Research Progress on the Removal of Contaminants from Wastewater by Constructed Wetland Substrate: A Review
Abstract
:1. Introduction
2. Typical/New Substrates for CWs
2.1. Natural Substrates
2.2. By-Products
2.2.1. Agricultural By-Products
2.2.2. Industrial By-Products
2.3. Artificial Synthetic Substrates
| Category | Substrate | Bases | Sources | Characteristic | Reference |
|---|---|---|---|---|---|
| Natural substrate | Gravel | SiO2 and Al2O3 | Rock or mineral detritus | ① Low cost ② Poor removal of contaminants | [28] |
| Natural substrate | Sand | SiO2 | Tiny stone particles | ① Availability and low cost ② Easily clog CWs | [29] |
| Natural substrate | Volcanics | SiO2, Fe2O3, CaO and Al2O3 | Cooling magma after a volcanic eruption | ① High porosity and specific surface area ② Poor contaminant removal | [30] |
| Natural substrate | Zeolite | SiO2 and Al2O3 | Mainly derived from alkaline groundwater and volcanic rocks | ① High ion exchange, low cost, can be regenerated ② Low adsorption rate, easy to be affected by the environment | [31,32] |
| By-product | Fly ash | Fe2O3, Al2O3, SiO2 and CaO | Waste from burning coal | ① Large specific surface area, good adsorption performance ② Can easily cause secondary pollution in the environment | [33] |
| By-product | Steel slag | CaO, SiO2 and Fe2O3 | From electric furnaces and refining furnaces during steelmaking | ① High phosphorus-removal performance ② Strong alkalinity, which makes it difficult for microorganisms and plants to survive | [34] |
| By-product | Construction solid waste | - | Waste generated during demolition, construction and maintenance in the construction industry | ① Low cost, high availability ② Poor reusability | [35] |
| By-product | Sludge | Organics, SiO2 and Al2O3 | Waste from drinking water treatment | ① Low cost, high capacity for P and HMs adsorption, easy to obtain ② Very sensitive to the environment, might leach toxic substances | [14] |
| By-product | Iron scraps | Fe | Waste from steel mills and machinery-processing plants | ① Low cost, large specific surface area | [36,37] |
| By-product | Woodchip | Cellulose, hemicellulose, lignin | Waste from wood processing | ① Low cost, excellent N removal ② May cause secondary pollution | [38] |
| By-product | Crushed glass | Na2SiO3, CaO and SiO2 | Waste from glass production and use | ① Wide range of sources, reusable, provides space for microbes to attach | [39] |
| Artificial synthetic substrate | Ceramsite | SiO2, Al2O3 and CaO | Made by roasting raw materials | ① Good adsorption performance, high strength ② High energy consumption | [40] |
| Artificial synthetic substrate | Activated carbon | Porous carbon | Made by carbonization of raw materials | ① Moderate adsorption effect ② High cost | [41] |
| Artificial synthetic substrate | Biochar | Porous carbon | Made by biomass pyrolysis | ① Excellent contaminant adsorption, stable ② Difficulty in preparation | [42] |
| Artificial synthetic substrate | Modified substrates | - | Further processing of materials | ① Excellent contaminant adsorption ② High cost and high energy cost | [43] |
3. Functions of Substrates Removal of Contaminants
3.1. Substrates Removes Conventional Contaminants
3.1.1. Organic
3.1.2. Nitrogen
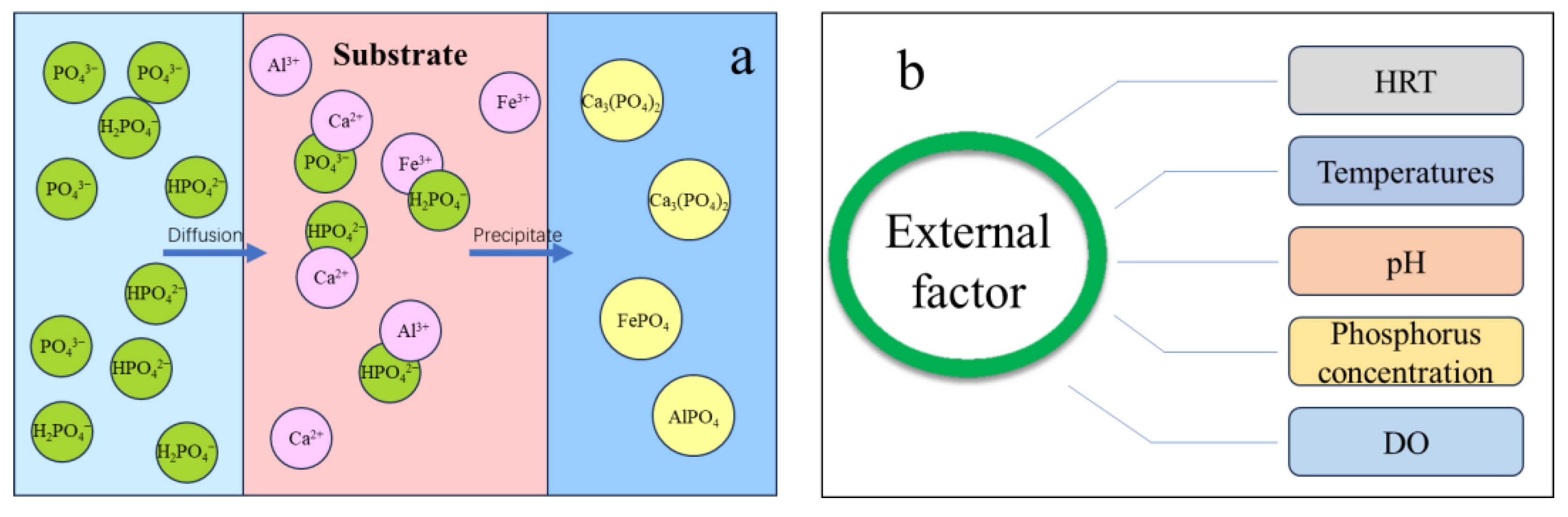
3.1.3. Phosphorus
3.2. HMs Removal
3.3. Fluoride Compounds Removal
3.4. PPCPs Removal
3.5. Microplastics Removal
3.6. Chapter Summary
4. Problems and Prospects
4.1. Clogging
4.2. Substrate Selection and Collocation
4.3. Combining CW Technology with Other Processes
5. Conclusions
- CWs have been widely used in wastewater treatment. At present, there are more than 11,000 articles related to CWs in the Web of Science database. The term “constructed wetland” was used as the key word to search the database. In the past five years (2019–2023), a total of 5140 relevant publications were found after deduplication. Afterwards, VOSviewer software was used to visually analyze these 5140 articles, and the results show that the current research on CWs is still mainly focused on the removal of traditional contaminants (e.g., nitrogen and phosphorus).
- According to the source of the substrates, they can be divided into natural substrates, by-products (agricultural by-products and industrial by-products) and artificially synthesized substrates. Different substrates exhibit significant disparities in performance. This paper systematically summarizes the characteristics of different substrates and their ability to remove various contaminants in CWs. For the purposes of practical engineering, this paper provides important reference information for the selection and configuration of substrates.
- CWs rely on the synergistic interaction of plants, microorganisms, and substrate to purify contaminants. Among them, the substrate plays an important role in CWs. It not only can help microbial proliferation and plant growth, but also directly participate in removing contaminants through filtration and interception, adsorption, chemical precision, redox reactions, and ion exchange.
- With the continuous improvement in requirements for contaminant removal, EPs have received extensive attention. At present, CWs have been proved to be an effective way to remove EPs. In the future, the mechanism of their removal from CWs should be studied systematically.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sandoval-Herazo, L.C.; Alvarado-Lassman, A.A.; Marín-Muñiz, J.L.; Méndez-Contreras, J.M.; Zamora-Castro, S.A. Effects of the Use of Ornamental Plants and Different Substrates in the Removal of Wastewater Pollutants through Microcosms of Constructed Wetlands. Sustainability 2018, 10, 1594. [Google Scholar] [CrossRef]
- Tian, T.; Yang, Q.; Wei, G.; Cheung, S.G.; Shin, P.K.; Wong, Y.S.; Li, Z.; Chen, Z.; Tam, N.F.Y. Changes of substrate microbial biomass and community composition in a constructed mangrove wetland for municipal wastewater treatment during 10-years operation. Mar. Pollut. Bull. 2020, 155, 111095. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.J.; Xu, S.G.; Zhang, Y.M.; Han, C.W. Molybdenum (VI) removal by using constructed wetlands with different filter media and plants. Water Sci. Technol. 2013, 67, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Xu, W.; Wang, H.; Zhao, D.; Ding, H. Nitrogen and Phosphorus Removal Efficiency and Denitrification Kinetics of Different Substrates in Constructed Wetland. Water 2022, 14, 1757. [Google Scholar] [CrossRef]
- Li, X.; Guo, Q.; Wang, Y.; Xu, J.; Wei, Q.; Chen, L.; Liao, L. Enhancing Nitrogen and Phosphorus Removal by Applying Effective Microorganisms to Constructed Wetlands. Water 2020, 12, 2443. [Google Scholar] [CrossRef]
- Negi, D.; Verma, S.; Singh, S.; Daverey, A.; Lin, J.-G. Nitrogen removal via anammox process in constructed wetland—A comprehensive review. Chem. Eng. J. 2022, 437, 135434. [Google Scholar] [CrossRef]
- Shehzadi, M.; Afzal, M.; Khan, M.U.; Islam, E.; Mobin, A.; Anwar, S.; Khan, Q.M. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res. 2014, 58, 152–159. [Google Scholar] [CrossRef]
- Türker, O.C.; Böcük, H.; Yakar, A. The phytoremediation ability of a polyculture constructed wetland to treat boron from mine effluent. J. Hazard. Mater. 2013, 252–253, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-L.; Nakano, K.; Taniguchi, T.; Nomura, M.; Nishimura, O. Estrogen removal from treated municipal effluent in small-scale constructed wetland with different depth. Bioresour. Technol. 2009, 100, 2945–2951. [Google Scholar] [CrossRef]
- Alsubih, M.; El Morabet, R.; Khan, R.A.; Khan, N.A.; Khan, A.R.; Khan, S.; Mubarak, N.M.; Dehghani, M.H.; Singh, L. Field performance investigation for constructed wetland clubbed with tubesettler for hospital wastewater treatment. J. Water Process. Eng. 2022, 49, 103147. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.; Dong, J.; Tan, S.K. Application of constructed wetlands for treating agricultural runoff and agro-industrial wastewater: A review. Hydrobiologia 2017, 805, 1–31. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Huang, M.; Chen, M.; Wang, M. Application of basalt fiber in vertical flow constructed wetland for different pollution loads wastewater: Performance, substrate enzyme activity and microorganism community. Bioresour. Technol. 2020, 318, 124229. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhao, Y.; Guo, J.; Ji, B.; García, A.P.; Núñez, A.E. Developing a novel lightweight substrate for constructed treatment wetland: The idea and the reality. J. Water Process. Eng. 2024, 57, 104587. [Google Scholar] [CrossRef]
- Li, H.Z.; Wang, S.; Ye, J.F.; Xu, Z.X.; Jin, W. A practical method for the restoration of clogged rural vertical subsurface flow constructed wetlands for domestic wastewater treatment using earthworm. Water Sci. Technol. 2011, 63, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Tang, W.; Pei, Y. Constructed wetland substrates: A review on development, function mechanisms, and application in contaminants removal. Chemosphere 2021, 286, 131564. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Lv, J.; Lu, S.; Wu, W.; Wu, S. Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresour. Technol. 2016, 210, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.Y.; Hu, X.J.; Cheng, S.Y.; Xie, H.J.; Hu, Z.; Wu, H.M.; Liang, S. Effects and mechanisms of constructed wetlands with different substrates on N2O emission in wastewater treatment. Environ. Sci. Pollut. Res. 2022, 29, 19045–19053. [Google Scholar] [CrossRef] [PubMed]
- Rehrah, D.; Reddy, M.; Novak, J.; Bansode, R.; Schimmel, K.; Yu, J.; Watts, D.; Ahmedna, M. Production and characterization of biochars from agricultural by-products for use in soil quality enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Saeed, T.; Muntaha, S.; Rashid, M.; Sun, G.; Hasnat, A. Industrial wastewater treatment in constructed wetlands packed with construction materials and agricultural by-products. J. Clean. Prod. 2018, 189, 442–453. [Google Scholar] [CrossRef]
- Nandhini, K.; Karthikeyan, J. Influence of Industrial and Agricultural by-Products as Cementitious Blends in Self-Compacting Concrete—A Review. Silicon 2021, 14, 2431–2452. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, Y.; Liang, S.; Hu, J.; Hou, H.; Quan, J.; Li, X.; Duan, H.; Yuan, S.; Yang, J. Environmentally sound management of industrial solid waste: A paradigm of proposed bi-tetrahedron. Resour. Conserv. Recycl. 2023, 198, 107212. [Google Scholar] [CrossRef]
- Samanlioglu, F. A multi-objective mathematical model for the industrial hazardous waste location-routing problem. Eur. J. Oper. Res. 2013, 226, 332–340. [Google Scholar] [CrossRef]
- Sahu, A.; Kumar, S.; Srivastava, A.; Pratap, B. Performance of recycled aggregate concrete using copper slag as fine aggregate. J. Build. Eng. 2024, 82, 108364. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Li, W.; Yu, Y.; Yan, K. Environmental assessment, management and utilization of red mud in China. J. Clean. Prod. 2014, 84, 606–610. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Tang, Y.; Jiang, Y.; Xie, S.; Zhang, Y.; Qin, Y. Selection and optimization of the substrate in constructed wetland: A review. J. Water Process. Eng. 2022, 49, 103140. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, Y.; Wang, H.; Tang, J.; Li, Y.; Wang, S. Removal of Cr(VI) from wastewater by artificial zeolite spheres loaded with nano Fe–Al bimetallic oxide in constructed wetland. Chemosphere 2020, 257, 127224. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, X.; Zheng, Y.; Dzakpasu, M.; Zhao, Y.; Xiong, J. Functions of slags and gravels as substrates in large-scale demonstration constructed wetland systems for polluted river water treatment. Environ. Sci. Pollut. Res. 2015, 22, 12982–12991. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Hu, N.; Wang, Q.H.; Chen, Y.C.; Huang, L. How to select substrate for alleviating clogging in the subsurface flow constructed wetland? Sci. Total Environ. 2022, 828, 154529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, J.; Liu, L.; Zhu, G.; Liu, C. Screening of phosphate-removing substrates for use in constructed wetlands treating swine wastewater. Ecol. Eng. 2013, 54, 57–65. [Google Scholar] [CrossRef]
- Cuomo, M.; König, R.; Zanardini, E.; Di Guardo, A.; Bianchi, G.; Ortona, A.; Principi, P. Using zeolite filters to reduce activated carbon use in micropollutant removal from wastewater. J. Water Process. Eng. 2023, 56, 104298. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhou, L.; Wang, W.; Li, N.; Zhang, F. Performance and mechanisms of fly ash for graphene oxide removal from aqueous solution. Environ. Sci. Pollut. Res. 2021, 29, 3773–3783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, Y.; Yu, X.; Ding, S.; Yan, J.; Min, Y. Vegetated Steel Slag Substrate Constructed Wetlands can Achieve High Efficiency Simultaneous Nitrogen and Phosphorus Removal. Front. Environ. Sci. 2022, 10, 947783. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.M.; Liu, C.; Guo, X.C. Enhanced P, N and C removal from domestic wastewater using constructed wetland employing construction solid waste (CSW) as main substrate. Water Sci. Technol. 2012, 66, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Dong, L.; Guo, Z.; Hu, Z.; Dai, P.; Zhang, J.; Wu, H. Highly efficient phosphorous removal in constructed wetland with iron scrap: Insights into the microbial removal mechanism. J. Environ. Manag. 2023, 347, 119076. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, D.; Yang, X.; Xing, W.; Li, J.; Zhang, Q. Iron [Fe(0)]-rich substrate based on iron–carbon micro–electrolysis for phosphorus adsorption in aqueous solutions. Chemosphere 2017, 168, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, M.V.; Larsen, S.E.; Kjærgaard, C.; Hoffmann, C.C. Reducing adverse side effects by seasonally lowering nitrate removal in subsurface flow constructed wetlands. J. Environ. Manag. 2019, 240, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Barquero, L.G.; Humeniuk, B.W.; Luong, K.H.; Cicek, N.; Wong, C.S.; Hanson, M.L. Crushed recycled glass as a substrate for constructed wetland wastewater treatment: A case study of its potential to facilitate pharmaceutical removal. Environ. Sci. Pollut. Res. 2021, 28, 52306–52318. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Han, Q.; Luo, H.; He, T.; Xue, F.; Ye, Z.; Chen, C.; Huang, S. Ceramsite Facilitated Microbial Degradation of Pollutants in Domestic Wastewater. Int. J. Environ. Res. Public Health 2020, 17, 4692. [Google Scholar] [CrossRef] [PubMed]
- Vispo, C.; Geronimo, F.K.; Jeon, M.; Kim, L.-H. Performance Evaluation of Various Filter Media for Multi-Functional Purposes to Urban Constructed Wetlands. Sustainability 2023, 16, 287. [Google Scholar] [CrossRef]
- Gupta, P.; Ann, T.-W.; Lee, S.-M. Use of biochar to enhance constructed wetland performance in wastewater reclamation. Environ. Eng. Res. 2016, 21, 36–44. [Google Scholar] [CrossRef]
- Yun, Y.; Zhou, X.; Li, Z.; Uddin, S.M.N.; Bai, X. Comparative research on phosphorus removal by pilot-scale vertical flow constructed wetlands using steel slag and modified steel slag as substrates. Water Sci. Technol. 2015, 71, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, B.; Xu, Y.; Guan, J.; Liu, S. Removal of nitrogen and COD in horizontal subsurface flow constructed wetlands under different influent C/N ratios. Ecol. Eng. 2014, 63, 58–63. [Google Scholar] [CrossRef]
- Xu, Q.; Cui, L. Removal of COD from synthetic wastewater in vertical flow constructed wetland. Water Environ. Res. 2019, 91, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Xu, H.; Liu, C.; Shen, Z.; Hu, K. Preparation of Ceramsite Based on Waterworks Sludge and Its Application as Matrix in Constructed Wetlands. Int. J. Environ. Res. Public Health 2019, 16, 2637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Wang, X.Y.; Teng, H.W.; Xu, J.L.; Sheng, L.X. Purification mechanism of city tail water by constructed wetland substrate with NaOH-modified corn straw biochar. Ecotoxicol. Environ. Saf. 2022, 238, 113597. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Sheng, L.; Teng, H. Study on treatment of city tail water by constructed wetland with corn straw biochar substrate. Environ. Technol. Innov. 2022, 28, 102855. [Google Scholar] [CrossRef]
- Kong, F.L.; Wang, J.R.; Hou, W.H.; Cui, Y.Q.; Yu, L.H.; Zhang, Y.; Wang, S. Influence of modified biochar supported sulfidation of nano-zero-valent-iron (S-nZVI/BC) on nitrate removal and greenhouse gas emission in constructed wetland. J. Environ. Sci. 2023, 125, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.-H.; Wang, H.-C.; Li, Z.-L.; Liang, B.; Sun, Y.-L.; Cheng, H.-Y.; Lu, S.-Y.; Wang, A.-J. Pyrite coupled with steel slag to enhance simultaneous nitrogen and phosphorus removal in constructed wetlands. Chem. Eng. J. 2023, 470, 143944. [Google Scholar] [CrossRef]
- Zheng, T.; Lin, X.; Xu, J.; Ren, J.; Sun, D.; Gu, Y.; Huang, J. Enhanced Nitrogen Removal of Steel Rolling Wastewater by Constructed Wetland Combined with Sulfur Autotrophic Denitrification. Sustainability 2021, 13, 1559. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, W.; Tan, Q.; Yang, L.; Chen, J.; Ma, L.; Zhang, Y.; Wu, Z.; He, F. Vertical-flow constructed wetland based on pyrite intensification: Mixotrophic denitrification performance and mechanism. Bioresour. Technol. 2022, 347, 126710. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Li, F.; Ji, X.; Hou, M. Intensified nitrogen removal in constructed wetlands by novel spray aeration system and different influent COD/N ratios. Bioresour. Technol. 2020, 306, 123008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, C.; Jia, L.; Feng, L.; Wang, R.; Wu, H. An innovative biochar-amended substrate vertical flow constructed wetland for low C/N wastewater treatment: Impact of influent strengths. Bioresour. Technol. 2018, 247, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J. The concept of “capacity” in zeolite ion-exchange systems. J. Colloid Interface Sci. 2005, 281, 68–79. [Google Scholar] [CrossRef]
- Alshameri, A.; Ibrahim, A.; Assabri, A.M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technol. 2014, 258, 20–31. [Google Scholar] [CrossRef]
- Tang, X.Q.; Huang, S.L.; Scholz, M. Comparison of phosphorus removal between vertical subsurface flow constructed wetlands with different substrates. Water Environ. J. 2009, 23, 180–188. [Google Scholar] [CrossRef]
- Jakubaszek, A. Nitrogen and Phosphorus Accumulation in Horizontal Subsurface Flow Constructed Wetland. Agronomy 2021, 11, 1317. [Google Scholar] [CrossRef]
- Le, T.; Ngo, H.; Guo, W.; Vu, N.; Tran, T.; Nguyen, T.; Nguyen, X.; Nguyen, V.; Pham, T. Hybrid use of coal slag and calcined ferralsol as wetland substrate for improving phosphorus removal from wastewater. Chem. Eng. J. 2022, 428, 132124. [Google Scholar] [CrossRef]
- Han, C.; Wang, Z.; Yang, W.; Wu, Q.; Yang, H.; Xue, X. Effects of pH on phosphorus removal capacities of basic oxygen furnace slag. Ecol. Eng. 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.; Zhang, B.; He, Y.; Kirumba, G. Comparison of quartz sand, anthracite, shale and biological ceramsite for adsorptive removal of phosphorus from aqueous solution. J. Environ. Sci. 2014, 26, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef]
- Stocker, K.; Ellersdorfer, M. Phosphate Fixation and P Mineralogy on Natural and Ca-Modified Zeolites During Simultaneous Nutrient Removal. Water Air Soil Pollut. 2022, 233, 41. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Le, T.V.; Nguyen, B.T. Heavy metal pollution in surface water bodies in provincial Khanh Hoa, Vietnam: Pollution and human health risk assessment, source quantification, and implications for sustainable management and development. Environ. Pollut. 2024, 343, 123216. [Google Scholar] [CrossRef] [PubMed]
- Bavandpour, F.; Zou, Y.; He, Y.; Saeed, T.; Sun, Y.; Sun, G. Removal of dissolved metals in wetland columns filled with shell grits and plant biomass. Chem. Eng. J. 2018, 331, 234–241. [Google Scholar] [CrossRef]
- Hafeznezami, S.; Kim, J.-L.; Redman, J. Evaluating Removal Efficiency of Heavy Metals in Constructed Wetlands. J. Environ. Eng. 2012, 138, 475–482. [Google Scholar] [CrossRef]
- Šíma, J.; Svoboda, L.; Pomijová, Z. Removal of Selected Metals from Wastewater Using a Constructed Wetland. Chem. Biodivers. 2016, 13, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Dehmani, Y.; Mohammed, B.B.; Oukhrib, R.; Dehbi, A.; Lamhasni, T.; Brahmi, Y.; El-Kordy, A.; Franco, D.S.; Georgin, J.; Lima, E.C.; et al. Adsorption of various inorganic and organic pollutants by natural and synthetic zeolites: A critical review. Arab. J. Chem. 2024, 17, 105474. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.-S. Competitive adsorption of heavy metals onto modified biochars: Comparison of biochar properties and modification methods. J. Environ. Manag. 2021, 299, 113651. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-F.; Zhou, H.; Tan, W.-T.; Huang, J.-G.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Adsorption Characteristics and Mechanisms of Fe-Mn Oxide Modified Biochar for Pb(II) in Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 8420. [Google Scholar] [CrossRef] [PubMed]
- Otal, E.H.; Kim, M.L.; Dietrich, S.; Takada, R.; Nakaya, S.; Kimura, M. Open-Source Portable Device for the Determination of Fluoride in Drinking Water. ACS Sensors 2021, 6, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Styburski, D.; Baranowska-Bosiacka, I.; Goschorska, M.; Chlubek, D.; Gutowska, I. Beer as a Rich Source of Fluoride Delivered into the Body. Biol. Trace Element Res. 2016, 177, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.D.; Hu, X.J.; Shen, X.T.; Xie, H.J.; Hu, Z.; Zhang, J.; Liang, S. Can calcium-based constructed wetlands improve fluoride removal performance? Chem. Eng. J. 2022, 450, 138314. [Google Scholar] [CrossRef]
- Rickard, B.P.; Overchuk, M.; Tulino, J.; Tan, X.; Ligler, F.S.; Bae-Jump, V.L.; Fenton, S.E.; Rizvi, I. Exposure to select PFAS and PFAS mixtures alters response to platinum-based chemotherapy in endometrial cancer cell lines. Environ. Health 2023, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kang, Y.; Li, M.; Dong, J.; Wang, Y.; Xiao, J.; Guo, Z. Enhancement of perfluorooctanoic acid and perfluorooctane sulphonic acid removal in constructed wetland using iron mineral: Performance and mechanisms. J. Hazard. Mater. 2023, 447, 130819. [Google Scholar] [CrossRef] [PubMed]
- Srain, H.S.; Beazley, K.F.; Walker, T.R. Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms. Environ. Rev. 2021, 29, 142–181. [Google Scholar] [CrossRef]
- Gilroy, E.A.M.; Klinck, J.S.; Campbell, S.D.; McInnis, R.; Gillis, P.L.; de Solla, S.R. Toxicity and bioconcentration of the pharmaceuticals moxifloxacin, rosuvastatin, and drospirenone to the unionid mussel Lampsilis siliquoidea. Sci. Total Environ. 2014, 487, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Krogh, J.; Lyons, S.; Lowe, C.J. Pharmaceuticals and Personal Care Products in Municipal Wastewater and the Marine Receiving Environment Near Victoria Canada. Front. Mar. Sci. 2017, 4, 415. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.-J.; Liu, Y.-S.; Hu, L.-X.; He, L.-Y.; Zhao, J.-L.; Wang, T.-T.; Ying, G.-G. Fate and removal of antibiotics and antibiotic resistance genes in hybrid constructed wetlands. Environ. Pollut. 2019, 249, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Lu, S.; Zhao, B.; Wang, Z.; Xi, B.; Guo, W. Adsorption and biodegradation of sulfamethoxazole and ofloxacin on zeolite: Influence of particle diameter and redox potential. Chem. Eng. J. 2019, 384, 123346. [Google Scholar] [CrossRef]
- Rodriguez, E.; Campinas, M.; Acero, J.L.; Rosa, M.J. Investigating PPCP Removal from Wastewater by Powdered Activated Carbon/Ultrafiltration. Water Air Soil Pollut. 2016, 227, 177. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Hamid, A.K.; Krebsbach, S.A.; He, J.; Wang, D. Critical review of microplastics removal from the environment. Chemosphere 2022, 293, 133557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Jin, R.X.; Chen, Y.H.; Zhang, J.H.; Tao, S.Y.; Liu, S.W.; Shen, M.C. Constructed wetlands as neglected fixed source of microplastics and antibiotic resistance genes in natural water bodies? Sci. Total Environ. 2023, 902, 166474. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T.; Hu, H.; Ao, H.; Xiong, X.; Shi, H.; Wu, C. Transport and fate of microplastics in constructed wetlands: A microcosm study. J. Hazard. Mater. 2021, 415, 125615. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, T.; Huang, W.; Song, B.; Zeng, G.; Zhang, Y. Removal of microplastics from wastewater with aluminosilicate filter media and their surfactant-modified products: Performance, mechanism and utilization. Chem. Eng. J. 2021, 421, 129918. [Google Scholar] [CrossRef]
- Miranda, S.T.; de Matos, A.T.; de Matos, M.P.; Saraiva, C.B.; Teixeira, D.L. Influence of the substrate type and position of plant species on clogging and the hydrodynamics of constructed wetland systems. J. Water Process. Eng. 2019, 31, 100871. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Xu, J.; Sheng, L. Study on clogging mechanisms of constructed wetlands from the perspective of wastewater electrical conductivity change under different substrate conditions. J. Environ. Manag. 2021, 292, 112813. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, T.; Yi, W.; Zhang, T.; Zhang, Y. The release mechanism of heavy metals from lab-scale vertical flow constructed wetlands treating road runoff. Environ. Sci. Pollut. Res. 2019, 26, 16588–16595. [Google Scholar] [CrossRef] [PubMed]
- Ury, E.A.; Arrumugam, P.; Herbert, E.R.; Badiou, P.; Page, B.; Basu, N.B. Source or sink? Meta-analysis reveals diverging controls of phosphorus retention and release in restored and constructed wetlands. Environ. Res. Lett. 2023, 18, 083002. [Google Scholar] [CrossRef]
- Ávila, C.; Bayona, J.M.; Martín, I.; Salas, J.J.; García, J. Emerging organic contaminant removal in a full-scale hybrid constructed wetland system for wastewater treatment and reuse. Ecol. Eng. 2015, 80, 108–116. [Google Scholar] [CrossRef]
- Jehawi, O.H.; Abdullah, S.R.S.; Kurniawan, S.B.; Ismail, N.; Idris, M.; Al Sbani, N.H.; Muhamad, M.H.; Abu Hasan, H. Performance of pilot Hybrid Reed Bed constructed wetland with aeration system on nutrient removal for domestic wastewater treatment. Environ. Technol. Innov. 2020, 19, 100891. [Google Scholar] [CrossRef]
- Sharma, P.K.; Rausa, K.; Rani, A.; Mukherjee, S.; Kumar, M. Biopurification of dairy farm wastewater through hybrid constructed wetland system: Groundwater quality and health implications. Environ. Res. 2021, 200, 111426. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, J.; Wang, H.; Wang, X. Study of the effect of pyrite and alkali-modified rice husk substrates on enhancing nitrogen and phosphorus removals in constructed wetlands. Environ. Sci. Pollut. Res. 2022, 29, 54234–54249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, P.; Patil, S.A.; Yadav, A.K. A comprehensive review on emerging constructed wetland coupled microbial fuel cell technology: Potential applications and challenges. Bioresour. Technol. 2021, 320, 124376. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-C.; Wang, Y.-H.; Lu, Y.-C. Treatment of polluted water for reclamation using photocatalysis and constructed wetlands. Catal. Today 2011, 175, 276–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Lin, Y.; Sun, L.; Guo, X.; Yang, S.; He, J. Enhanced Swine Wastewater Treatment by Constructed Wetland—Microbial Fuel Cell Systems. Water 2022, 14, 3930. [Google Scholar] [CrossRef]
- Xiao, E.-R.; Liang, W.; He, F.; Cheng, S.-P.; Wu, Z.-B. Performance of the combined SMBR–IVCW system for wastewater treatment. Desalination 2010, 250, 781–786. [Google Scholar] [CrossRef]
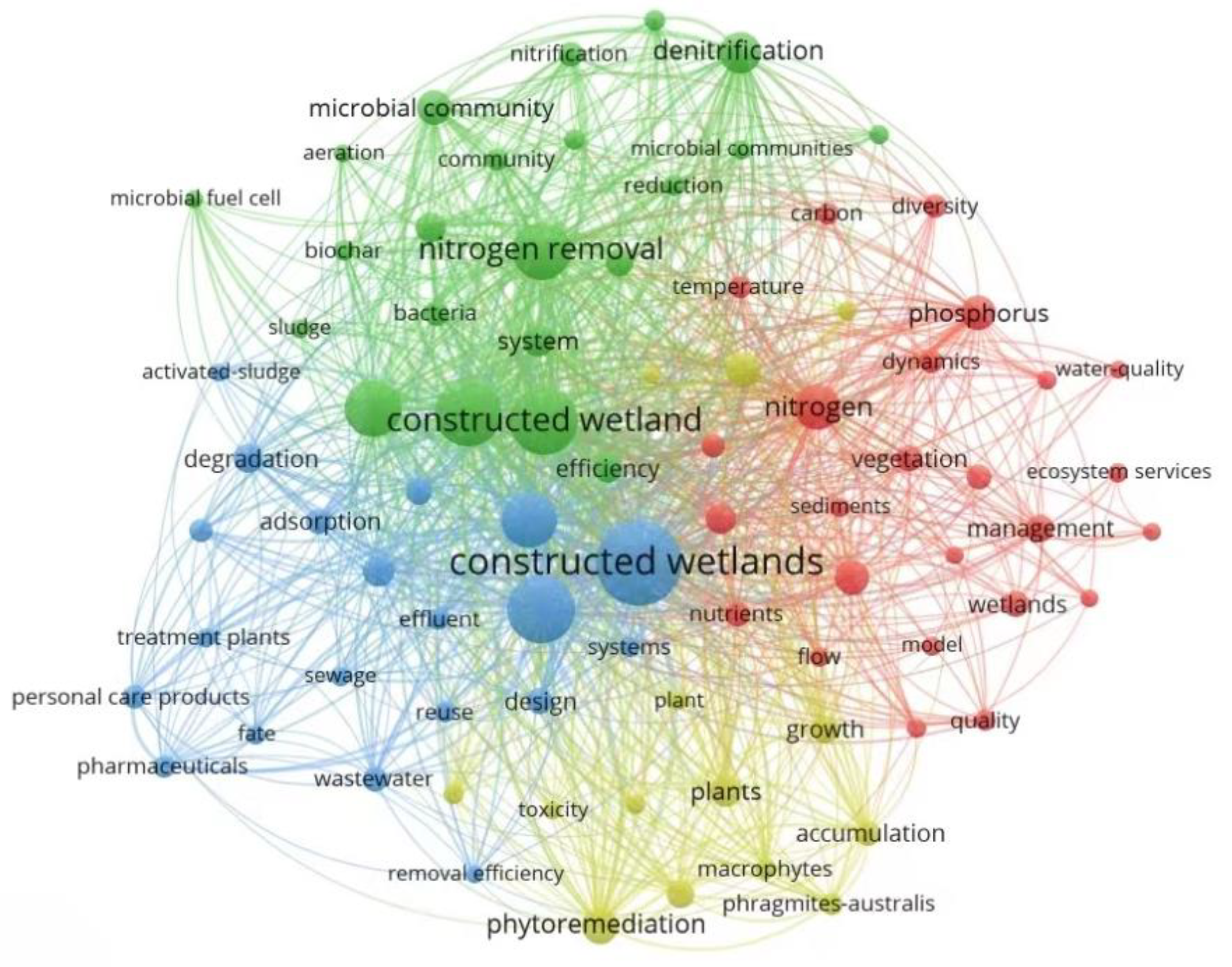
| Sort | Keywords | Frequency |
|---|---|---|
| 1 | Constructed wetlands | 1546 |
| 2 | Constructed wetland | 1029 |
| 3 | Removal | 1019 |
| 4 | Performance | 993 |
| 5 | Nitrogen removal | 796 |
| 6 | Waste-water treatment | 772 |
| 7 | Waste-water | 712 |
| 8 | Nitrogen | 530 |
| 9 | Denitrification | 446 |
| 10 | Phytoremediation | 350 |
| 11 | Nutrient removal | 333 |
| 12 | Phosphorus | 331 |
| Contaminants Type | COD | N | P | Heavy Metals | Fluoride | PPCPs | Microplastics |
|---|---|---|---|---|---|---|---|
| Gravel | + | + | ++ | ++ | - | + | + |
| Sand | + | + | + | + | - | ++ | ++ |
| Volcanics | + | ++ | + | + | ++ | - | - |
| Zeolite | ++ | +++ | + | ++ | ++ | - | ++ |
| Fly ash | + | ++ | +++ | - | + | - | - |
| Steel slag | ++ | ++ | ++ | - | - | - | - |
| Construction solid waste | + | ++ | +++ | - | - | - | - |
| Sludge | ++ | + | +++ | - | - | - | - |
| Iron scraps | + | +++ | +++ | + | - | - | - |
| Woodchip | +++ | ++ | +++ | - | - | - | |
| Crushed glass | - | - | - | - | - | ++ | - |
| Ceramsite | +++ | + | +++ | ++ | ++ | + | - |
| Activated carbon | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Biochar | +++ | +++ | +++ | +++ | ++ | ++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ma, L.; Wang, J.; Zhao, X.; Jing, Y.; Liu, C.; Xiao, Y.; Li, C.; Jiao, C.; Xu, M. Research Progress on the Removal of Contaminants from Wastewater by Constructed Wetland Substrate: A Review. Water 2024, 16, 1848. https://doi.org/10.3390/w16131848
Wang L, Ma L, Wang J, Zhao X, Jing Y, Liu C, Xiao Y, Li C, Jiao C, Xu M. Research Progress on the Removal of Contaminants from Wastewater by Constructed Wetland Substrate: A Review. Water. 2024; 16(13):1848. https://doi.org/10.3390/w16131848
Chicago/Turabian StyleWang, Liyan, Leihui Ma, Junke Wang, Xia Zhao, Yushu Jing, Changqing Liu, Yihua Xiao, Cang Li, Chen Jiao, and Mengchen Xu. 2024. "Research Progress on the Removal of Contaminants from Wastewater by Constructed Wetland Substrate: A Review" Water 16, no. 13: 1848. https://doi.org/10.3390/w16131848
APA StyleWang, L., Ma, L., Wang, J., Zhao, X., Jing, Y., Liu, C., Xiao, Y., Li, C., Jiao, C., & Xu, M. (2024). Research Progress on the Removal of Contaminants from Wastewater by Constructed Wetland Substrate: A Review. Water, 16(13), 1848. https://doi.org/10.3390/w16131848







