A Mini-Review on Safe Treatment and Valorization of Salt Waste in Chemical Production Processes in China
Abstract
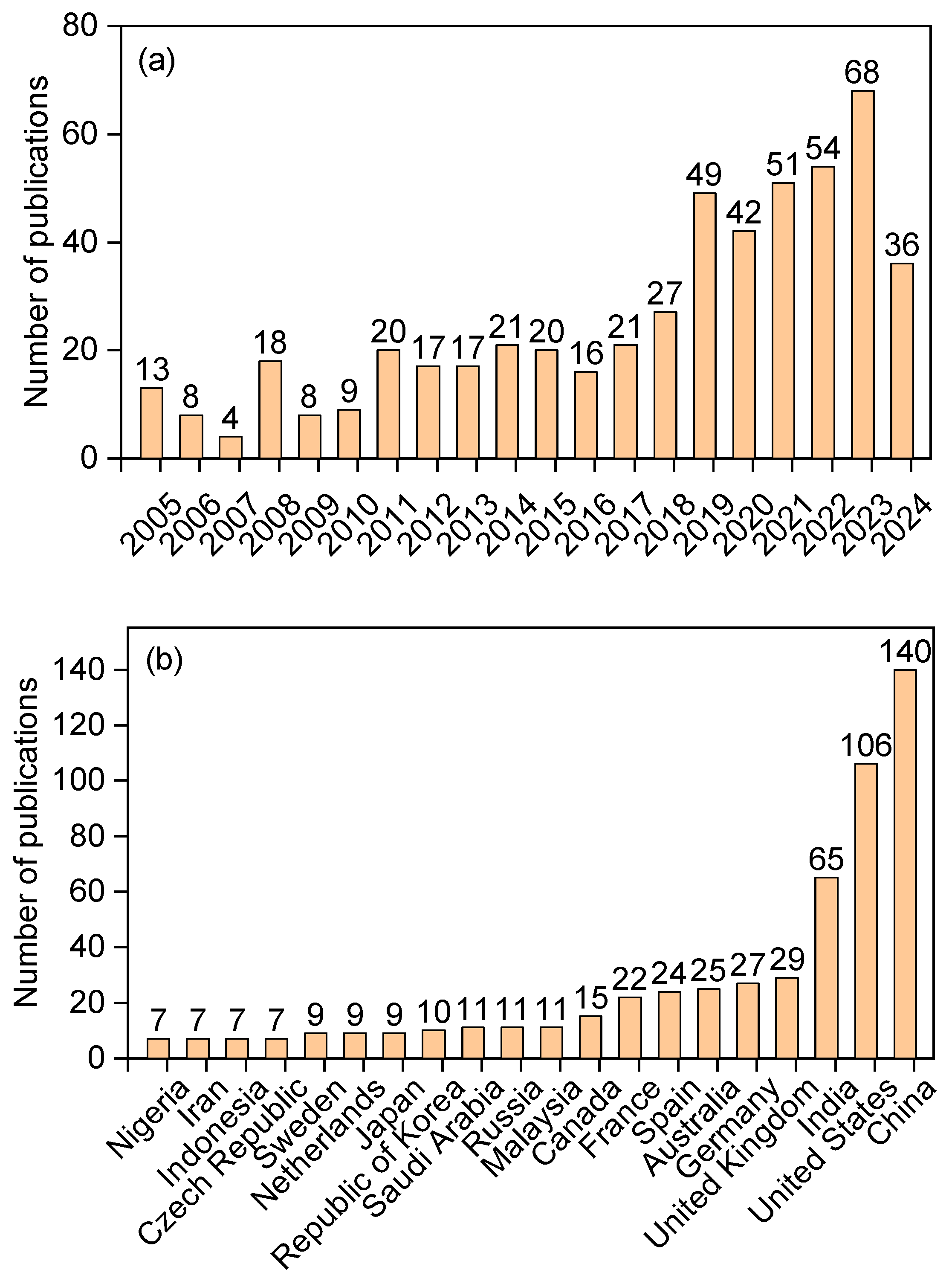
:1. Introduction
2. Technologies for the Treatment and Valorization of Chemical Industrial Salt Waste
2.1. Harmless Treatment
2.2. Valorization
2.2.1. Thermal Treatment
2.2.2. Oxidation Method
2.2.3. Metathesis
2.2.4. Washing Separation Method
2.2.5. Precipitation Method
2.2.6. Evaporation Crystallization Method
2.2.7. Other Valorization Technologies
3. Discussion and Outlook
4. Conclusions
- For salt wastes with low and uniform organic content: salt washing + impurity removal (removing impurities and separating salts) + crystallization to produce salt that meets national product standards.
- For salt wastes with high organic contents and large-scale and mixed salts: stable high-temperature oxidation + impurity removal (removing impurities and separating salts) + crystallization to produce salt that meets national product standards.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine management in desalination industry: From waste to resources generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Simpson, M.F. Projected Salt Waste Production from a Commercial Pyroprocessing Facility. Sci. Technol. Nucl. Install. 2013, 2013, 945858. [Google Scholar] [CrossRef]
- Xie, G.; Liu, L.; Suo, Y.; Zhu, M.; Yang, P.; Sun, W. Study on the green disposal of industrial high salt water and its performance as activator to prepare magnesium-coal based solid waste backfill material for mine. J. Clean. Prod. 2024, 452, 141933. [Google Scholar] [CrossRef]
- Crum, J.V.; Sundaram, S.K.; Riley, B.J.; Matyas, J.; Arreguin, S.A.; Vienna, J.D. Alternative Waste Forms for Electro-Chemical Salt Waste; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2009. [Google Scholar]
- Ruan, X.; Song, M.; Fang, Z.; Wang, H.; Zhang, C.; Chen, W. Methods to treat industrial salted waste: A review. Environ. Chem. Lett. 2024, 59, 9760–9774. [Google Scholar] [CrossRef]
- Xue, T.; Yang, C.; Li, Y.; Shi, X.; Ma, H.; Wei, X.; Liu, Z.; Deng, J. Disposal of drilling waste in salt mines in China. Sci. Total Environ. 2024, 912, 168746. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, J.; Peng, X.; Dai, Z.; Liu, W.; Deng, H.; Xi, B.; Lin, Z. NaCl recovery from organic pollutants-containing salt waste via dual effects of aqueous two-phase systems (ATPS) and crystal regulation with acetone. J. Clean. Prod. 2020, 260, 121044. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Dahmardeh, H.; Akhlaghi Amiri, H.A.; Nowee, S.M. Evaluation of mechanical vapor recompression crystallization process for treatment of high salinity wastewater. Chem. Eng. Process.-Process Intensif. 2019, 145, 107682. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Treatment technologies for reverse osmosis concentrate volume minimization: A review. Sep. Purif. Technol. 2014, 122, 472–489. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Gao, J.; Qiu, X.; Dai, J. The current situation and development trend of salt waste recycling in the chemical industry. Technol. Rev. 2021, 39, 17. [Google Scholar]
- Li, Y.; Wu, H. Mesavannah river high-level radioactive salt waste liquid treatment facility approved for hot testing. Foreign Nucl. News 2020, 9, 21. [Google Scholar]
- Panagopoulos, A. Techno-economic assessment of minimal liquid discharge (MLD) treatment systems for saline wastewater (brine) management and treatment. Process Saf. Environ. Prot. 2021, 146, 656–669. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani, J.J.; Báez, S.O.P. Saline waste disposal reuse for desalination plants for the chlor-alkali industry The particular case of pozo izquierdo SWRO desalination plant. Desalination Int. J. Sci. Technol. Desalt. Water Purif. 2011, 281, 35–41. [Google Scholar]
- Li, G.; Zeng, Y. Experience in the resource utilization of industrial waste salt and the use of waste salt electrolysis. China Chlor-Alkali 2021, 1, 5–10. [Google Scholar]
- Shen, J.; Huang, J.; Ruan, H.; Wang, J.; Bart, V.D.B. Techno-economic analysis of resource recovery of glyphosate liquor by membrane technology. Desalination 2014, 342, 118–125. [Google Scholar] [CrossRef]
- Zhou, H.; Bao, Y.; Bao, J.; Zhu, J. Research progress on the treatment and disposal of industrial waste salt. Environ. Technol. 2020, 33, 6. [Google Scholar]
- Gao, J.; Pang, Q.; Li, Q. Research on the preparation of high-purity yuan powder from salt lake brine in winter nitrate. Inorg. Salt Ind. 2011, 43, 54. [Google Scholar]
- Reig, M.; Casas, S.; Aladjem, C.; Valderrama, C.; Gibert, O.; Valero, F.; Centeno, C.M.; Larrotcha, E.; Cortina, J.L. Concentration of NaCl from seawater reverse osmosis brines for the chlor-alkali industry by electrodialysis. Desalination 2014, 342, 107–117. [Google Scholar] [CrossRef]
- Shi, J.; Huang, W.; Han, H.; Xu, C. Pollution control of wastewater from the coal chemical industry in China: Environmental management policy and technical standards. Renew. Sustain. Energy Rev. 2021, 143, 110883. [Google Scholar] [CrossRef]
- Shi, H.; Xu, C. Review on treatment technology of salt wastewater in coal chemical industry of China. Desalination 2020, 493, 114640. [Google Scholar] [CrossRef]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2017, 342, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.C.; Linga, P.; Park, K.N.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Li, W.; Xu, Y.; Lei, G.; Huang, Z.; Liu, Y.; Huang, Q.; Zheng, S. Characteristics and applicability of heat treatment of typical pesticide salt waste. Environ. Sci. Res. 2018, 10, 1779–1786. [Google Scholar]
- Zhang, L. Research on Pyrolysis Characteristics and Comprehensive Utilization Technology of Organic Industrial Salts. Master’s Thesis, China University of Petroleum, Beijing, China, 2018. [Google Scholar]
- Chen, L. Research on Thermal Treatment of Sodium Chloride Salt Waste as a Byproduct in the Glyphosate Industry. Master’s Thesis, Hefei Polytechnic University, Hefei, China, 2021. [Google Scholar]
- Hu, W.; He, Z.; Zhu, W.; Peng, A.; Zhuang, X. Harmless treatment and utilization of pesticide by-product waste salt residue. Fine Chem. Intermed. 2013, 43, 48–50. [Google Scholar]
- Ding, Z.; Guo, J.; Lu, C. Experimental research on harmless treatment of chemical salt waste. Inorg. Salt Ind. 2020, 52, 58–61. [Google Scholar]
- Yang, W. Development status of chemical waste salt treatment and resource utilization technology. In Proceedings of the 2019 Academic Annual Conference of the Chinese Society of Environmental Sciences, Xi’an, China, 23–25 August 2019; Volume 31, pp. 1–5. [Google Scholar]
- Jiang, T. Exploration of the application of waste salt resource utilization and thermochemical treatment technology. Sichuan Chem. Ind. 2022, 2, 1–6. [Google Scholar]
- Li, X.; Liu, H.; Chen, S.; Wang, Y.; Mu, G.; Liu, J. Fluidization behavior of industrial waste salt. Chem. Ind. Eng. Prog. 2017, 36, 81–90. [Google Scholar]
- Lin, C.; Chi, Y.; Jin, Y.; Jiang, X.; Buekens, A.; Zhang, Q.; Chen, J. Molten salt oxidation of organic hazardous waste with high salt content. Waste Manag. Res. 2018, 36, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, S.; Zuo, Y.; Qi, S.; Wang, D. Resource treatment of epoxy resin high salt content wastewater. Ind. Water Treat. 2021, 41, 126–129. [Google Scholar]
- Wang, L. Research on the Resource Utilization and Disposal Technology of Industrial Waste Salt. Master’s Thesis, Zhejiang Gongshang University, Hangzhou, China, 2020. [Google Scholar]
- Zheng, X.; Shang, H.; Ma, J.; Zhang, L. Research on the process of producing sodium dihydrogen phosphate from industrial waste salt. In Proceedings of the Green Chemistry Branch of the 26th Annual Conference of the Chinese Chemical Society, Tianjin, China, 13–16 July 2008; Volume 1, pp. 11–20. [Google Scholar]
- Li, C. Development of Industrial Waste Salt Preparation Process for Soda Ash. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2016. [Google Scholar]
- Han, M. Research on the Process of Resource Utilization of High Salt Wastewater Using Combined Alkali Production Method. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2017. [Google Scholar]
- Chen, X.; Bai, F.; Shi, Y.; Dong, S.; Liao, E.; Li, Y.; Miao, S. Experimental study on preparation of potassium sulfate by metathesis of high salt wastewater in coal chemical industry. Inorg. Chem. Ind. 2019, 51, 57–61. [Google Scholar]
- Li, N.; Sun, X.; Zou, M.; Sun, X.; Han, W.; Wang, L. Research on purification of phosphorus containing waste salt from pharmaceutical byproducts. Inorg. Salt Ind. 2021, 3, 73–77. [Google Scholar]
- Yao, X. Recycling and utilization of by-product salt residue from hydrated traps. China Chlor-Alkali 2006, 29, 40–42. [Google Scholar]
- Ning, W.; Gong, D. Experimental study on the recovery and utilization of organic matter from furan phenol etherification waste salt residue. Fine Chem. Ind. 2010, 40, 63–65. [Google Scholar]
- Peng, J.; Li, L.; Jiang, J.; Yang, T.; Yang, Y. Study on the removal of high concentration chloride ions from wastewater by co-precipitation method. Water Purif. Technol. 2019, 38, 95–101. [Google Scholar]
- Yang, H.; Sun, T.; Liu, L.; Hao, H. Research on preparation of MnCO3 powder from titanium white salt waste. J. Bohai Univ. (Nat. Sci. Ed.) 2012, 33, 345–349. [Google Scholar]
- Zou, M.; Li, Q.; Li, N.; Han, W.; Sun, X.; Wang, L. Application of hydroxyapatite precipitation method in the resource utilization of waste phosphate in pharmaceutical intermediates. Environ. Pollut. Prev. Control. 2020, 10, 2–7. [Google Scholar]
- Chen, Q.; Wei, J. Typical process and prospect analysis of industrial salt waste resource utilization. Research 2021, 6, 78–80. [Google Scholar]
- Hao, H.; Lu, H.; Su, N.; Yin, Q.; Xie, C.; Wang, Z.; Hou, B.; Huang, X.; Wang, Y.; Bao, Y. Fractional Crystallization Method for Extracting High-Purity Sodium Sulfate and Sodium Chloride from High-Salt Wastewater That Can Be Used as a Resource. China Patent CN201710994394.0, 3 April 2018. [Google Scholar]
- Han, S.; Yin, Z. Talking about the process route of salt treatment of coal chemical wastewater. East China Sci. Technol. 2017, 2, 1–5. [Google Scholar]
- Guo, P.; Li, Z.; Chen, L. Research on refinement and purification technology of salt waste in pesticide industry. Zhejiang Chem. Ind. 2022, 2, 1–7. [Google Scholar]
- Murcia, M.D.; Vershinin, N.O.; Briantceva, N.; Gomez, M.; Gomez, E.; Cascales, E.; Hidalgo, A.M. Development of a kinetic model for the UV/H2O2 photodegradation of 2,4-dichlorophenoxiacetic acid. Chem. Eng. J. 2015, 266, 356–367. [Google Scholar] [CrossRef]
- Miao, S. Research on Resource Utilization of Salt Waste during PPS Production. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2019. [Google Scholar]
- Riley, B. Electrochemical salt wasteform development: A review of salt treatment and immobilization options. Ind. Eng. Chem. Res. 2020, 59, 9760–9774. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, Y.; Chen, X.; Liu, Y.; Zhang, X.; Cheng, L.; Hu, Y. Experimental study on high temperature oxidation removal of impurities in cyanide containing industrial salt waste. Inorg. Salt Ind. 2020, 52, 62–64. [Google Scholar]
- News, S. Breakthrough in Comprehensive Utilization Technology of Industrial Salt Waste in China. 2021. Available online: https://www.sohu.com/a/454742291_100012935 (accessed on 8 March 2021).
- Environment, Z.E. Building a “Waste Free City” and Composing a New Chapter of Sustainable Development. 2019. Available online: https://www.chinacace.org/news/view?id=10454 (accessed on 9 April 2019).
- Bureau, S.E.E. Announcement on the Cross Provincial Transfer of Solid Waste from Shaoxing Yuexin Environmental Protection Technology Co., Ltd. 2024. Available online: https://sxepb.sx.gov.cn/art/2024/3/14/art_1229602247_59021417.html (accessed on 14 March 2024).
- Ma, J. Research on Evaporation Crystallization and Fluidized Bed Incineration of Salt-Containing High-Concentration Organic Waste Liquid. Master’s Thesis, Zhejiang University, Hangzhou, China, 2006. [Google Scholar]
- Qian, P.; Chi, Z.; Wang, J.; Zhang, G.; Zhang, M. Pyrolysis kinetics and carbonization characteristics of salt-containing organic waste liquid. J. Chem. Eng. Chin. Univ. 2018, 32, 1450–1457. [Google Scholar]
- Tang, H.; Xu, M.; Hu, H.; Yang, F.; Yang, Y.; Liu, H.; Li, X.; Yao, H. In-situ removal of sulfur from high sulfur solid waste during molten salt pyrolysis. Fuel 2018, 231, 489–494. [Google Scholar] [CrossRef]

| Type of Salt Waste | Valorization Application | Main Conclusions |
|---|---|---|
| NaCl | Used in the chlor-alkali industry as a raw material [14] or as a deicing agent (must meet the GB/T 23851 standard (https://www.mee.gov.cn), with very strict requirements for heavy metals in the product [15]) or as a raw material for bipolar membranes to produce acids and bases [16] and as a coal additive [17] | Currently, expanding chlor-alkali projects using industrial salt waste as a raw material is not restricted by production capacity policies. However, as chlor-alkali projects are high-energy-consuming endeavors, while they facilitate the environmentally friendly disposal of industrial salt waste, they are subject to constraints such as energy assessments and carbon emission audits. This imposes significant limitations and restrictions on the expansion of chlor-alkali projects using industrial salt waste |
| Na2SO4 | Used as soda ash [18] or as a raw material for bipolar membrane production for acid–base production [19] | / |
| A mixture of NaCl and Na2SO4 | Used to produce soda ash or reused after fractional crystallization [20,21] | / |
| CaCl2 | Used as a raw material for gypsum [22] or as an additive [23] | / |
| FeCl3 | Used as a water purifier | / |
| Standard Name | Implementation Date (Day Month Year) | Main Contents |
|---|---|---|
| Standard for glyphosate by-product industrial salts (HG/T 5531.1-2019, https://www.mee.gov.cn) | 1 January 2020 | Specifies that by-product NaCl must have a purity of more than 94%, with total phosphorus less than 0.15% and total organic carbon less than 0.03% |
| Coal chemical by-product industrial sodium sulfate (T/CCT 001-2019, https://www.mee.gov.cn) | 1 January 2020 | After refining high-salt wastewater, the by-product industrial NaCl must have a purity of more than 96% and grade A Na2SO4 must have a purity of more than 97% |
| Coal chemical by-product industrial sodium sulfate (T/CCT 002-2019, https://www.mee.gov.cn) | 1 January 2020 | |
| Industrial salts (GB/T 5462-2015, https://www.mee.gov.cn) | 1 May 2016 | Sets physicochemical benchmarks for refined industrial salts, including dry and wet salts, as well as sun-dried salts |
| Industrial anhydrous sodium sulfate (GB/T 6009-2014, https://www.mee.gov.cn) | 1 December 2014 | Specifies product grades and other requirements |
| Management of hazardous solid waste in heat-treatment salt baths (GB/T 27945.1-201, https://www.mee.gov.cn) | 1 October 2012 | Standardizes methods for the harmless treatment of residues such as barium salts, nitrate salts, and cyanide salts |
| Iron and steel enterprises use mixed wastewater by-product industrial salt (T/CISA 225-2022, https://www.mee.gov.cn) | 1 August 2022 | Establishes physicochemical standards for industrial salts derived as by-products from mixed wastewater of steel companies |
| Industrial salt as a by-product of coking wastewater (T/CISA 227-2022, https://www.mee.gov.cn) | 1 August 2022 | Sets physicochemical benchmarks for industrial salts obtained as by-products from coking wastewater |
| Technical specification for pollution control and treatment of high-salt wastewater in textile printing and dyeing industry (DB 37/T 3536-2019, https://www.mee.gov.cn) | 2 May 2019 | Outlines regulations for the solidification and recycling use of salts |
| Treatment Method | Principle | Applicable Scope | Advantages and Limitations | |
|---|---|---|---|---|
| Thermal treatment | Pyrolysis | Generally, the process is conducted in a controlled oxygen environment and at temperatures below the melting point of the salt waste. By heating the salt waste, some organic materials in the salt waste vaporize into gas and move into subsequent treatment units, while other organic materials are converted into ash | Single-stage carbonization process: suitable for treating industrial salt wastes with relatively simple structures. Multi-stage carbonization process: suitable for salt wastes containing long carbon chains and heterocyclic organic compounds [56] | Using this process effectively treats industrial salt waste and reduces the content of toxic and hazardous substances in the salt waste [57,58]. Practice has shown that variations in pyrolysis temperature and other process parameters significantly affect the final treatment outcome |
| High-temperature melting | This refers to treating salt waste at higher temperatures, typically requiring calcination temperatures between 800 °C and 1200 °C. Since the calcination temperature is often higher than the melting point of the salt waste, the salt is in a molten state during treatment, allowing for the thorough removal of organic materials from the salt waste | Suitable for treating salt wastes with high organic contents and complex functional groups | Although high-temperature melting thoroughly removes organic materials from salt waste, impurities such as oxidized carbon (C), sulfur (S), and phosphorus (P) may still be present, requiring further treatment to meet reuse standards. Additionally, the high temperature of the molten salt can cause salt components to volatilize under ventilated conditions, which then crystallize in subsequent cooling units, potentially clogging pipes. The refining process of the salt after melting treatment still requires the involvement of evaporation units, resulting in high energy consumption throughout this part of the process | |
| High-temperature fluidized bed | Salt waste containing organic materials | A large contact area and uniform mixing prevent the clumping of salt waste and enhance thermal efficiency. The fluidization condition within the bed layer must be monitored in real time during production, and appropriate measures should be taken to promptly intervene | ||
| Oxidation method (such as advanced oxidation, catalytic wet oxidation, hydrothermal oxidation techniques) | The oxidation method uses oxidizers to oxidize organic impurities in salt waste, thereby purifying the inorganic salts. Advanced oxidation is characterized by the production of hydroxyl radicals (•OH) with strong oxidative capabilities, transforming large, difficult-to-degrade organic molecules into less toxic or non-toxic smaller molecules. This method is a green, pollution-free, and efficient water treatment technology | Used for salt wastes with few organic impurities that are easily oxidized, this method does not produce secondary pollution and does not introduce new impurities after the reaction | The effectiveness of the oxidation method is closely related to the properties of the organic materials present. The types of organic compounds in salt waste can vary significantly between different products and production processes, making it difficult to determine operational parameters such as the amount of oxidizer needed and the treatment time. This variability restricts the application of the oxidation method | |
| Washing and separation method | A method of purifying salt by dissolving the salt to be purified in water or organic solvents, allowing some organic substances and heavy metal ions in the salt to remain in the solution, thereby achieving the purpose of purifying the salt waste | It generally applies only to salt waste with single components and few impurities | The solution produced by this method is difficult to handle, and, often, a large amount of water or solvent is used during the process to ensure the effectiveness of salt washing, which can lead to secondary pollution. In practice, it has been found that the quality of salt waste fluctuates significantly, making it difficult to accurately calculate the required amount of water or solvent, resulting in resource wastage. Some impurities may be trapped within the salt particles and cannot be thoroughly cleaned | |
| Precipitation method | The precipitation method involves dissolving salt waste in water and adding chemical reagents to remove certain characteristic pollutants from the salt waste. While this method offers relatively stable results in treating salt waste, it may also lead to secondary pollution | The precipitation method is only suitable for treating salt waste with a relatively stable and uniform composition. Additionally, the waste residue after precipitation also requires secondary treatment | Removing organic substances is challenging, and the process is lengthy | |
| Evaporation crystallization | Evaporation crystallization is a method where a mixture of salts containing one or more impurities are dissolved in water. Then, by adjusting the temperature or evaporating water, one component reaches a saturated state and crystallizes, thereby achieving the separation and purification of salts | It is suitable for high-salinity wastewater containing components with different solubilities | High energy consumption and the need for further treatment of the source liquor after system processing are required. It is often combined with other processes for further treatment of salt waste | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Wang, Y.; Zhang, D.; Wu, C.; Zhang, J.; Zhao, Z.; Nabi, M.; Luo, X.; Xiao, K. A Mini-Review on Safe Treatment and Valorization of Salt Waste in Chemical Production Processes in China. Water 2024, 16, 1620. https://doi.org/10.3390/w16111620
Lv Y, Wang Y, Zhang D, Wu C, Zhang J, Zhao Z, Nabi M, Luo X, Xiao K. A Mini-Review on Safe Treatment and Valorization of Salt Waste in Chemical Production Processes in China. Water. 2024; 16(11):1620. https://doi.org/10.3390/w16111620
Chicago/Turabian StyleLv, Yang, Yi Wang, Dapeng Zhang, Chaoyue Wu, Jun Zhang, Zehua Zhao, Mohammad Nabi, Xuan Luo, and Keke Xiao. 2024. "A Mini-Review on Safe Treatment and Valorization of Salt Waste in Chemical Production Processes in China" Water 16, no. 11: 1620. https://doi.org/10.3390/w16111620





