Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sediment Sampling
- (1)
- The groundwater from water-filling aquifers was the origin of mine water. A rock roadway is a roadway with a rock area higher than 80% in the excavation section and is mainly used for ventilation, transportation equipment and materials, etc. The 4 rock roadway sampling points passed through the L3 limestone aquifer (RRs: RR1, RR2, RR3, RR4). Thus, most of the rock roadway water originated from the L3 limestone groundwater and collected in the drainage ditches of the rock roadways, except for RR2 which collected the drainage of coal roadways and rock roadways.
- (2)
- A coal roadway is a roadway with a coal area higher than 80% in an excavation section including a panel, which was mainly used for mining and transporting coal. The main water-filling aquifer of coal roadways was the S3 sandstone aquifer, and the sediment contained much coal (CRs: CR1, CR2).
- (3)
- After mining is finished for a certain panel, a wall is built to close it for safety; the panel is called the goaf at this time. The continuous entry of groundwater gradually raises the water level in the goaf; coupled with the consumption of biochemical reactions, this results in a gradual reduction in the level of O2. At this time, the goaf becomes a key zone where physical, chemical and biological changes occur. Thus, the goafs whose water supply source aquifers were all S3 sandstone groundwater were selected for this study (goafs: G1, G2, G3, G4). Here, we had to substitute space for time in the experimental design (the goafs were closed in 2021, 2012, 2010 and 2009) because the microbial community and geochemical characteristics of goafs closed several years ago were not detected.
- (4)
- A water sump is used to temporarily store underground mine water and sediment from the whole mine, and it collects the water and sediment from groundwater aquifers, rock roadways, coal roadways, goafs, etc. Five water sump samples (sumps: WS1, WS2, WS3, WS4, WS5) were collected.
- (5)
- The mine water in the water sumps is conveyed to the surface. Then, some treatment processes should be performed to treat the mine water before it is discharged into the Zhushui River. Three sample points were designed so as to analyze the contribution of mine drainage to the microbial habitat and community in rivers. The sampling points were: the intersection of Zhushui River and mine drainage (SW1), the upstream section of Zhushui River (SW2) and the downstream section of Zhushui River (SW3).
2.3. Sediment Geochemical Property Determination
2.4. DNA Extraction and 16S rRNA Gene Sequencing
2.5. Processing and Statistical Analyses of Sequence Data
3. Results and Discussion
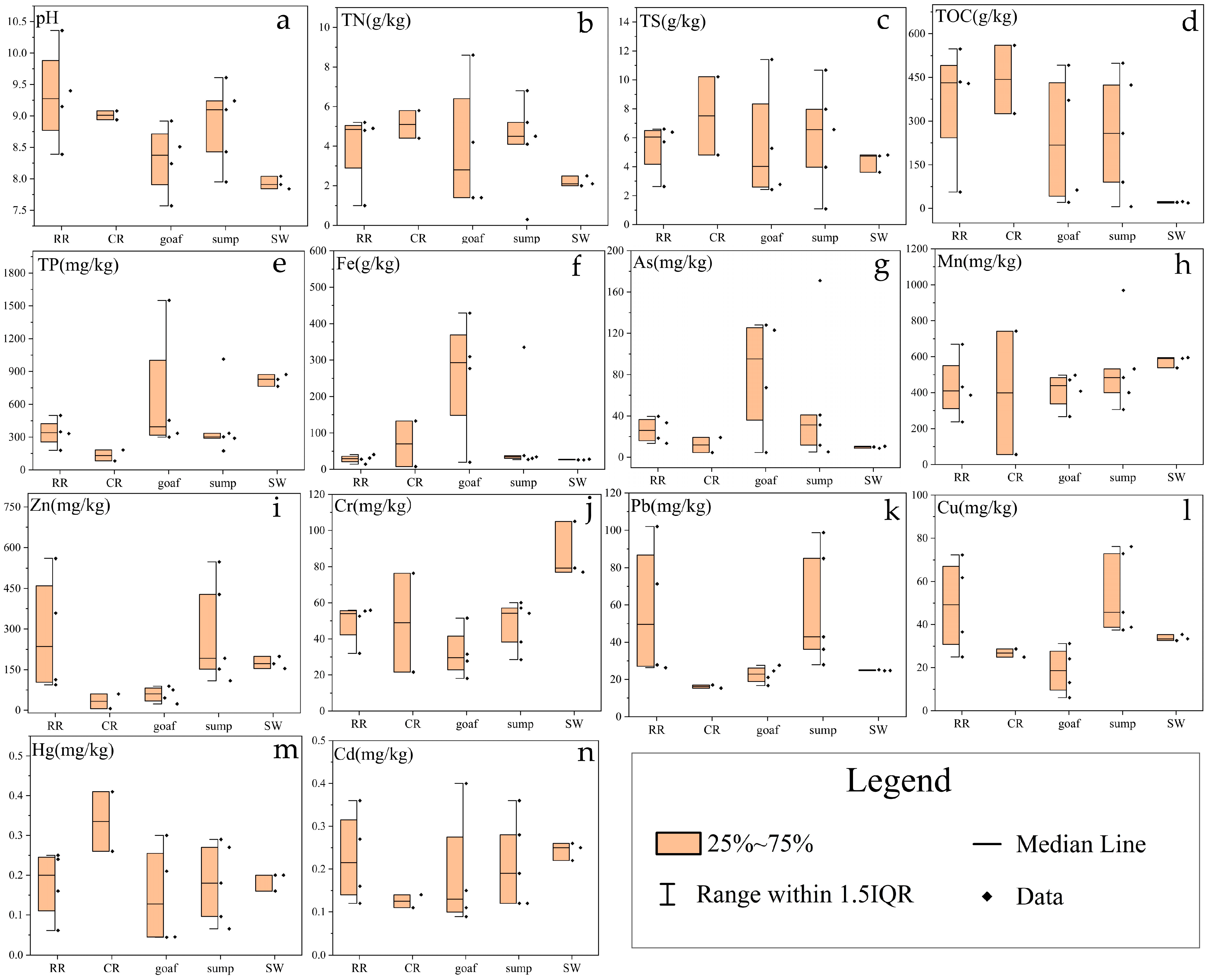
3.1. Geochemical Variation Characteristic of Sediments across Five Different Zones
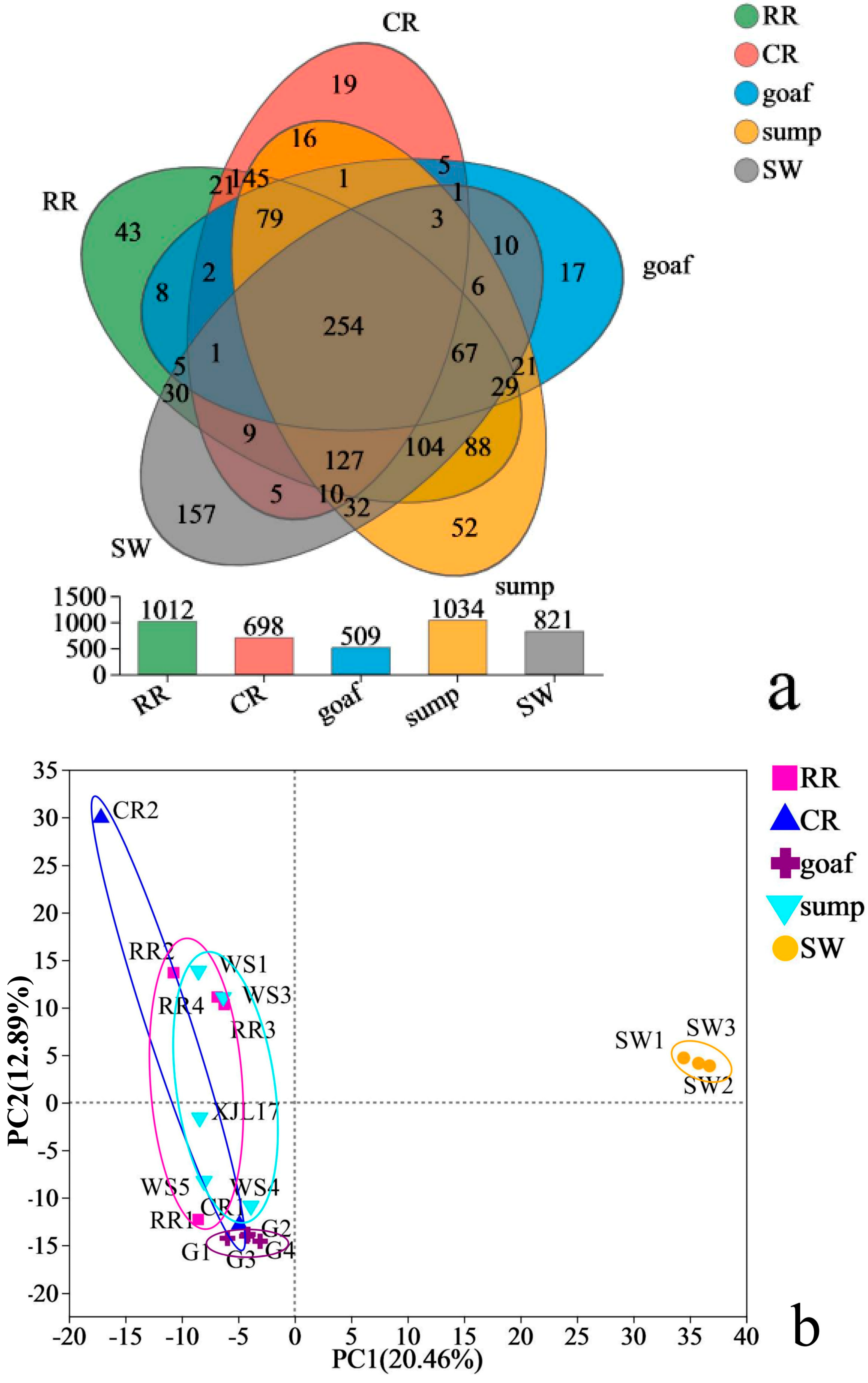
3.2. Overall Microbial Diversity and Taxonomic Composition Changes in Different Zones
3.2.1. The Alpha Diversity Analysis of Sediment Sample
3.2.2. Microbial Community Characteristics of Sediments in Five Zones
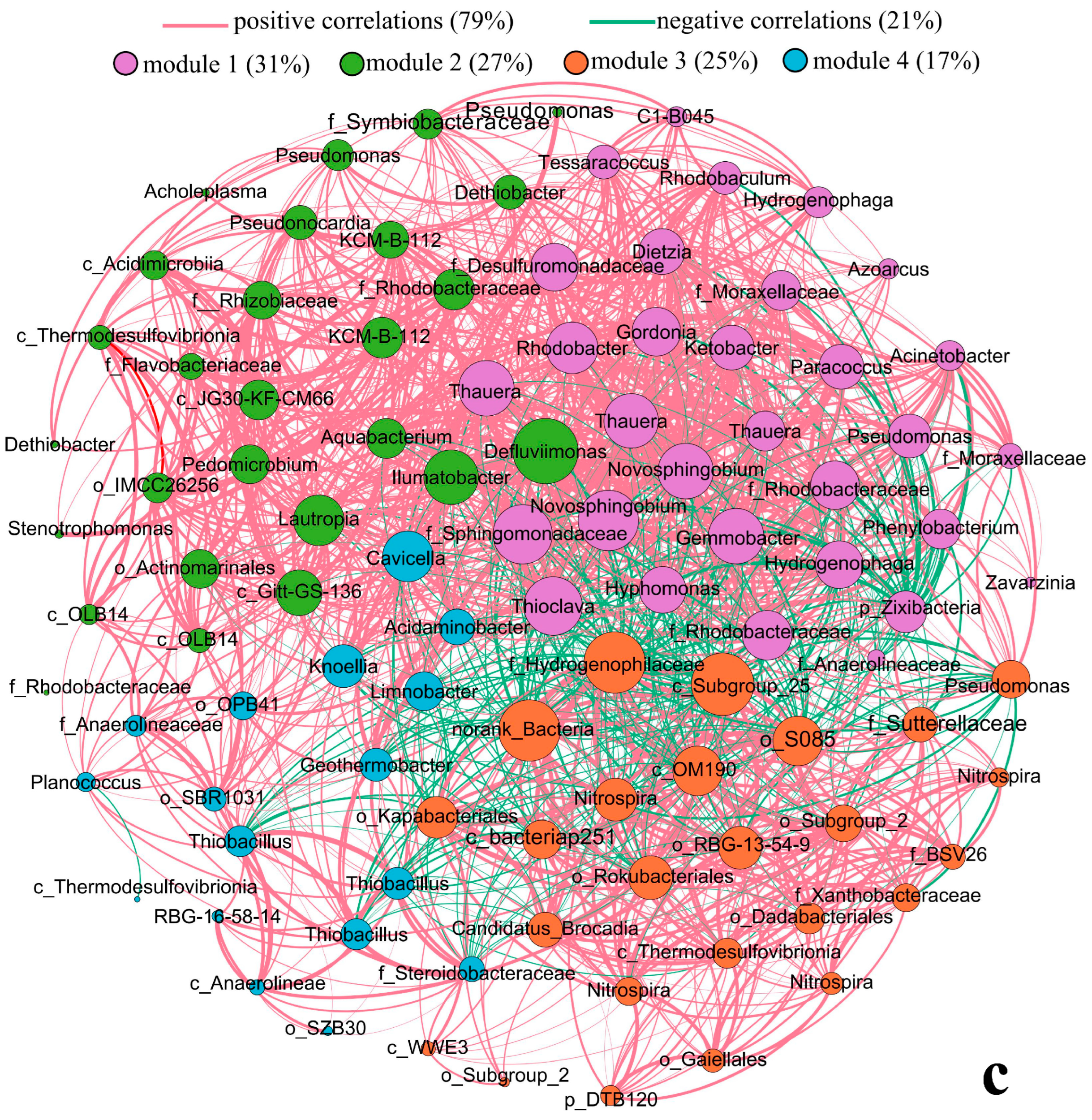
3.3. Correlation between Microbial Communities and Environmental Variables
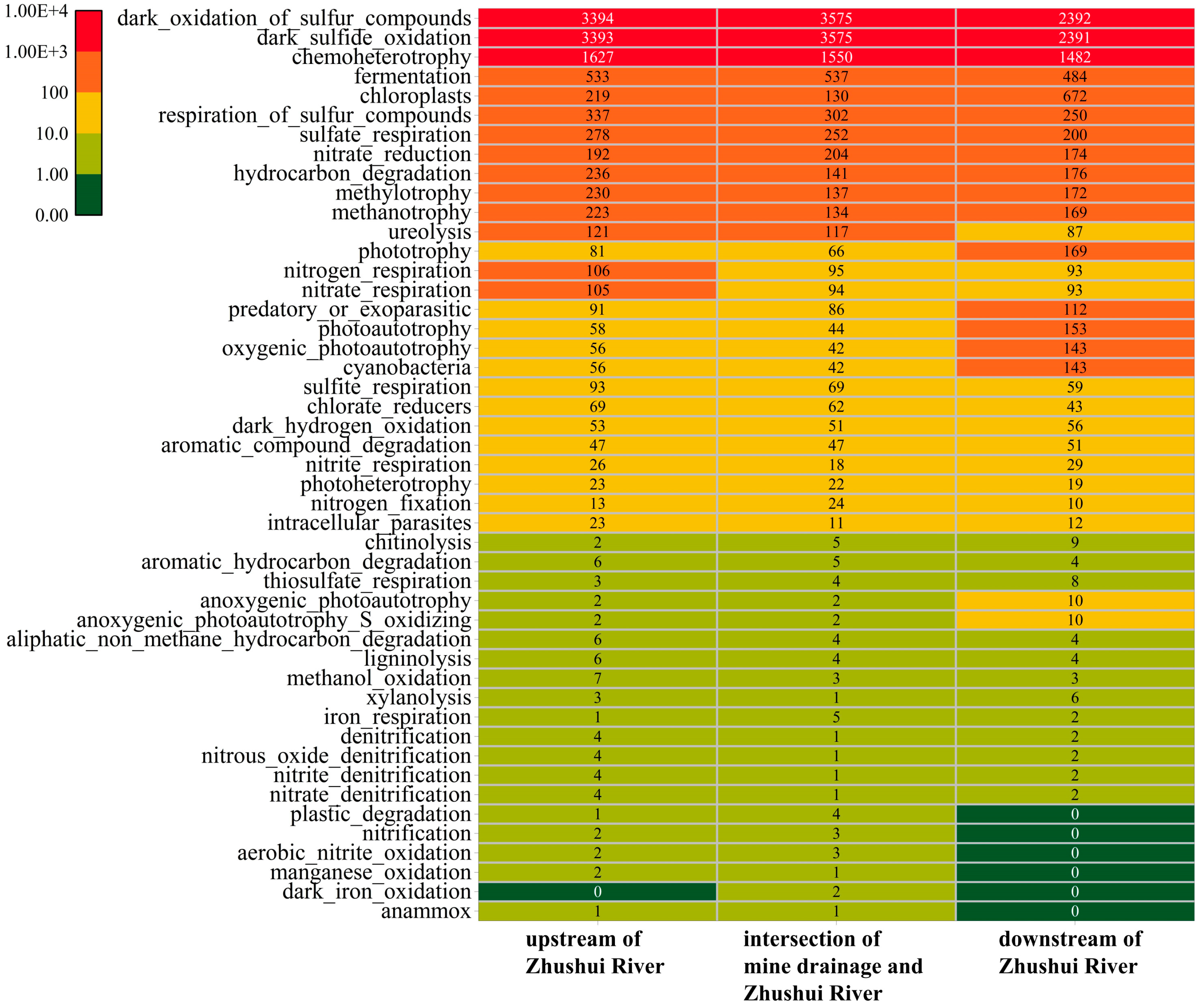
3.4. Mechanism of Microbial Community Variation in Goafs with Increasing Goaf Closure Time
4. Conclusions
- (1)
- The geochemical compounds of sediments differed obviously in the samples collected from different functional zones of the coal mine. The concentrations of TOC, TS and TN in the coal mine sediments were higher than those in river sediments, but the concentrations of TN, TS and TOC in goafs were lower. The concentrations of As, Fe and TP in the goafs was distinctly higher than those in other underground zones, and the pH values of goafs were the lowest, indicating that in the initial stage of mine water entering the goaf, the water level gradually rose, and the pyrite oxidation reaction became more complete.
- (2)
- The microbial community richness and diversity were ranked as follows: surface water > rock roadways > sumps > coal roadways ≥ goafs. The microbial community composition in the different functional zones were eminently different. Surface water sediments and underground coal mine samples belong to two branches in the sample clustering. Thioclava, which can accelerate the oxidation of pyrite, had a higher abundance in coal roadways. Bacteria related to SO42− reduction (i.e., c_Thermodesulfovibrionia, Desulfomicrobium and Geothermobacter) and nitrification (i.e., Nitrospira) accounted for higher proportions in goafs. Cyanobacteriota and Spirochaetota, which perform oxygen-producing photosynthesis, were only found in surface water sediments.
- (3)
- The relationships between microbial communities and geochemical characteristics were illustrated by CCA, Spearman correlation and co-occurrence network analysis, which demonstrated that microbial communities were sensitive and closely related to hydrochemical processes. The surface sediment samples clustered separately from the underground samples. The distribution of microbial communities in the underground mine was closely related not only to nutrient elements such as C, S, P and N, but also to redox-sensitive substances such as Fe and As. The correlation of most of the top 50 microbial species with Fe and As was opposite to that with pH, TOC, TS and TN, possibly due to the coupling relationship between iron transformation and the nutrient compound cycle. Co-occurrence network analysis, with 79% positive correlations, proved that the bacterial genera associated with elemental cycles (S, N, C, P) exhibit interrelationships and modify geochemical characteristics through their metabolic activities.
- (4)
- Goafs were the critical zones of mine water pollution prevention and control. Compared with a tunneling panel, the bacteria with a sulfate reduction function (SRB) had relatively higher proportions in goafs, and the functions of sulfate respiration and respiration of sulfur compounds were highest in the goaf closed in 2021. In addition, with the increase in goaf closure time, the concentration of the characteristic pollutant SO42− in water samples decreased, while As and Fe in sediments increased, suggesting that goafs were able to purify themselves. The small-molecule organic matter released from coal could also provide a continuous carbon source for SRB. Therefore, microbial treatment technologies such as artificial enhancement of SRB reducing sulfate could be applied to remediate groundwater pollution in coal mine areas.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Xiao, Y.; Li, Q.; Deng, J.; Wang, K. Free radicals, apparent activation energy, and functional groups during low-temperature oxidation of Jurassic coal in Northern Shaanxi. Int. J. Min. Sci. Technol. 2018, 28, 469–475. [Google Scholar] [CrossRef]
- Wang, R.; Xu, S.; Jiang, C.; Zhang, Y.; Bai, N.; Zhuang, G.; Bai, Z.; Zhuang, X. Impacts of human activities on the composition and abundance of sulfate-reducing and sulfur-oxidizing microorganisms in polluted river sediments. Front. Microbiol. 2019, 10, 408327. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, B.; Li, L.; Zhao, Y.; Shi, Y.; Jiang, Q.; Jia, L. Analysis of microbial community structure and diversity in surrounding rock soil of different waste dump sites in fushun western opencast mine. Chemosphere 2021, 269, 128777. [Google Scholar] [CrossRef]
- Martiny, J.B.; Eisen, J.A.; Penn, K.; Allison, S.D.; Horner-Devine, M.C. Drivers of bacterial β-diversity depend on spatial scale. Proc. Natl. Acad. Sci. USA 2011, 108, 7850–7854. [Google Scholar] [CrossRef]
- Bier, R.L.; Voss, K.A.; Bernhardt, E.S. Bacterial community responses to a gradient of alkaline mountaintop mine drainage in Central Appalachian streams. ISME J. 2015, 9, 1378–1390. [Google Scholar] [CrossRef]
- De Quadros, P.D.; Zhalnina, K.; Davis-Richardson, A.G.; Drew, J.C.; Menezes, F.B.; De Camargo, F.A.; Triplett, E.W. Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 2016, 98, 195–203. [Google Scholar] [CrossRef]
- Chen, H.; Kimyon, O.; Ramandi, H.L.; Manefield, M.; Kaksonen, A.; Morris, C.; Crosky, A.; Saydam, S. Microbiologically influenced corrosion of cable bolts in underground coal mines: The effect of Acidithiobacillus ferrooxidans. Int. J. Min. Sci. Technol. 2021, 31, 357–363. [Google Scholar] [CrossRef]
- Gao, P.; Sun, X.; Xiao, E.; Xu, Z.; Li, B.; Sun, W. Characterization of iron-metabolizing communities in soils contaminated by acid mine drainage from an abandoned coal mine in Southwest China. Environ. Sci. Pollut. Res. 2019, 26, 9585–9598. [Google Scholar] [CrossRef]
- Brantner, J.S.; Senko, J.M. Response of soil-associated microbial communities to intrusion of coal mine-derived acid mine drainage. Environ. Sci. Technol. 2014, 48, 8556–8563. [Google Scholar] [CrossRef]
- Jin, L.; Gerson, J.R.; Rocca, J.D.; Bernhardt, E.S.; Simonin, M. Alkaline mine drainage drives stream sediment microbial community structure and function. Sci. Total Environ. 2022, 805, 150189. [Google Scholar] [CrossRef]
- Zhao, G. Experimental Study on Anaerobic Degradation of Organic Matter in Coal Desulphurization of Coal Water. Master’s Thesis, Hefei University of Technology, Hefei, China, 2016. [Google Scholar]
- Rudakov, D.; Sobolev, V. A mathematical model of gas flow during coal outburst initiation. Int. J. Min. Sci. Technol. 2019, 29, 791–796. [Google Scholar] [CrossRef]
- Onifade, M.; Lawal, A.; Abdulsalam, J.; Genc, B.; Bada, S.; Said, K.; Gbadamosi, A. Development of multiple soft computing models for estimating organic and inorganic constituents in coal. Int. J. Min. Sci. Technol. 2021, 31, 483–494. [Google Scholar] [CrossRef]
- Krake, N.; Birgel, D.; Smrzka, D.; Zwicker, J.; Huang, H.; Feng, D.; Bohrmann, G.; Peckmann, J.O. Molecular and isotopic signatures of oil-driven bacterial sulfate reduction at seeps in the southern Gulf of Mexico. Chem. Geol. 2022, 595, 120797. [Google Scholar] [CrossRef]
- Gao, B. Study on Occurrence and Biodegradation Mechanism of PAHs in Closed Coal Mine. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2019. [Google Scholar]
- Zhang, Y. Experimental Study on the Degradation of Polycyclic Aromatic Hydrocarbons in Groundwater by Obligate Mixed Bacteria. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2019. [Google Scholar]
- Zhang, X.; Ding, S.; Lv, H.; Cui, G.; Yang, M.; Wang, Y.; Guan, T.; Li, X.D. Microbial controls on heavy metals and nutrients simultaneous release in a seasonally stratified reservoir. Environ. Sci. Pollut. Res. 2022, 29, 1937–1948. [Google Scholar] [CrossRef]
- Liu, L.; Sun, F.; Zhao, H.; Mi, H.; He, S.; Chen, Y.; Liu, Y.; Lan, H.; Zhang, M.; Wang, Z. Compositional changes of sedimentary microbes in the Yangtze River Estuary and their roles in the biochemical cycle. Sci. Total Environ. 2021, 760, 143383. [Google Scholar] [CrossRef]
- Dai, L.; Liu, C.; Peng, L.; Song, C.; Li, X.; Tao, L.; Li, G. Different distribution patterns of microorganisms between aquaculture pond sediment and water. J. Microbiol. 2021, 59, 376–388. [Google Scholar] [CrossRef]
- Lv, B.; Shi, J.; Li, T.; Ren, L.; Tian, W.; Lu, X.; Han, Y.; Cui, Y.; Jiang, T. Deciphering the characterization, ecological function and assembly processes of bacterial communities in ship ballast water and sediments. Sci. Total Environ. 2022, 816, 152721. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Sun, Y.; Gao, Y.; Zhu, L. Coal mining activities driving the changes in microbial community and hydrochemical characteristics of underground mine water. Int. J. Environ. Res. Public Health 2022, 19, 13359. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Sun, Y.; Zhu, L.; Chen, G.; Gao, Y.; Zhao, X. Hydrochemistry and microbial community characteristics and environmental response in different functional zones of a typical coal mine in Ordos. Coal Sci. Technol. 2023, 51, 180–196. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, T.; Sun, M.; Dong, Y.; Ning, Z.; Xiao, E.; Tang, S.; Li, J. Diversity of the sediment microbial community in the Aha watershed (Southwest China) in response to acid mine drainage pollution gradients. Appl. Environ. Microbiol. 2015, 81, 4874–4884. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Sheng, Y.; Sun, X.; Shi, Z.; Xu, Q.; Mu, W. Temperature governs the distribution of hot spring microbial community in three hydrothermal fields, Eastern Tibetan Plateau Geothermal Belt, Western China. Sci. Total Environ. 2020, 720, 137574. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiao, E.; Krumins, V.; Dong, Y.; Xiao, T.; Ning, Z.; Chen, H.; Xiao, Q. Characterization of the microbial community composition and the distribution of Fe-metabolizing bacteria in a creek contaminated by acid mine drainage. Appl. Microbiol. Biotechnol. 2016, 100, 8523–8535. [Google Scholar] [CrossRef]
- De Menezes, A.B.; Prendergast-Miller, M.T.; Richardson, A.E.; Toscas, P.; Farrell, M.; Macdonald, L.M.; Baker, G.; Wark, T.; Thrall, P.H. Network analysis reveals that bacteria and fungi form modules that correlate independently with soil parameters. Environ. Microbiol. 2015, 17, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.H. Direct multi-scale ordination with canonical correspondence analysis. Ecology 2004, 85, 342–351. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Krumins, V.; Dong, Y.; Li, B.; Deng, J.; Wang, Q.; Xiao, T.; Liu, J. Comparative analyses of the microbial communities inhabiting coal mining waste dump and an adjacent acid mine drainage creek. Microb. Ecol. 2019, 78, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Akai, J.; Izumi, K.; Fukuhara, H.; Masuda, H.; Nakano, S.; Yoshimura, T.; Ohfuji, H.; Anawar, H.M.; Akai, K. Mineralogical and geomicrobiological investigations on groundwater arsenic enrichment in Bangladesh. Appl. Geochem. 2004, 19, 215–230. [Google Scholar] [CrossRef]
- Bortoluzzi, E.C.; Pérez, C.A.; Ardisson, J.D.; Tiecher, T.; Caner, L. Occurrence of iron and aluminum sesquioxides and their implications for the P sorption in subtropical soils. Appl. Clay Sci. 2015, 104, 196–204. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, N.; Du, H.; Cai, X.; Cui, Y. The fate of arsenic adsorbed on iron oxides in the presence of arsenite-oxidizing bacteria. Chemosphere 2016, 151, 108–115. [Google Scholar] [CrossRef]
- An, X.; Baker, P.; Li, H.; Su, J.; Yu, C.; Cai, C. The patterns of bacterial community and relationships between sulfate-reducing bacteria and hydrochemistry in sulfate-polluted groundwater of Baogang rare earth tailings. Environ. Sci. Pollut. Res. 2016, 23, 21766–21779. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, T.; Chen, N.; Feng, C. Changes in microbial community diversity, composition, and functions upon nitrate and Cr (VI) contaminated groundwater. Chemosphere 2022, 288, 132476. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Dong, L.; Han, C.; Li, M.; Wu, H. Effects of biochar dosage on treatment performance, enzyme activity and microbial community in aerated constructed wetlands for treating low C/N domestic sewage. Environ. Technol. Innov. 2021, 24, 101919. [Google Scholar] [CrossRef]
- Graham, E.D.; Tully, B.J. Marine Dadabacteria exhibit genome streamlining and phototrophy-driven niche partitioning. ISME J. 2021, 15, 1248–1256. [Google Scholar] [CrossRef]
- Jokanović, S.; Kajan, K.; Perović, S.; Ivanić, M.; Mačić, V.; Orlić, S. Anthropogenic influence on the environmental health along Montenegro coast based on the bacterial and chemical characterization. Environ. Pollut. 2021, 271, 116383. [Google Scholar] [CrossRef]
- Oren, A. On validly published names, correct names, and changes in the nomenclature of phyla and genera of prokaryotes: A guide for the perplexed. NPJ Biofilms Microbiomes 2024, 10, 20. [Google Scholar] [CrossRef]
- Umezawa, K.; Kojima, H.; Kato, Y.; Fukui, M. Dissulfurispira thermophila gen. nov., sp. nov., a thermophilic chemolithoautotroph growing by sulfur disproportionation, and proposal of novel taxa in the phylum Nitrospirota to reclassify the genus Thermodesulfovibrio. Syst. Appl. Microbiol. 2021, 44, 126184. [Google Scholar] [CrossRef]
- Pratte, B.S.; Thiel, T. Cross-activation of two nitrogenase gene clusters by CnfR1 or CnfR2 in the cyanobacterium Anabaena variabilis. Microbiol. Spectrum 2021, 9, e01060. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, F.; Wu, Y.; Xu, G.; Liu, H.; Zhang, H.; Yang, F.; Xu, Y. Small-sized salt-tolerant denitrifying and phosphorus removal aerobic granular sludge cultivated with mariculture waste solids to treat synthetic mariculture wastewater. Biochem. Eng. J. 2022, 181, 108396. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Liu, H.B.; Liu, S.J.; He, X.S.; Dang, F.; Tang, Y.Y.; Xi, B.D. Effects of landfill refuse on the reductive dechlorination of pentachlorophenol and speciation transformation of heavy metals. Sci. Total Environ. 2021, 760, 144122. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Chen, Y.; Zhang, D.; Zhao, M.; Li, H.; Guo, P. Microbiome analysis reveals microecological balance in the emerging rice—Crayfish integrated breeding mode. Front. Microbiol. 2021, 12, 669570. [Google Scholar] [CrossRef]
- He, H.; Ma, H.; Liu, L. Combined photocatalytic pre-oxidation reactor and sequencing batch bioreactor for advanced treatment of industrial wastewater. J. Water Process Eng. 2020, 36, 101259. [Google Scholar] [CrossRef]
- He, Q.; Dasi, E.A.; Cheng, Z.; Talla, E.; Main, K.; Feng, C.; Ergas, S.J. Wood and sulfur-based cyclic denitrification filters for treatment of saline wastewaters. Bioresour. Technol. 2021, 328, 124848. [Google Scholar] [CrossRef]
- Li, R.; Ding, H.; Guo, M.; Shen, X.; Zan, Q. Do pyrene and Kandelia obovata improve removal of BDE-209 in mangrove soils? Chemosphere 2020, 240, 124873. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Y.; Wang, J.; Zhang, L.; Jiang, X.; Shen, J. Coupled biodegradation of p-nitrophenol and p-aminophenol in bioelectrochemical system: Mechanism and microbial functional diversity. J. Environ. Sci. 2021, 108, 134–144. [Google Scholar] [CrossRef]
- Xin, X.; Liu, S.; Qin, J.; Ye, Z.; Liu, W.; Fang, S.; Yang, J. Performances of simultaneous enhanced removal of nitrogen and phosphorus via biological aerated filter with biochar as fillers under low dissolved oxygen for digested swine wastewater treatment. Bioprocess. Biosyst. Eng. 2021, 44, 1741–1753. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, L.; Li, Y.; Qian, Y. Structural, metabolic, and functional characteristics of soil microbial communities in response to benzo [a] pyrene stress. J. Hazard. Mater. 2022, 431, 128632. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Wu, B.; Liu, F.; Fang, W.; Yang, T.; Chen, G.H.; He, Z.; Wang, S. Microbial sulfur metabolism and environmental implications. Sci. Total Environ. 2021, 778, 146085. [Google Scholar] [CrossRef]
- Gao, Y. Microbial Driving Mechanism of Mine Water Quality Formation and Evolution in Xinjulong Coal Mine. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2023. [Google Scholar]
- Sui, X.; Zhang, R.; Xu, N.; Liu, Y.; Ni, H.; Lei, J.; Li, M. Diversity of soil acidobacterial community of different land use types in the Sanjiang Plain, northeast of China. Int. J. Agric. Biol. 2017, 19, 1279–1285. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J.; Li, S.; Ji, G. Long-term sulfide input enhances chemoautotrophic denitrification rather than DNRA in freshwater lake sediments. Environ. Pollut. 2021, 270, 116201. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Ren, H. Intelligent and ecological coal mining as well as clean utilization technology in China: Review and prospects. Int. J. Min. Sci. Technol. 2019, 29, 161–169. [Google Scholar] [CrossRef]







| Parameter | Method | Reference | Limit of Quantification |
|---|---|---|---|
| TS | Ignition iodimetry and EDTA interconnect titration method | LY/T 1255-1999 | — |
| TN | Kjeldahl distillation–volumetric method | DZ/T 0279.29-2016 | 0.02 g/kg |
| TP | Alkali fusion Mo-Sb Anti spectrophotometric method | HJ 632-2011 | 10 mg/kg |
| TOC | Dry combustion method | Rock and Mineral Analysis (2011) 84.2.37 | — |
| pH | Potentiometry | GB/T 50123-2019 | 0.01 |
| As | Atomic fluorescence spectrometry | GB/T 22105.2-2008 | 0.01 mg/kg |
| Hg | Atomic fluorescence spectrometry | GB/T 22105.1-2008 | 0.001 mg/kg |
| Fe, Mn, Gr, Pb, Cu, Zn | Inductively coupled plasma atomic emission spectrometry | DZ/T 0279.2-2016 | 6.3, 0.02, 0.2, 0.7, 0.5 and 0.03 mg/kg |
| Cd | Atomic absorption spectrophotometry | Rock and Mineral Analysis (2011) 84.2.6 | — |
| Sampling Zone | Sample Name | Sequence | OTUs | ACE | Chao1 | Shannon | Simpson | Coverage (%) |
|---|---|---|---|---|---|---|---|---|
| Surface water | SW1 | 67,147 | 2441 | 3111.66 | 3118.89 | 5.98 | 0.02 | 97.57% |
| SW2 | 73,065 | 2400 | 3155.00 | 3098.21 | 5.92 | 0.02 | 97.50% | |
| SW3 | 68,161 | 2413 | 3013.80 | 2980.90 | 6.05 | 0.01 | 97.72% | |
| Coal roadways | CR1 | 55,401 | 210 | 332.24 | 277.00 | 1.61 | 0.48 | 99.78% |
| CR2 | 60,792 | 1652 | 2251.87 | 2272.90 | 5.70 | 0.01 | 98.21% | |
| Rock roadways | RR1 | 50,151 | 633 | 811.11 | 795.90 | 3.76 | 0.07 | 99.40% |
| RR2 | 59,355 | 1691 | 2265.65 | 2222.67 | 5.39 | 0.02 | 98.18% | |
| RR3 | 56,099 | 2015 | 2821.82 | 2730.45 | 5.58 | 0.03 | 97.70% | |
| RR4 | 61,858 | 2212 | 3063.54 | 2959.39 | 5.76 | 0.02 | 97.48% | |
| Goafs | G1 | 38,821 | 395 | 409.32 | 413.60 | 4.25 | 0.04 | 99.90% |
| G2 | 54,471 | 349 | 442.19 | 455.31 | 3.39 | 0.09 | 99.69% | |
| G3 | 53,805 | 355 | 459.87 | 472.86 | 3.22 | 0.09 | 99.67% | |
| G4 | 58,729 | 455 | 562.46 | 586.10 | 3.68 | 0.07 | 99.62% | |
| Water sumps | WS1 | 55,563 | 2010 | 2584.42 | 2541.66 | 5.94 | 0.01 | 98.02% |
| WS2 | 38,178 | 837 | 875.14 | 877.79 | 4.56 | 0.06 | 99.71% | |
| WS3 | 56,680 | 2121 | 2949.83 | 2836.15 | 5.65 | 0.03 | 97.61% | |
| WS4 | 60,168 | 785 | 1343.55 | 1148.83 | 3.02 | 0.27 | 99.02% | |
| WS5 | 41,930 | 765 | 873.83 | 875.61 | 3.31 | 0.16 | 99.47% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhang, L.; Gao, Y.; Tan, X.; Sun, Y.; Chen, W. Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine. Water 2024, 16, 1836. https://doi.org/10.3390/w16131836
Xu Z, Zhang L, Gao Y, Tan X, Sun Y, Chen W. Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine. Water. 2024; 16(13):1836. https://doi.org/10.3390/w16131836
Chicago/Turabian StyleXu, Zhimin, Li Zhang, Yating Gao, Xianfeng Tan, Yajun Sun, and Weixiao Chen. 2024. "Effects of Coal Mining Activities on the Changes in Microbial Community and Geochemical Characteristics in Different Functional Zones of a Deep Underground Coal Mine" Water 16, no. 13: 1836. https://doi.org/10.3390/w16131836





