Heavy Metal Concentrations in Wild and Cultured Oplegnathus fasciatus from the East China Sea and Associated Health Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Metal Content
2.3. Evaluation of Heavy Metal Pollution Levels in Samples
2.4. Health Risk Assessment of Heavy Metal Exposure
2.4.1. Estimated Daily Intake (EDI)
2.4.2. Non-Carcinogenic Risk Evaluation
2.5. The Stepwise Discriminant Analysis
2.6. Statistical Analysis
3. Results
3.1. Analysis of Heavy Metal Concentrations in Wild and Cultured Oplegnathus fasciatus
3.2. Assessment of Heavy Metal Pollution Levels in Oplegnathus fasciatus
3.3. Evaluation of Health Risks
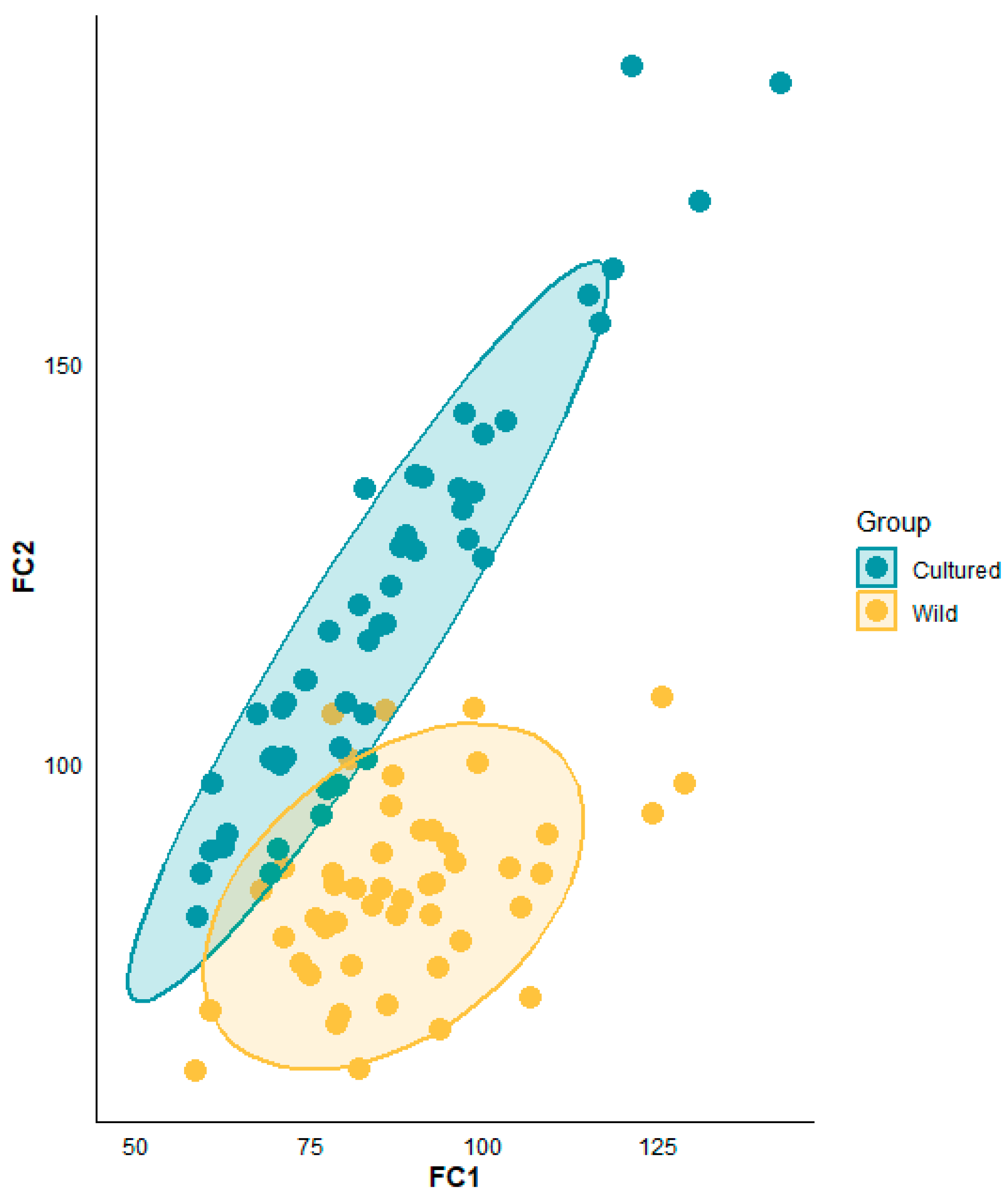
3.4. Discriminant Analysis of Wild and Cultured Oplegnathus fasciatus
4. Discussion
4.1. Heavy Metal Accumulation in Wild and Cultured Oplegnathus fasciatus
4.2. Heavy Metal Concentrations and Safety Evaluation of Wild and Cultured Oplegnathus fasciatus
4.3. Heavy Metals for Distinguishing Wild and Cultured Oplegnathus fasciatus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Wang, S.; Shen, X.; Jiang, M. Ecological risk assessment of heavy metal pollution in the water of China’s coastal shellfish culture areas. Environ. Sci. Pollut. Res. 2020, 27, 18392–18402. [Google Scholar] [CrossRef]
- Li, M.; Bao, K.; Wang, H.; Dai, Y.; Wu, S.; Yan, K.; Liu, S.; Yuan, Q.; Lu, J. Distribution and Ecological Risk Assessment of Nutrients and Heavy Metals in the Coastal Zone of Yantai, China. Water 2024, 16, 760. [Google Scholar] [CrossRef]
- Omar, W.A.; Zaghloul, K.H.; Abdel-Khalek, A.A.; Abo-Hegab, S. Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch. Environ. Contam. Toxicol. 2013, 65, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Emenike, E.C.; Iwuozor, K.O.; Anidiobi, S.U. Heavy metal pollution in aquaculture: Sources, impacts and mitigation techniques. Biol. Trace Elem. Res. 2022, 200, 4476–4492. [Google Scholar] [CrossRef] [PubMed]
- Sauliutė, G.; Svecevičius, G. Heavy metal interactions during accumulation via direct route in fish: A review. Zool. Ecol. 2015, 25, 77–86. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Monroy, M.; de Sostoa, A. Metal bioaccumulation in the Mediterranean barbel (Barbus meridionalis) in a Mediterranean River receiving effluents from urban and industrial wastewater treatment plants. Ecotoxicol. Environ. Saf. 2012, 76, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Losada, V.; Prego, R. Distribution of lipids and trace minerals in different muscle sites of farmed and wild turbot (Psetta maxima). Int. J. Food Sci. Technol. 2007, 42, 1456–1464. [Google Scholar] [CrossRef]
- FLallah, A.A.; Siavash Saei-Dehkordi, S.; Nematollahi, A. Comparative assessment of proximate composition, physicochemical parameters, fatty acid profile and mineral content in farmed and wild rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Technol. 2011, 46, 767–773. [Google Scholar] [CrossRef]
- López-Mas, L.; Claret, A.; Reinders, M.J.; Banovic, M.; Krystallis, A.; Guerrero, L. Farmed or wild fish? Segmenting European consumers based on their beliefs. Aquaculture 2021, 532, 735992. [Google Scholar] [CrossRef]
- Chanpiwat, P.; Sthiannopkao, S.; Widmer, K.; Himeno, S.; Miyataka, H.; Vu, N.U.; Tran, V.V. Assessment of metal and bacterial contamination in cultivated fish and impact on human health for residents living in the Mekong Delta. Chemosphere 2016, 163, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Viscardi, V.; Fasano, E.; Farina, A.; Amodio-Cocchieri, R. Polychlorinated biphenyls, organochlorine pesticides, and polycyclic aromatic hydrocarbons in wild, farmed, and frozen marine seafood marketed in Campania, Italy. J. Food Prot. 2009, 72, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- GB 2762-2022; National Food Safety Standard Maximum Levels of Contaminants in Foods. State Administration for Market Regulation, National Health Commission: Beijing, China, 2022.
- Bortey-Sam, N.; Nakayama, S.M.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Yohannes, Y.B.; Mizukawa, H.; Ishizuka, M. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. Environ. Saf. 2015, 111, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Su, J.; Miao, X. Fish Taxonomy; China Agriculture Press: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Chen, L.; Wang, H.; Xu, K.; Lv, Z.; Huang, B.; Li, Z.; Liang, J.; Zhou, Y.; Li, P.; Liu, L. Analysis of Current Situation of Proliferation and Release of Island and Reef Fishes in the Coastal Waters of Zhejiang. J. Zhejiang Ocean Univ. (Nat. Sci.) 2022, 41, 459–465+472. (In Chinese) [Google Scholar]
- Hu, L.; Li, J.; Qu, Y.; Cai, C. Analysis of Nutrient Components and Evaluation of Nutritive Quality in the Muscle of Oplegnathus fasciatus. J. South China Agric. Univ. 2010, 31, 71–75. (In Chinese) [Google Scholar]
- Jiang, R.; Wu, Y.; Huang, X.; Liu, S.; Chen, Q. Heavy metal enrichment characteristics and risk assessment of typical fishes in tropical seagrass beds. South China Fish. Sci. 2023, 19, 48–57. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Y.; Liang, H.; Huang, S.; Liu, J.; Lin, C.; Feng, J. Heavy metals concentrations of marine commercial species and health risk estimation in Huizhou. Ecol. Sci. 2020, 39, 95–103. (In Chinese) [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. Assessment of heavy metals (Cu, Ni, Fe, Co, Mn, Cr, Zn) in rivulet water, their accumulations and alterations in hematology of fish Channa punctatus. Afr. J. Biotechnol. 2014, 13, 492–501. [Google Scholar] [CrossRef]
- Mills, N.; Weber, M.J.; Pierce, C.L.; Cashatt, D. Factors influencing fish mercury concentrations in Iowa rivers. Ecotoxicology 2019, 28, 229–241. [Google Scholar] [CrossRef]
- Cammilleri, G.; Galluzzo, F.G.; Fazio, F.; Pulvirenti, A.; Vella, A.; Lo Dico, G.M.; Macaluso, A.; Ciaccio, G.; Ferrantelli, V. Mercury detection in benthic and pelagic fish collected from western Sicily (Southern Italy). Animals 2019, 9, 594. [Google Scholar] [CrossRef]
- Yang, F. Feeding Ecology of Three Enhancement and Releasing Species in Zhoushan Islands; Zhejiang Ocean University: Zhoushan, China, 2023; (In Chinese). [Google Scholar] [CrossRef]
- Schartup, A.T.; Thackray, C.P.; Qureshi, A.; Dassuncao, C.; Gillespie, K.; Hanke, A.; Sunderland, E.M. Climate change and overfishing increase neurotoxicant in marine predators. Nature 2019, 572, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Yuan, Q.; Ping, X.; Shen, X.; Wang, Y.; Chao, M. Time series analysis and potential ecological risk assessment of heavy metals in sediments from the Yangtze Estuary and Hangzhou Bay. Mar. Environ. Sci. 2023, 50, 252–258. (In Chinese) [Google Scholar] [CrossRef]
- Tu, N.P.C.; Agusa, T.; Ha, N.N.; Tuyen, B.C.; Tanabe, S.; Takeuchi, I. Stable isotope-guided analysis of biomagnication profiles of arsenic species in a tropical mangrove ecosystem. Mar. Pollut. Bull. 2011, 63, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Du, Z.; Zhang, F. A GIS-based health assessment of the offshore marine ecosystem in north Zhejiang Province. Acta Ecol. Sin. 2016, 36, 8183–8193. (In Chinese) [Google Scholar]
- Popowich, A.; Zhang, Q.; Le, X. Arsenobetaine: The ongoing mystery. Natl. Sci. Rev. 2017, 3, 451–458. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Song, D.; Du, S.; Zhang, L. Arsenic speciation in wild marine organisms and a health risk assessment in a subtropical bay of China. Sci. Total Environ. 2018, 626, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Clowes, L.A.; Francesconi, K.A. Uptake and elimination of arsenobetaine by the mussel Mytilus edulis is related to salinity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 35–42. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Wang, W. Prey-specific determination of arsenic bioaccumulation and transformation in a marine benthic fish. Sci. Total Environ. 2017, 586, 296–303. [Google Scholar] [CrossRef]
- Hightower, J.M.; Moore, D. Mercury levels in high-end consumers of fish. Environ. Health Perspect. 2003, 111, 604–608. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Clickner, R.P.; Bodurow, C.C. Blood organic mercury and dietary mercury intake: National health and nutrition examination survey, 1999 and 2000. Environ. Health Perspect. 2004, 112, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Huang, D.M.; Cai, Y.Q.; Meng, X.J.; Shi, Y.F.; Kong, C.; Huang, X.Y.; Tang, Y.Y.; Zhang, X.; Yang, G.X. Geographical Origin Traceability of Chinese Mitten Crabs Based on Mineral Elements and Stable Isotopes. Food Sci. 2020, 41, 125–130. (In Chinese) [Google Scholar]
- Zitek, A.; Sturm, M.; Waidbacher, H.; Prohaska, T. Discrimination of wild and hatchery trout by natural chronological patters of elements and isotopes in otoliths using LA-ICP-MS. Fish. Manag. Ecol. 2010, 17, 435–445. [Google Scholar] [CrossRef]
- Yamashita, Y.; Omura, Y.; Okazaki, E. Distinct regional profiles of trace element content in muscle of Japanese eel Anguilla japonica from Japan, Taiwan, and China. Fish. Sci. 2006, 72, 1109–1113. [Google Scholar] [CrossRef]
- Anderson, K.A.; Hobbie, K.A.; Smith, B.W. Chemical profiling with modeling differentiates wild and farm-raised salmon. J. Agric. Food Chem. 2010, 58, 11768–11774. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, C.; Wang, Y.; Li, Z.; Xue, Y.; Xu, J. The classification of sea cucumber (Apostichopus japonicus) according to region of origin using multi-element analysis and pattern recognition techniques. Food Control 2012, 23, 522–527. [Google Scholar] [CrossRef]
- Varrà, M.O.; Ghidini, S.; Zanardi, E.; Badiani, A.; Ianieri, A. Authentication of European sea bass according to production method and geographical origin by light stable isotope ratio and rare earth elements analyses combined with chemometrics. Ital. J. Food Saf. 2019, 8, 7872. [Google Scholar] [CrossRef]
- Gopi, K.; Mazumder, D.; Sammut, J.; Saintilan, N.; Crawford, J.; Gadd, P. Isotopic and elemental profiling to trace the geographic origins of farmed and wild-caught Asian seabass (Lates calcarifer). Aquaculture 2019, 502, 56–62. [Google Scholar] [CrossRef]
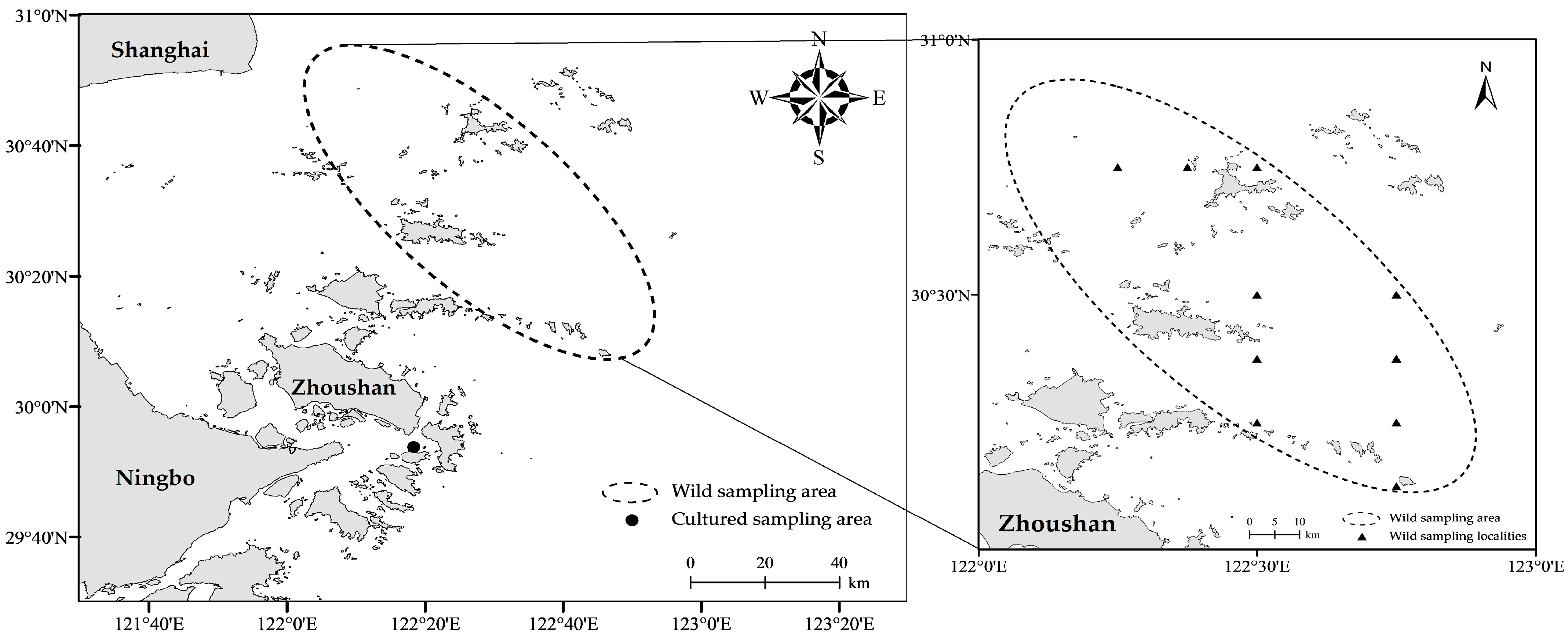
| Species | Number | Body Mass (g) | Body Length (mm) | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Wild | 44 | 92.93 ± 34.86 | 62.90~206.50 | 129.82 ± 17.57 | 106~200 |
| Cultured | 44 | 71.56 ± 26.70 | 22.24~108.20 | 103.70 ± 18.90 | 68~131 |
| Index Value | Degree of Pollution |
|---|---|
| SFI < 0.2 | No contamination |
| 0.2 < SFI < 0.6 | Low contamination |
| 0.6 < SFI < 1.0 | Medium contamination |
| SFI > 1.0 | High contamination |
| Index Value | Degree of Pollution |
|---|---|
| MPI < 2 | Not impacted |
| 2 < MPI < 5 | Very low contamination |
| 5 < MPI < 10 | Low contamination |
| 10 < MPI < 20 | Medium contamination |
| 20 < MPI < 50 | High contamination |
| 50 < MPI < 100 | Very high contamination |
| MPI > 100 | Extreme contamination |
| Heavy Metals | Wild | Cultured | p | ||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | ||
| Fe | 2.801~32.896 | 8.018 ± 6.593 | 2.676~65.721 | 6.676 ± 9.056 | 0.523 |
| Mn | 0.093~0.376 | 0.171 ± 0.059 | 0.093~0.402 | 0.167 ± 0.058 | 0.735 |
| Cu | 0.107~1.01 | 0.398 ± 0.199 | 0.231~1.007 | 0.445 ± 0.133 | 0.102 |
| Zn | 1.737~3.358 | 2.512 ± 0.407 | 2.156~6.08 | 3.051 ± 0.738 | <0.001 |
| Cr | 0.004~0.146 | 0.037 ± 0.028 | 0.003~0.202 | 0.036 ± 0.035 | 0.816 |
| Cd | 0.0005~0.018 | 0.003 ± 0.003 | 0.0002~0.003 | 0.001 ± 0.0007 | <0.001 |
| As | 0.596~3.57 | 1.494 ± 0.659 | 0.213~1.137 | 0.594 ± 0.215 | <0.001 |
| Hg | 0.0007~0.058 | 0.014 ± 0.011 | 0.021~0.106 | 0.042 ± 0.016 | <0.001 |
| Class Limits | Species | SFI | Degree of Pollution | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Cr | Cd | As | Hg | Mean | |||
| First | Wild | 0.04 | 0.13 | 0.07 | 0.02 | 1.49 | 0.29 | 0.34 | No contamination |
| Cultured | 0.04 | 0.15 | 0.07 | 0.01 | 0.59 | 0.85 | 0.29 | No contamination | |
| Second | Wild | 0.02 | 0.05 | 0.02 | 0.00 | 0.30 | 0.15 | 0.09 | No contamination |
| Cultured | 0.02 | 0.06 | 0.02 | 0.00 | 0.12 | 0.43 | 0.11 | No contamination | |
| Species | THQ | HI | ||||||
|---|---|---|---|---|---|---|---|---|
| Mn | Cu | Zn | Cr | Cd | As | Hg | ||
| Wild | 0.0002 | 0.0016 | 0.0014 | 0.0020 | 0.0006 | 0.8050 | 0.0079 | 0.8186 |
| Cultured | 0.0002 | 0.0018 | 0.0016 | 0.0020 | 0.0002 | 0.3205 | 0.0230 | 0.3493 |
| Stepwise Discriminant Analysis | Species | Species | Total | Accuracy (%) | Overall Accuracy (%) | |
|---|---|---|---|---|---|---|
| Wild | Cultured | |||||
| Initial discrimination | Wild | 45 | 4 | 49 | 91.8 | 96.0 |
| Cultured | 0 | 50 | 50 | 100 | ||
| Cross-validation | Wild | 44 | 5 | 49 | 89.8 | 94.9 |
| Cultured | 0 | 50 | 50 | 100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Qian, W.; Zhu, K.; Xu, K. Heavy Metal Concentrations in Wild and Cultured Oplegnathus fasciatus from the East China Sea and Associated Health Risks. Water 2024, 16, 1957. https://doi.org/10.3390/w16141957
Lu K, Qian W, Zhu K, Xu K. Heavy Metal Concentrations in Wild and Cultured Oplegnathus fasciatus from the East China Sea and Associated Health Risks. Water. 2024; 16(14):1957. https://doi.org/10.3390/w16141957
Chicago/Turabian StyleLu, Kexiang, Weiguo Qian, Kai Zhu, and Kaida Xu. 2024. "Heavy Metal Concentrations in Wild and Cultured Oplegnathus fasciatus from the East China Sea and Associated Health Risks" Water 16, no. 14: 1957. https://doi.org/10.3390/w16141957
APA StyleLu, K., Qian, W., Zhu, K., & Xu, K. (2024). Heavy Metal Concentrations in Wild and Cultured Oplegnathus fasciatus from the East China Sea and Associated Health Risks. Water, 16(14), 1957. https://doi.org/10.3390/w16141957





