The Status of Research on the Root Exudates of Submerged Plants and Their Effects on Aquatic Organisms
Abstract
:1. Introduction
2. Submerged Plant Exudates
2.1. Definition and Classification
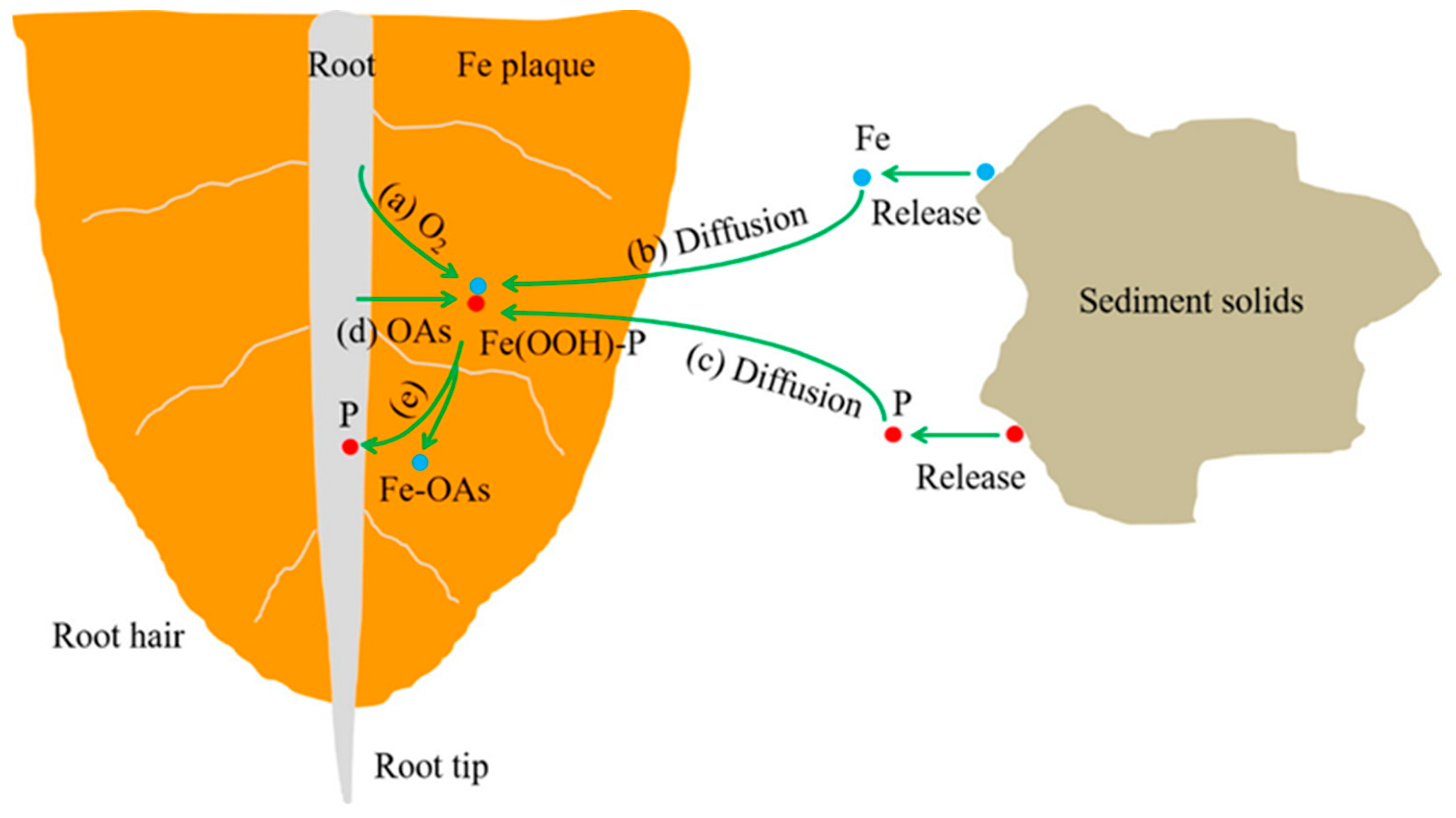
2.2. Production Pathway and Mechanism
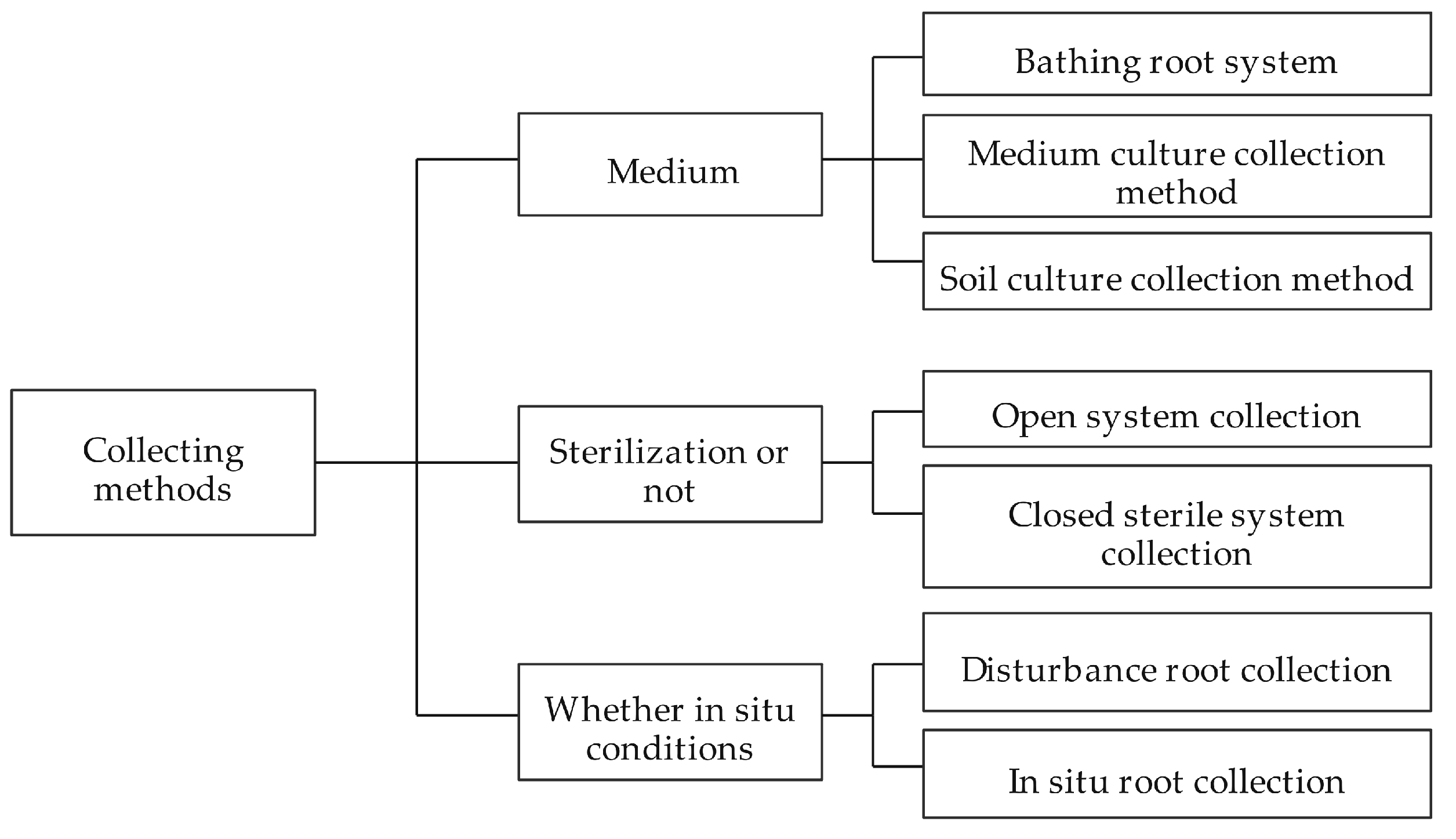
2.3. Collection and Analysis
3. Influencing Factors of Root Exudates
3.1. Plant Species and Growth Stages
3.2. Environmental Factors
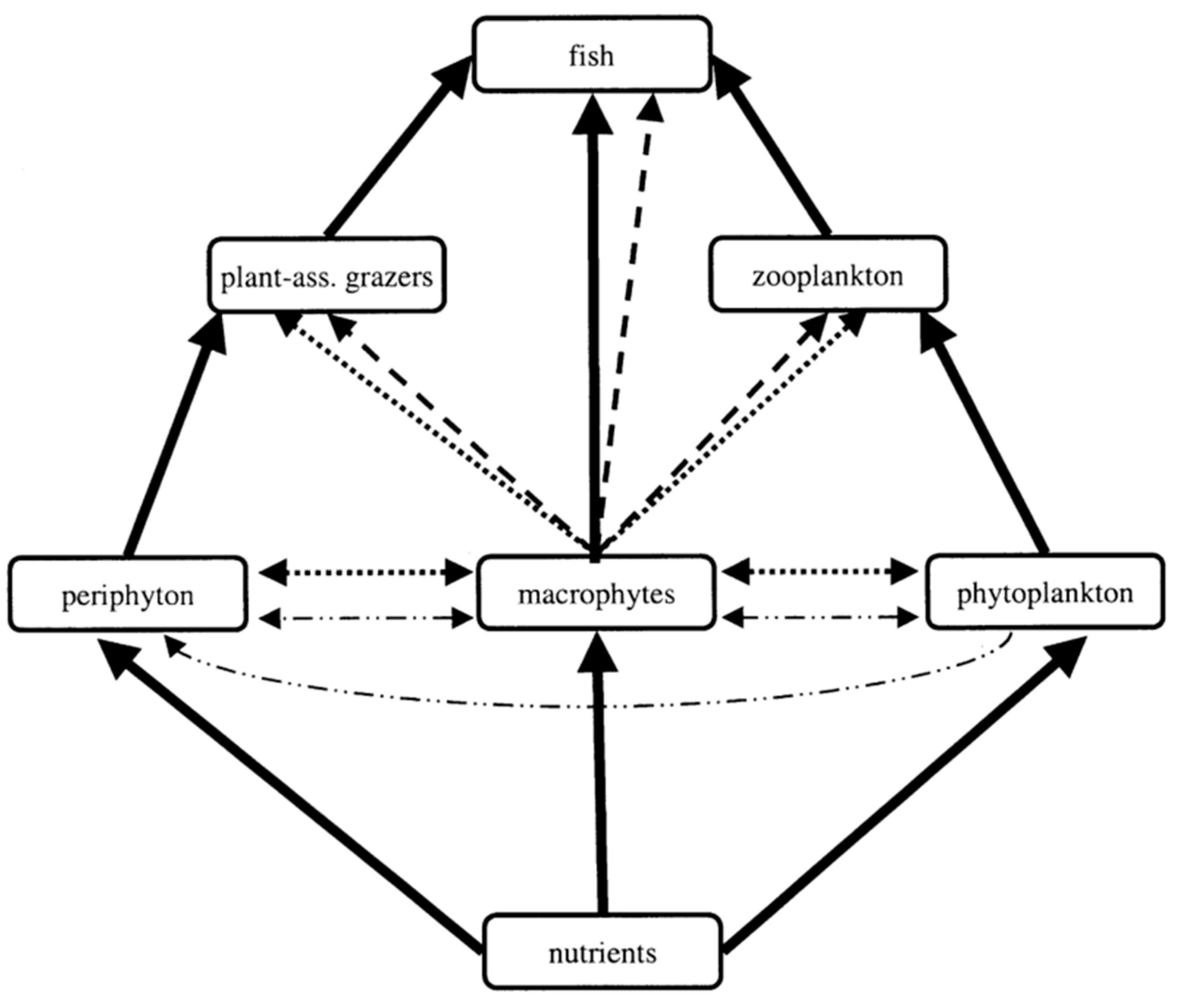
4. Effects of Root Exudates on Aquatic Organisms
4.1. Effects on Phytoplankton
4.2. Allelopathy on Zooplankton
4.3. Effects on Microorganisms
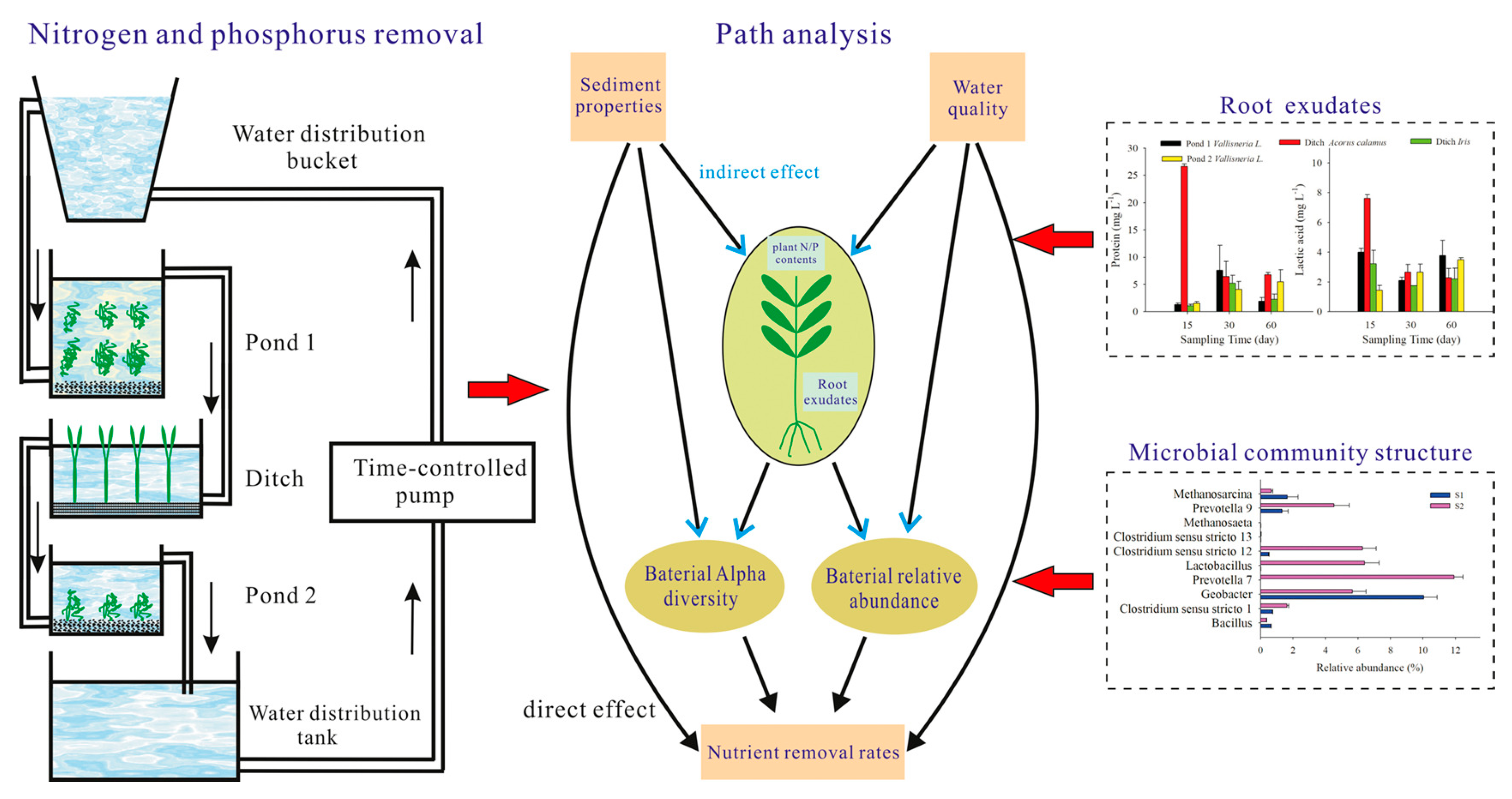
4.4. Possibility of Using Root Exudates of Submerged Plants for Water Restoration
5. Conclusions
6. Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| MC | Microcystin |
| PGPR | Plant-growth-promoting rhizobacteria |
| ABC | ATP-binding cassette |
| MATE | Multidrug and toxic compound extrusion |
| MFS | Major facilitator superfamily |
| ALMT | Aluminum-activated malate transporter |
| GC | Gas Chromatography |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| UPLC | Ultra Performance Liquid Chromatography |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| APCI-MS | Atmosphere Pressure Chemical Ionization Mass Spectrometry |
| UV-B | Ultraviolet B |
| DOC | Dissolved organic carbon |
| DOM | Dissolved organic matter |
| PS | Photosynthesis System |
| NIRS | Nuclear Information and Resource Service |
References
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil. 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Curl, E.A.; Truelove, B. The Rhizosphere; Springer: Berlin/Heidelberg, Germany; Springer: New York, NY, USA; Springer: Tokyo, Japan, 1986. [Google Scholar]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends. Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef]
- Hu, M.; Ma, R.; Xue, K.; Cao, Z.; Xiong, J.; Loiselle, S.A.; Shen, M.; Hou, X. Eutrophication evolution of lakes in China: Four decades of observations from space. J. Hazard. Mater. 2024, 470, 134225. [Google Scholar] [CrossRef]
- Ceng, P. Study on the Preparation and Application of High Efficiency Phosphorus Removing Flocculant. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2011. [Google Scholar]
- Wang, X.; Song, W.; Li, N.; Lu, J.; Niu, X.; Ma, Y.; Ding, J.; Wang, M. Ultraviolet-B radiation of Haematococcus pluvialis for enhanced biological contact oxidation pretreatment of black odorous water in the symbiotic system of algae and bacteria. Biochem. Eng. J. 2020, 157, 107553. [Google Scholar] [CrossRef]
- Yu, S.; Miao, C.; Song, H.; Huang, Y.; Chen, W.; He, X. Efficiency of nitrogen and phosphorus removal by six macrophytes from eutrophic water. Int. J. Phytoremediat. 2019, 21, 643–651. [Google Scholar] [CrossRef]
- Dinakar, C.; Djilianov, D.; Bartels, D. Photosynthesis in desiccation tolerant plants: Energy metabolism and antioxidative stress defense. Plant Sci. 2012, 182, 29–41. [Google Scholar] [CrossRef]
- Long, M.H.; McGlathery, K.J.; Zieman, J.C.; Berg, P. The role of organic acid exudates in liberating phosphorus from seagrass-vegetated carbonate sediments. Limnol. Oceanogr. 2008, 53, 2616–2626. [Google Scholar] [CrossRef]
- Xu, R.; Wu, F.; Hilt, S.; Wu, C.; Wang, X.; Chang, X. Recovery limitation of endangered Ottelia acuminata by allelopathic interaction with cyanobacteria. Aquat. Ecol. 2015, 49, 333–342. [Google Scholar] [CrossRef]
- Tamire, G.; Mengistou, S.; Degefe, G. Potential allelopathic impact of Potamogeton schweinfurthii on phytoplankton in Lake Ziway, Ethiopia. Inland Waters 2016, 6, 336–342. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Li, Y.; Li, Q.; Yan, W.; Zhang, Y.; Wu, Z.; Zhou, Q. Plant growth-promoting rhizobacteria isolation from rhizosphere of submerged macrophytes and their growth-promoting effect on Vallisneria natans under high sediment organic matter load. Microb. Biotechnol. 2021, 14, 726–736. [Google Scholar] [CrossRef]
- Myrold, D.D. The rhizosphere: Biochemistry and organic substances at the soil-plant interface, second edition. Soil Sci. Soc. Am. J. 2008, 72, 339–353. [Google Scholar] [CrossRef]
- Rovira, A.D. Plant root exudates. Bot. Rev. 1969, 35, 35–57. [Google Scholar] [CrossRef]
- Meng, H.; Yan, Z.; Li, X. Effects of exogenous organic acids and flooding on root exudates, rhizosphere bacterial community structure, and iron plaque formation in Kandelia obovata seedlings. Sci. Total Environ. 2022, 830, 154695. [Google Scholar] [CrossRef]
- Dietz, S.; Herz, K.; Doell, S.; Haider, S.; Jandt, U.; Bruelheide, H.; Scheel, D. Semi-polar root exudates in natural grassland communities. Ecol. Evol. 2019, 9, 5526–5541. [Google Scholar] [CrossRef]
- Haichar, F.E.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot. 2012, 63, 3445–3454. [Google Scholar] [CrossRef]
- Selmar, D.; Abouzeid, S.; Radwan, A.; Hijazin, T.; Kleinwchter, M. Horizontal Natural Product Transfer: A Novel Attribution in Allelopathy. In Co-Evolution of Secondary Metabolites, 3rd ed.; Springer: Cham, Switzerland, 2020; pp. 429–439. [Google Scholar]
- Hijazin, T.; Lewerenz, L.; Yahyazadeh, M.; Selmar, D. Horizontal Natural Product Transfer: A Phenomenon Which Is Responsible for the Widespread Alkaloidal Contaminations of Herbal Products. In Environmental Challenges and Medicinal Plants, 3rd ed.; Springer: Cham, Switzerland, 2022; pp. 183–201. [Google Scholar]
- Lewerenz, L.; Hijazin, T.; Abouzeid, S.; Haensch, R.; Selmar, D. Pilot study on the uptake and modification of harmaline in acceptor plants: An innovative approach to visualize the interspecific transfer of natural products. Phytochemistry 2020, 174, 112362. [Google Scholar] [CrossRef]
- Hijazin, T.; Radwan, A.; Lewerenz, L.; Abouzeid, S.; Selmar, D. The uptake of alkaloids by plants from the soil is determined by rhizosphere pH. Rhizosphere 2020, 15, 100234. [Google Scholar] [CrossRef]
- Tu, S.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant Soil. 2004, 258, 9–19. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Timergalina, L.N.; Arkhipova, T.N.; Shendel, G.V.; Kuz’mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Bioch. 2014, 83, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Pierre, P.; Alex, W.; Anne, C.; Alexander, E.M.; Stephen, A.R.; Jurriaan, T. Metabolite profiling of non-sterile rhizosphere soil. Plant J. 2017, 92, 147–162. [Google Scholar]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Herz, K.; Dietz, S.; Gorzolka, K.; Haider, S.; Jandt, U.; Scheel, D.; Bruelheide, H. Linking root exudates to functional plant traits. PLoS ONE 2019, 14, e0204128. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.E.; Matamoros, V. Analytical challenges and solutions for performing metabolomic analysis of root exudates. Trends Environ. Anal. Chem. 2021, 31, e00130. [Google Scholar] [CrossRef]
- Robinson, M.D.; De Souza, D.P.; Keen, W.W.; Saunders, E.C.; McConville, M.J.; Speed, T.P.; Likic, V.A. A dynamic programming approach for the alignment of signal peaks in multiple gas chromatography-mass spectrometry experiments. BMC Bioinform. 2007, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Song, Q.; Liu, Y.; Li, J.; Wan, J.B.; Wang, Y.; Jiang, Y.; Tu, P. Integrated work-flow for quantitative metabolome profiling of plants, Peucedani Radix as a case. Anal. Chim. Acta 2017, 953, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, F.; Lai, L.; Gika, H.; Theodoridis, G.; Wilson, I. UPLC-MS-based analysis of human plasma for metabonomics using solvent precipitation or solid phase extraction. J. Proteome Res. 2009, 8, 2114–2121. [Google Scholar] [CrossRef]
- Plumb, R.S.; Johnson, K.A.; Rainville, P.; Smith, B.W.; Wilson, I.D.; Castro-Perez, J.M.; Nicholson, J.K. UPLC/MSE; a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Commun. Mass Spectrom. 2006, 20, 1989–1994. [Google Scholar] [CrossRef]
- Zheng, X.B.; Liu, S.H.; Wen, B.Y.; Peng, W.; Li, H.M.; Kathiresan, M.; Zhang, Y.J.; Jin, S.Z.; Li, J.F. Rapid detection and whole class control of quinolone antibiotics in pork based on surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2023, 54, 468–476. [Google Scholar] [CrossRef]
- Oledzka, I.; Baczek, T. Urinary steroids measured by modern separation techniques and applied as biomarkers in stress studies. Curr. Pharm. Anal. 2010, 6, 151–163. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, X.X.; Wu, F.; Liu, J.Y.; Zheng, G.L. Studies on allelopathy of aquatic macrophytes on Synahocystis sp. J. Yunnan Univ. 2008, 30, 535–540. [Google Scholar]
- Zhang, S.H.; Sun, P.S.; Ge, F.J.; Wu, Z.B. Different sensitivities of Selenastrum capricornutum and toxic strain Microcystis aeruginosa to exudates from two Potamogeton species. Pol. J. Environ. Stud. 2011, 20, 1359–1366. [Google Scholar]
- Xing, X.; Ding, S.; Liu, L.; Chen, M.; Yan, W.; Zhao, L.; Zhang, C. Direct evidence for the enhanced acquisition of phosphorus in the rhizosphere of aquatic plants: A case study on Vallisneria natans. Sci. Total Environ. 2018, 616, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F.M.; Sim, L.; Bearda, J.; Davis, J. Allelopathic inhibition of microalgae by the freshwater stonewort, Chara australis, and a. submerged angiosperm, Potamogeton crispus. Aquat. Bot. 2013, 110, 24–30. [Google Scholar] [CrossRef]
- Mulderij, G.; Van, D.E.; Roelofs, J.G.M. Differential sensitivity of green algae to allelopathic substances from Chara. Hydrobiologia 2003, 491, 261–271. [Google Scholar] [CrossRef]
- Wang, W.; Jia, T.; Qi, T.; Li, S.; Degen, A.A.; Han, J.; Bai, Y.; Zhang, T.; Qi, S.; Huang, M.; et al. Root exudates enhanced rhizobacteria complexity and microbial carbon metabolism of toxic plants. iScience 2022, 25, 105243. [Google Scholar] [CrossRef]
- Gross, E.M. Differential response of tellimagrandin II and total bioactive hydrolysable tannins in an aquatic angiosperm to changes in light and nitrogen. Oikos 2003, 103, 497–504. [Google Scholar] [CrossRef]
- Erhard, D.; Gross, E.M. Do environmental factors influence composition of potential allelochemicals in the submersed freshwater macrophyte Elodea nuttallii (Hydrocharitaceae)? Int. Assoc. Theor. Appl. Limnoloy 2005, 29, 287–291. [Google Scholar] [CrossRef]
- Martin, B.C.; Statton, J.; Siebers, A.R.; Grierson, P.F.; Ryan, M.H.; Kendrick, G.A. Colonizing tropical seagrasses increase root exudation under fluctuating and continuous low light. Limnol. Oceanogr. 2018, 63, S381–S391. [Google Scholar] [CrossRef]
- Bressan, M.; Roncato, M.A.; Bellvert, F.; Comte, G.; Haichar, F.E.Z.; Achouak, W.; Berge, O. Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME J. 2009, 3, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.Y.; Wyatt, K.H. Light availability regulates the response of algae and heterotrophic bacteria to elevated nutrient levels and warming in a northern boreal peatland. Freshw. Biol. 2016, 61, 1442–1453. [Google Scholar] [CrossRef]
- Gu, S.; Qian, Y.; Jiao, Y.; Li, Q.; Pinay, G.; Gruau, G. An innovative approach for sequential extraction of phosphorus in sediments: Ferrous iron P as an independent P fraction. Water Res. 2016, 103, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.M.; Meyer, H.; Schilling, G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry 1996, 41, 133–138. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere. A critical review. Plant Soil. 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Taghipour, M.; Jalali, M. Effect of low-molecular-weight organic acids on kinetics release and fractionation of phosphorus in some calcareous soils of western Iran. Environ. Monit. Assess. 2013, 185, 5471–5482. [Google Scholar] [CrossRef] [PubMed]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and Their Various Applications. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Filzgerald, G.P. Some factors in the competition or antagonism among bacteria, algae, and aquatic weeds. J. Phycol. 1969, 5, 351–359. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M.; Hupfer, M.; Morscheid, H.; Maehlmann, J.; Melzer, A.; Poltz, J.; Sandrock, S.; Scharf, E.M.; Schneider, S.; et al. Restoration of submerged vegetation in shallow eutrophic lakes—A guideline and state of the art in Germany. Limnologica 2006, 36, 155–171. [Google Scholar] [CrossRef]
- Li, B.; Yin, Y.; Kang, L.; Feng, L.; Liu, Y.; Du, Z.; Tian, Y.; Zhang, L. A review: Application of allelochemicals in water ecological restoration--algal inhibition. Chemosphere 2021, 267, 128869. [Google Scholar] [CrossRef] [PubMed]
- Declerck, S.; Vanderstukken, M.; Pals, A.; Muylaert, K.; de Meester, L. Plankton biodiversity along a gradient of productivity and its mediation by macrophytes. Ecology 2007, 88, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Svanys, A.; Paskauskas, R.; Hilt, S. Effects of the allelopathically active macrophyte Myriophyllum spicatum on a natural phytoplankton community: A mesocosm study. Hydrobiologia 2014, 737, 57–66. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, A.; Zhang, S.; Cheng, S.; Wu, X. Short-term effects of drawing water for connectivity of rivers and lakes on zooplankton community structure. J. Environ. Sci. 2008, 20, 419–423. [Google Scholar] [CrossRef]
- Hilt, S.; Ghobrial, M.G.N.; Gross, E.M. In situ allelopathic potential of Myriophyllum verticillatum (Haloragaceae) against selected phytoplankton species. J. Phycol. 2006, 42, 1189–1198. [Google Scholar] [CrossRef]
- Rojo, C.; Segura, M.; Rodrigo, M.A. The allelopathic capacity of submerged macrophytes shapes the microalgal assemblages from a recently restored coastal wetland. Ecol. Eng. 2013, 58, 149–155. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef] [PubMed]
- Leu, E.; Krieger-Liszkay, A.; Goussias, C.; Gross, E.M. Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum inhibit photosystem II. Plant Physiol. 2002, 130, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef]
- Van Donk, E.; van de Bund, W.J. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Cerbin, S.; van Donk, E.; Gulati, R.D. The influence of Myriophyllum verticillatum and artificial plants on some life history parameters of Daphnia magna. Aquat. Ecol. 2007, 41, 263–271. [Google Scholar] [CrossRef]
- Espinosa-Rodriguez, C.A.; Rivera-De la Parra, L.; Martinez-Tellez, A.; Gomez-Cabral, G.C.; Sarma, S.S.S.; Nandini, S. Allelopathic interactions between the macrophyte Egeria densa and plankton (alga, Scenedesmus acutus and cladocerans, Simocephalus spp.): A laboratory study. J. Limnol. 2016, 75, 151–160. [Google Scholar] [CrossRef]
- Espinosa-Rodriguez, C.A.; Sarma, S.S.S.; Nandini, S. Effect of the allelochemicals from the macrophyte Egeria densa on the competitive interactions of pelagic and littoral cladocerans. Chem. Ecol. 2017, 33, 247–256. [Google Scholar] [CrossRef]
- Wolters, J.W.; Reitsema, R.E.; Verdonschot, R.C.M.; Schoelynck, J.; Verdonschot, P.F.M.; Meire, P. Macrophyte-specific effects on epiphyton quality and quantity and resulting effects on grazing macroinvertebrates. Freshwater. Biol. 2019, 64, 1131–1142. [Google Scholar] [CrossRef]
- Bai, G.; Liu, Y.; Liu, Z.; Kong, L.; Tang, Y.; Ding, Z.; Zou, Y.; Wang, C.; Zhang, C.; Chen, D.; et al. Effects of lake geo-engineering on plankton in a typical shallow urban lake: Evidence based on 10-year data. ACS EST Eng. 2022, 3, 105–120. [Google Scholar] [CrossRef]
- Vanderstukken, M.; Declerck, S.A.J.; Decaestecker, E.; Muylaert, K. Long-term allelopathic control of phytoplankton by the submerged macrophyte Elodea nuttallii. Freshwater Biol. 2014, 59, 930–941. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Williams, A.; Stringer, F.; Willcocks, R.; McEwing, R.; Langridge, H.; Straathof, A.L. Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 2019, 224, 132–145. [Google Scholar] [CrossRef]
- Landi, L.; Valori, F.; Ascher, J.; Renella, G.; Falchini, L.; Nannipieri, P. Root exudate effects on the bacterial communities, CO2 evolution, nitrogen transformations and ATP content of rhizosphere and bulk soils. Soil Biol. Biochem. 2006, 38, 509–516. [Google Scholar] [CrossRef]
- Yin, X.; Lu, J.; Wang, Y.; Liu, G.; Hua, Y.; Wan, X.; Zhao, J.; Zhu, D. The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere 2020, 240, 124903. [Google Scholar] [CrossRef]
- Ma, L.; Yang, L.; Liu, W.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. Effects of root exudates on rhizosphere bacteria and nutrient removal in pond-ditch circulation systems (PDCSs) for rural wastewater treatment. Sci. Total Environ. 2021, 785, 147282. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.C.; Gleeson, D.; Statton, J.; Siebers, A.R.; Grierson, P.; Ryan, M.H.; Kendrick, G.A. Low light availability alters root exudation and reduces putative beneficial microorganisms in seagrass roots. Front. Microbiol. 2018, 8, 2667. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, M.; Gu, X.; Yan, P.; He, S.; Chachar, A. New insight and enhancement mechanisms for Feammox process by electron shuttles in wastewater treatment-A systematic review. Bioresour. Technol. 2023, 369, 128495. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dong, J.; Fu, Q.; Wang, Y.; Chen, C.; Li, J.; Li, R.; Zhou, C. Allelopathic effects of submerged macrophytes on phytoplankton. Allelopath. J. 2017, 40, 1–22. [Google Scholar] [CrossRef]
- Eigemann, F. Allelopathic Effects of Submerged Macrophytes on Phytoplankton: Determining the Factors of Phytoplankton Sensitivity and Detection of New Modes of Action. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2013; pp. 77–100. [Google Scholar]
- Peng, X.; Lin, Q.; Liu, B.; Huang, S.; Yan, W.; Zhang, L.; Ge, F.; Zhang, Y.; Wu, Z. Effect of submerged plant coverage on phytoplankton community dynamics and photosynthetic activity in situ. J. Environ. Manag. 2022, 301, 113822. [Google Scholar] [CrossRef]
- Müller, N.; Hempel, M.; Philipp, B.; Gross, E.M. Degradation of gallic acid and hydrolysable polyphenols is constitutively activated in the freshwater plant-associated bacterium Matsuebacter sp. FB25. Aquat. Microb. Ecol. 2007, 47, 83–90. [Google Scholar] [CrossRef]





| Class | Representative Compounds | Major Functions |
|---|---|---|
| Saccharide | Glucose, fructose, galactose, rhamnose, ribose, raffinose, xylose, sucrose, lactose, maltose, and arabinose | Promoting rhizosphere microbial growth, regulating soil properties, and affecting rhizosphere microbial community structures |
| Organic acids | Oxalic acid, tartaric acid, pyruvic acid, malic acid, malonic acid, lactic acid, catalpol, succinic acid, fumaric acid, formic acid, acetic acid, propionic acid, butyric acid, valeric acid, and salicylic acid | Changing the soil's pH value, activating soil nutrients, and improving nutrient absorption by plants |
| Amino acid | Aspartic acid, threonine, serine, glutamic acid, glycine, alanine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, γ-aminobutyric acid, lysine, histidine, arginine, aspartic acid, threonine, serine, glutamic acid, glycine, alanine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, γ-aminobutyric acid, lysine, histidine, arginine, and proline | Promoting plant growth and development, improving plant stress resistance, and regulating the soil's microbial community |
| Long-chain fatty acid | Stearic acid, palmitic acid, oleic acid, and linoleic acid | Promoting plant defense against foliar pathogens, enhancing plant resilience, regulating plant–microbial interactions, and acting as a nutrient source for microorganisms |
| Steroid | Cholesterol and stigmasterol | Acting as nutrient sources for microorganisms and enhancing the growth potential and stress resistance of plants |
| Growth hormone | Biotin, vitamin, choline, inositol, and phytohormone | Promoting cell growth, differentiation, division, and biosynthesis |
| Proteins and enzymes | Amylase, DNA enzyme, phosphatase, polygalacturonase, protease, RNA enzyme, invertase, urease, xylanase, PR protein, etc. | Promoting the absorption and conversion of nutrients and catalyzing the degradation of organic pollutants |
| Other compounds | Flavonoids, nucleosides, glycosides, and polysaccharides | Genetic information transfer, energy storage and conversion, signal transduction, and storage and transport of substances |
| Analysis and Test Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| GC | Analysis of the substances with a low boiling point, good thermal stability, high volatility, and stable retention time, which can directly identify the structure | Unsuitable for analyzing some substances that need pretreatment with a high boiling point and poor thermal stability via direct injection | [29,30] |
| GC-MS | Accurate characterization of the substances with a large database | Insufficient software for analyzing data | [31] |
| LC-MS | A wide range of analysis, strong separation ability, low detection limit, and high degree of automation | Lack of a standard database to identify the structure | [32] |
| UPLC | Fast analysis speed, short time, and high separation efficiency | Short service life of the chromatographic column and demanding laboratory conditions | [33,34,35] |
| HPLC | High separation efficiency, good selectivity, high detection sensitivity, automatic operation, and wide application range | High operating cost and long analysis time | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, X.; Zhao, M.; Zheng, X.; Gu, J.; Wang, Z.; Fan, C.; Gu, W. The Status of Research on the Root Exudates of Submerged Plants and Their Effects on Aquatic Organisms. Water 2024, 16, 1920. https://doi.org/10.3390/w16131920
Shi Y, Zhang X, Zhao M, Zheng X, Gu J, Wang Z, Fan C, Gu W. The Status of Research on the Root Exudates of Submerged Plants and Their Effects on Aquatic Organisms. Water. 2024; 16(13):1920. https://doi.org/10.3390/w16131920
Chicago/Turabian StyleShi, Yahan, Xu Zhang, Min Zhao, Xiangyong Zheng, Jianya Gu, Zhiquan Wang, Chunzhen Fan, and Wenwen Gu. 2024. "The Status of Research on the Root Exudates of Submerged Plants and Their Effects on Aquatic Organisms" Water 16, no. 13: 1920. https://doi.org/10.3390/w16131920
APA StyleShi, Y., Zhang, X., Zhao, M., Zheng, X., Gu, J., Wang, Z., Fan, C., & Gu, W. (2024). The Status of Research on the Root Exudates of Submerged Plants and Their Effects on Aquatic Organisms. Water, 16(13), 1920. https://doi.org/10.3390/w16131920






