imai, T. Effect of biogas sparging on the performance of bio-hydrogen reactor over a long-term operation. PLoS ONE 2017, 12, 13. [Google Scholar] [CrossRef]
- Wahid, R.; Horn, S.J. The effect of mixing rate and gas recirculation on biological CO2 methanation in two-stage CSTR systems. Biomass Bioenergy 2021, 144, 105918. [Google Scholar] [CrossRef]
- Salomoni, C.; Caputo, A.; Bonoli, M.; Francioso, O.; Rodriguez-Estrada, M.T.; Palenzona, D. Enhanced methane production in a two-phase anaerobic digestion plant, after CO2 capture and addition to organic wastes. Bioresour. Technol. 2011, 102, 6443–6448. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.H.; Selvam, A.; Wong, J.W.C. Innovative method for increased methane recovery from two-phase anaerobic digestion of food waste through reutilization of acidogenic off-gas in methanogenic reactor. Bioresour. Technol. 2016, 217, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Alkasrawi, M. Process enhancement of hydrogen and methane production from palm oil mill effluent using two-stage thermophilic and mesophilic fermentation. Int. J. Hydrogen Energy 2016, 41, 12888–12898. [Google Scholar] [CrossRef]
- Ma, S.J.; Ma, H.J.; Hu, H.D.; Ren, H.Q. Effect of mixing intensity on hydrolysis and acidification of sewage sludge in two-stage anaerobic digestion: Characteristics of dissolved organic matter and the key microorganisms. Water Res. 2019, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Fitamo, T.; Treu, L.; Boldrin, A.; Sartori, C.; Angelidaki, I.; Scheutz, C. Microbial population dynamics in urban organic waste anaerobic co-digestion with mixed sludge during a change in feedstock composition and different hydraulic retention times. Water Res. 2017, 118, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Mand, T.D.; Metcalf, W.W. Energy Conservation and Hydrogenase Function in Methanogenic Archaea, in Particular the Genus Methanosarcina. Microbiol. Mol. Biol. Rev. 2019, 83, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.F.; Zhao, L.; Li, C.X.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.J.; Zhu, G.F. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Jehlee, A.; Khongkliang, P.; Suksong, W.; Rodjaroen, S.; Waewsak, J.; Reungsang, A.; O-Thong, S. Biohythane production from Chlorella sp. biomass by two-stage thermophilic solid-state anaerobic digestion. Int. J. Hydrogen Energy 2017, 42, 27792–27800. [Google Scholar] [CrossRef]
- Braga, J.K.; Motteran, F.; Sakamoto, I.K.; Varesche, M.B.A. Bacterial and archaeal community structure involved in biofuels production using hydrothermal- and enzymatic-pretreated sugarcane bagasse for an improvement in hydrogen and methane production. Sustain. Energy Fuels 2018, 2, 2644–2660. [Google Scholar] [CrossRef]
- Gaby, J.C.; Zamanzadeh, M.; Horn, S.J. The effect of temperature and retention time on methane production and microbial community composition in staged anaerobic digesters fed with food waste. Biotechnol. Biofuels 2017, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kong, Z.; Qin, Y.; Wu, J.; Zhu, A.J.; Xiao, B.Y.; Ni, J.L.; Kubota, K.; Li, Y.Y. Temperature-phased anaerobic co-digestion of food waste and paper waste with and without recirculation: Biogas production and microbial structure. Sci. Total Environ. 2020, 724, 138168. [Google Scholar] [CrossRef] [PubMed]


| (a) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Single-Stage Process | Two-Stage System | Reference | ||||||||
| H2 Stage | CH4 Stage | The System | |||||||||
| CH4 Yield (mL/g-CODadded) | Reactor Type and Key Variables | COD Removal (%) | Energy Recovery (as Biogas, kJ/g-CODadded) | H2 Yield (mL/g-CODadded) | Reactor Type and Key Variables | CH4 Yield (mL/g-CODadded) | Reactor Type and Key Variables | COD Removal (%) | Energy Recovery (as Biogas, kJ/g-CODadded) | ||
| Synthetic wastewater | 227.43 | Packed bed reactor (PBR); 37 °C; HRT = 12 h | 74.0 | 8.15 | 61.81 | PBR; 37 °C; HRT = 12 h | 263.58 | PBR; 37 °C; HRT = 12 h | 97.3 | 10.09 | [9] |
| Synthetic wastewater | 275.25 | Upflow anaerobic sludge blanket (UASB); 37 °C; HRT = 12 h | 95.4 | 9.85 | 157.45 | UASB; 37 °C; HRT = 12 h | 234.43 | UASB; 37 °C; HRT = 12 h | 97.8 | 10.07 | [9] |
| Paper mill effluent (PME) | 213.5 | Batch; Initial pH = 7; 37 °C | 58.87 | 7.66 2 | 31.8 | Batch; Initial pH = 5; 37 °C | >400 | Batch; Initial pH = 7; 37 °C | 78.05 | Not available (N/A) | [10] |
| Palm oil mill effluent (POME) | 227 | UASB; 35 °C; HRT = 17 d | 84 | 9.08 | 210 | Anaerobic sequencing batch reactor (ASBR); 55 °C; HRT = 2 d | 315 | UASB; 28–34 °C; HRT = 15 d | H2 stage: 38 CH4-stage: 95 | 15.34 | [11] |
| Cheese whey | 312.1 | Sequencing batch reactor (SBR); 55°C; HRT = 12.5 d | N/A | 11.1 2 | 7.3 | Semi-continuously-fed reactor; 35 °C; HRT = 1.5 d | 340.4 | SBR; 55 °C; HRT = 12.5 d | N/A | 12.1 2 | [12] |
| Starchy wastewater | 111.8–134.8 | Batch; 40 °C | N/A | 5.0 | 92.0 | Batch; 55 °C | 138.0 | Batch; 40 °C | N/A | 8.2 | [13] |
| Agricultural wastewater | 203.7–245.6 | Batch; 40 °C | N/A | 6.1–7.3 | N/A | Batch; 55 °C | 114.1 | Batch; 40 °C | N/A | 2.1–6.2 | [13] |
| Dairy wastewater | 260.8–325.3 | Batch; 40 °C | N/A | 9.4–11.7 | 5.7 | Batch; 55 °C | 227.6–294.9 | Batch; 40 °C | N/A | 10.5–11.1 | [13] |
| Sugary wastewater | 160.8–182.9 | Batch; 40 °C | N/A | 6.2–6.7 | N/A | Batch; 55 °C | 82.8–107.3 | Batch; 40 °C | N/A | 2.8–3.3 | [13] |
| (b) | |||||||||||
| Substrate | Single-Stage Process | Two-Stage System | Reference | ||||||||
| H2 Stage | CH4 Stage | The System | |||||||||
| CH4 Yield (mL/g-VSadded) | Reactor Type and Key Variables | VS Removal (%) | Energy Recovery (as Biogas, kJ/g-VSadded) | H2 Yield (mL/g-VSadded) | Reactor Type and Key Variables | CH4 Yield (mL/g-VSadded) | Reactor Type and Key Variables | VS Removal (%) | Energy Recovery (as Biogas, kJ/g-VSadded) | ||
| Sewage sludge | 105.2 | Batch; 35 °C | N/A | 3.76 | 38.8 | Batch; 35 °C | 96.9 | Batch; 35 °C | N/A | 3.88 | [14] |
| Waste activated sludge (WAS) | 101.2 | Continuous stirred tank reactor (CSTR); 37 °C; HRT = 15 d | N/A | 4.02 | 74.5 | CSTR; 55 °C; HRT = 5 d | 150.7 | CSTR; 37 °C; HRT = 10 d | N/A | 7.00 | [15] |
| 6.7 | CSTR; 37 °C; HRT = 5 d | 127.8 | CSTR; 37 °C; HRT = 10 d | N/A | 5.16 | [15] | |||||
| Activated sludge + food waste (FW) | N/A | CSTR; 37 °C; HRT ≈ 17 d | 61.0 | N/A | N/A | CSTR; 37 °C; HRT ≈ 12 d | N/A | CSTR; 37 °C; HRT = 3 d | 71.5 | N/A | [16] |
| WAS + OFMSW 3 | 490 | CSTR; 55 °C; HRT = 20 d | 86.92 | 17.59 2 | 24 | CSTR; 55 °C; HRT = 3 d | 570 | CSTR; 55 °C; HRT = 17 d | 87.69 | 20.72 2 | [17] |
| Sewage sludge + wine vinasse | 120.20 | CSTR; 35 °C; HRT = 5 d | 38.32 | 4.32 2 | 22.90 | CSTR; 55 °C; HRT = 1 d | 212.56 | CSTR; 35 °C; HRT = 4 d | 53.19 | 7.88 2 | [18] |
| WAS + FW | 79.9 | Reactor; 37 °C; HRT = 12 d | 44.6 | 2.9 | 64.5 | Reactor; 55 °C; HRT = 1.1 d | 154.1 | Reactor; 37 °C; HRT = 6 d | 54.3 | 6.2 | [19] |
| Sewage sludge + rice straw | 171 | Batch; 55 °C | 43.8 | 5.5 | 21 | Batch; 55 °C | 266 | Batch; 55 °C | 60.4 | 8.8 | [20] |
| Fruit and vegetable waste + WAS + olive mill wastewater + cattle manure | 340 | ASBR; 37 °C; HRT = 20 d | 73.6 | 12.7 | 79.4 | CSTR; HRT = 5.33 d | 463 | ASBR; HRT = 8 d | 90.9 | 13.44 | [21] |
| 79.4 | CSTR; HRT = 5.33 d | 730 | ASBR; HRT = 8 d; biogas from H2 stage recirculated to CH4 stage | 91.15 | 21.06 | [21] | |||||
| Sole Substrate-a | Sole Substrate-b | Co-Substrates | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| Substrate | H2 Yield (mL/g-VSadded) | CH4 Yield (mL/g-VSadded) | Substrate | H2 Yield (mL/g-VSadded) | CH4 Yield (mL/g-VSadded) | H2 Yield (mL/g-VSadded) | CH4 Yield (mL/g-VSadded) | |
| Chlorella (Ch) | 36.40 | 166.18 | Molasses | N/A | N/A | 90.12 | 319.52 | [38] |
| Glycerol waste | N/A | N/A | 39.80 | 577.33 | ||||
| POME | N/A | N/A | 53.70 | 545.35 | ||||
| Napier grass | N/A | N/A | 39.98 | 401.69 | ||||
| Empty fruit bunch | N/A | N/A | 22.02 | 466.35 | ||||
| Empty fruit bunch | 35.3 | 236.9 | POME | 53.1 | 259.1 | 16.6 | 348.6 | [34] |
| Decanter cake | 35.9 | 251.2 | 50.9 | 247.8 | ||||
| Palm press fiber | 39.6 | 279.1 | 60.9 | 247.5 | ||||
| Oil palm frond | 66 | 202.7 | 23.4 | 299.5 | ||||
| Oil palm trunk | 28.1 | 216.4 | 18.3 | 364.3 | ||||
| Groundnut deoiled cake | N/A | N/A | Distillery effluent | 55.13 2 | 24.95 2 | 150.7 2 | 64.06 2 | [33] |
| Mustard deoiled cake | N/A | N/A | 109.1 2 | 43.13 2 | ||||
| Distillers’ dried grain with solubles | N/A | N/A | 144.2 2 | 63.84 2 | ||||
| Algal biomass | N/A | N/A | 116.1 2 | 38.88 2 | ||||
| FW | 47.37 | 498.06 | Glyceryl trioleate | 0.60 | 520.37 | 23.98 | 555.89 | [28] |
| FW | 149.3 | 270.9 | Sewage sludge | 17.9 | 70.6 | 174.6 | 264.1 | [39] |
| FW | 29.38 | N/A | Pulp & paper sludge | 2.294 | N/A | 64.48 | 432.3 | [40] |
| Sewage sludge | 1.0 | 122.1 | Cornstalk | N/A | N/A | 13.4 | 172.6 | [37] |
| Substrate | Biogas Yield (mL/g-CODadded) | Dominant Bacteria in H2 Stage | Dominant Bacteria in CH4 Stage | Dominant Archaea in CH4 Stage | Reference |
|---|---|---|---|---|---|
| Synthetic wastewater | H2: 61.81–157.45 CH4: 263.58–234.43 | Spirochaetaceae Bacteroidaceae Clostridiaceae Erysipelotrichaceae | Spirochaetaceae Synergistaceae Thermotogaceae Porphyromonadaceae | Methanosaetaceae | [9] |
| POME | H2: 210 CH4: 310 | Thermoanaerobacterium acidoterolans, Thermoanaerobacterium thermosaccharolyticum, Thermococalles sp., Clostridium acetobutylicum | N/A | Methanobrevibacter sp. Methanosarcina sp. Methanoculeus sp. Sulfolobus sp. Aeropyrum sp. | [96] |
| POME | H2: 53.1 1 CH4: 259.1 1 | Clostridium sp. | N/A | Methanocorpusculum sp. Thermococcus sp. | [34] |
| POME | H2: 60–188 CH4: 200–345 | Clostridium sp., Enterococcus sp., Marinomonas sp., Thermoanaerobacterium sp. | Clostridium sp. Fervidobacterium sp. Ruminococcus sp. | Methanosarcina sp. Methanoculleus sp. | [29] |
| Sewage sludge | H2: N/A CH4: N/A | Firmicutes, Proteobacteria, Bacteroidetes, Acidobacteria, Chloroflexi, Nitrospira, Actinobacteria | N/A | N/A | [97] |
| Waste activated sludge | H2: 6.7–74.5 1 CH4: 127.8–150.7 1 | Actinobacteria Nitrospirae Proteobacteria | Actinobacteria Proteobacteria | Methanoculleus | [15] |
| POME + various kinds of solid wastes | H2: 16.6–60.9 1 CH4: 247.5–364.3 1 | Enterobacter sp. Clostridium sp. Megasphaera sp. | N/A | Methanocorpusculum sp. Methanomicrobium sp. Thermococcus sp. Methanosphaera sp. | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Li, R. Critical Review on Two-Stage Anaerobic Digestion with H2 and CH4 Production from Various Wastes. Water 2024, 16, 1608. https://doi.org/10.3390/w16111608
Zheng X, Li R. Critical Review on Two-Stage Anaerobic Digestion with H2 and CH4 Production from Various Wastes. Water. 2024; 16(11):1608. https://doi.org/10.3390/w16111608
Chicago/Turabian StyleZheng, Xinyi, and Ruying Li. 2024. "Critical Review on Two-Stage Anaerobic Digestion with H2 and CH4 Production from Various Wastes" Water 16, no. 11: 1608. https://doi.org/10.3390/w16111608
APA StyleZheng, X., & Li, R. (2024). Critical Review on Two-Stage Anaerobic Digestion with H2 and CH4 Production from Various Wastes. Water, 16(11), 1608. https://doi.org/10.3390/w16111608









