Modern Treatment Using Powdered Chlorella vulgaris for Adsorption of Heavy Metals from Freshwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject of Research: Algal Biomass
2.2. Analysis of Algal Biomass Properties
2.2.1. Determination of Point of Zero Charge (PZC) of Chlorella vulgaris
2.2.2. Scanning Electron Microscopy with Back-Scattered Electron (SEM-BSE)
2.2.3. Fourier Transform Infrared Spectroscopy FT-IR
2.3. Batch Studies of the Adsorption Process
2.3.1. Equilibrium Studies
2.3.2. Determination of Cu(II) and Co(II) Concentration by Flame Atomic Absorption Spectrometry (F-AAS)
2.3.3. Adsorption Isotherm Models in a Single-Component and Binary System
2.3.4. Kinetic Studies
3. Results and Discussion
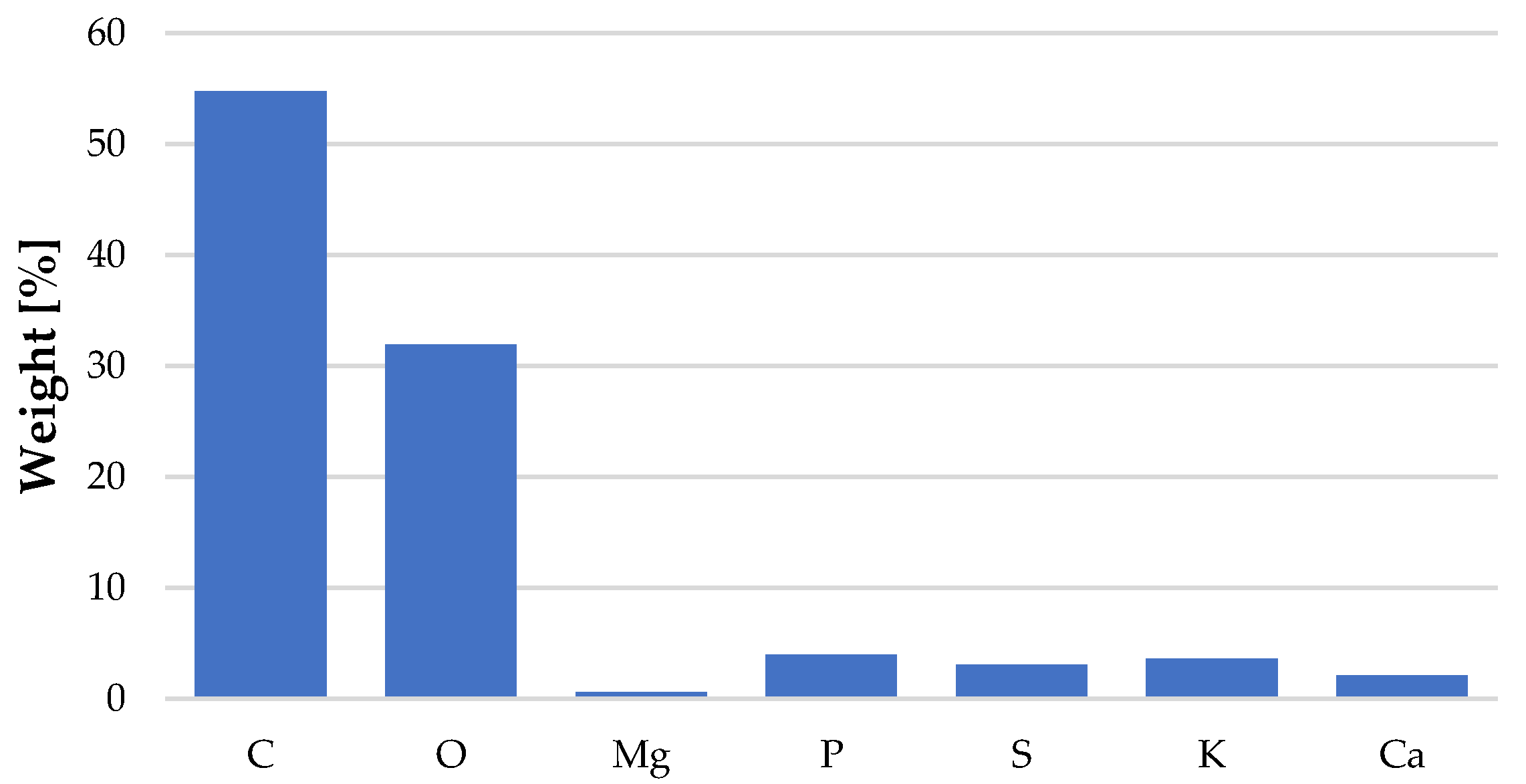
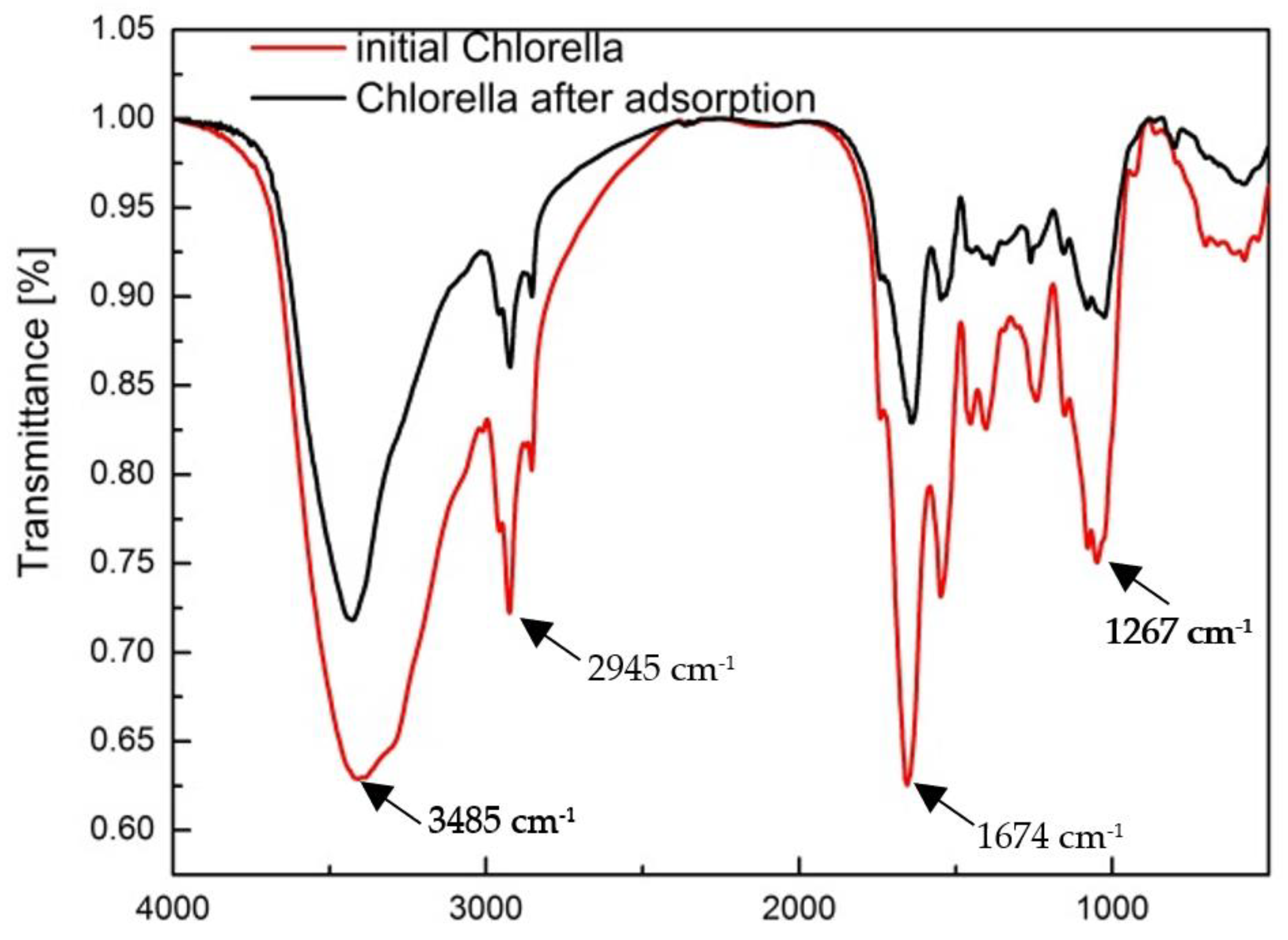
3.1. Characterization of the Biosorbent
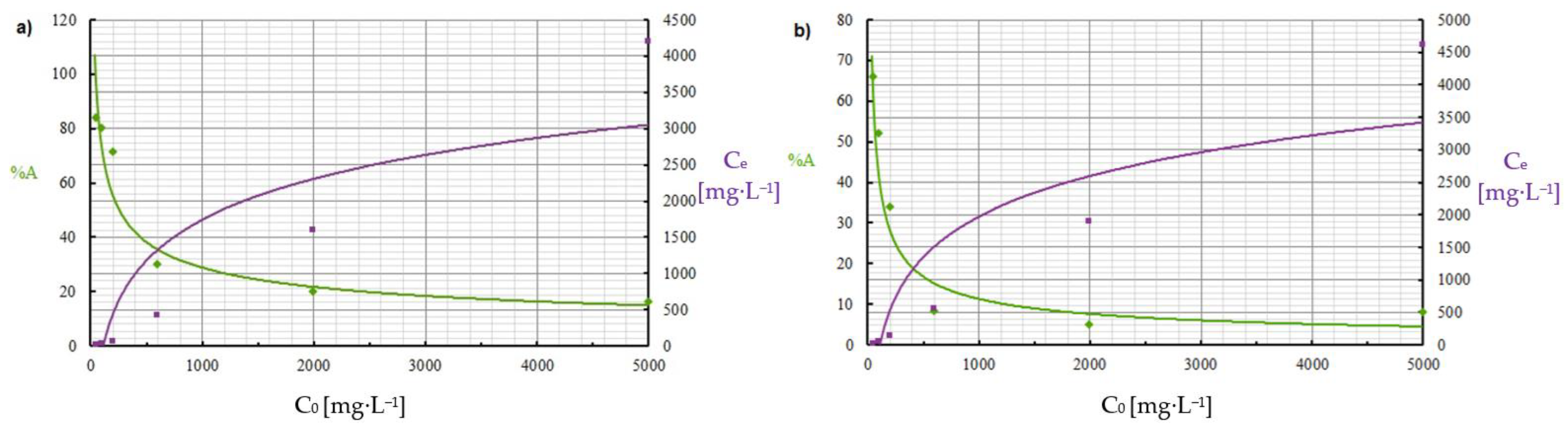
3.2. Sorption Batch Model
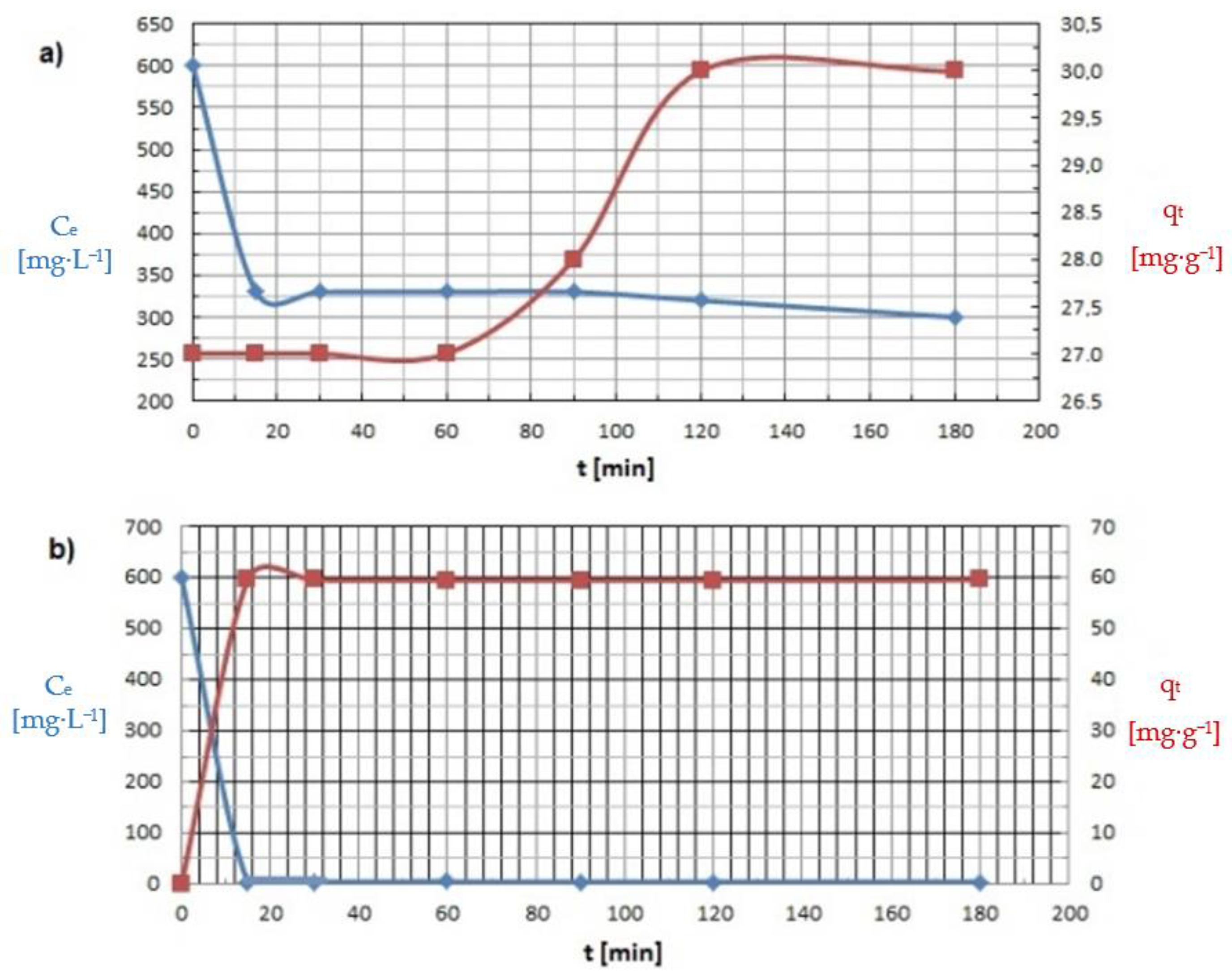
3.3. Kinetic Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Hadi, B.; El-Naas, M.H. Biosorption of Heavy Metals: Potential and Applications of Yeast Cells for Cadmium Removal. In Environmental Contaminants: Ecological Implications and Management; Bharagava, R.N., Ed.; Microorganisms for Sustainability; Springer: Singapore, 2019; Volume 14, pp. 237–271. [Google Scholar]
- Chen, X.; Tian, Z.; Cheng, H.; Xu, G.; Zhou, H. Adsorption Process and Mechanism of Heavy Metal Ions by Different Components of Cells, Using Yeast (Pichia Pastoris) and Cu2+ as Biosorption Models. RSC Adv. 2021, 11, 17080–17091. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; Fernandez-Pezua, M.C.; Gamarra-Gómez, F.; Sacari-Sacari, E.; Angeles-Suazo, J.; Dávalos-Prado, J.Z. Single and Binary Removals of Pb(II) and Cd(II) with Chemically Modified Opuntia ficus indica Cladodes. Molecules 2023, 28, 4451. [Google Scholar] [CrossRef] [PubMed]
- Hansda, A.; Kumar, V.; Anshumali. A Comparative Review towards Potential of Microbial Cells for Heavy Metal Removal with Emphasis on Biosorption and Bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Giráldez, I.; Vega, J.M.; Vigara, J. Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquat. Toxicol. 2021, 239, 105941. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Dutta, S.; Banerjee, I.; Pohrmen, C.B.; Kumar, V. Photosynthetic Microalgae–Based Carbon Sequestration and Generation of Biomass in Biorefinery Approach for Renewable Biofuels for a Cleaner Environment. Biomass Conv. Bioref. 2023, 13, 7403–7421. [Google Scholar] [CrossRef]
- Panahi, Y.; Darvishi, B.; Jowzi, N.; Beiraghdar, F.; Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr. Pharm. Des. 2016, 22, 164–173. [Google Scholar] [CrossRef]
- Adamakis, I.-D.; Lazaridis, P.A.; Terzopoulou, E.; Torofias, S.; Valari, M.; Kalaitzi, P.; Rousonikolos, V.; Gkoutzikostas, D.; Zouboulis, A.; Zalidis, G.; et al. Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ. Sci. Pollut. Res. 2018, 25, 23018–23032. [Google Scholar] [CrossRef]
- Kua, T.L.; Kooh, M.R.R.; Dahri, M.K.; Zaidi, N.A.H.M.; Lu, Y.; Lim, L.B.L. Aquatic Plant, Ipomoea Aquatica, as a Potential Low-Cost Adsorbent for the Effective Removal of Toxic Methyl Violet 2B Dye. Appl. Water Sci. 2020, 10, 243. [Google Scholar] [CrossRef]
- Liu, D.; Yang, W.; Lv, Y.; Li, S.; Qv, M.; Dai, D.; Zhu, L. Pollutant removal and toxic response mechanisms of freshwater microalgae Chlorella sorokiniana under exposure of tetrabromobisphenol A and cadmium. Chem. Eng. J. 2023, 461, 142065. [Google Scholar] [CrossRef]
- Tarlan, E.; Dilek, F.B.; Yetis, U. Effectiveness of Algae in the Treatment of a Wood-Based Pulp and Paper Industry Wastewater. Bioresour. Technol. 2002, 84, 1–5. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, L.; Liu, T.; Xu, X. Degradation of disperse blue 2BLN by oleaginous C. sorokiniana XJK. RSC Adv. 2016, 6, 106935–106944. [Google Scholar] [CrossRef]
- Ramesh, B.; Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Thamarai, P.; Shaji, A.; Rangasamy, G. A review on algae biosorption for the removal of hazardous pollutants from wastewater: Limiting factors, prospects and recommendations. Environ. Pollut. 2023, 327, 121572. [Google Scholar] [CrossRef]
- Goher, M.E.; Abd El-Monem, A.M.; Abdel-Satar, A.M.; Ali, M.H.; Hussian, A.-E.M.; Napiórkowska-Krzebietke, A. Biosorption of Some Toxic Metals from Aqueous Solution Using Non-Living Algal Cells of Chlorella Vulgaris. J. Elem. 2016, 21, 703–714. [Google Scholar]
- Lapeñas, L.A.; Peña-Bahamonde, J.; Faria, L.U.S.; De Luna, M.D.G.; Rodrigues, D.F. Removing Heavy Metal Ions from Wastewater by Chlorella Sorokiniana Coupled to Manganese-Doped Magnetic Ferrite Nanoparticles. J. Hazard. Mater. Lett. 2023, 4, 100082. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions from Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Alharbi, R.M.; Sholkamy, E.N.; Alsamhary, K.I.; Abdel-Raouf, N.; Ibraheem, I.B.M. Optimization Study of the Capacity of Chlorella Vulgaris as a Potential Bio-Remediator for the Bio-Adsorption of Arsenic (III) from Aquatic Environments. Toxics 2023, 11, 439. [Google Scholar] [CrossRef]
- Mohammad Ali, M.; Hossain, D.; Al-Imran, A.; Khan, M.S.; Begum, M.; Hasan Osman, M. Environmental Pollution with Heavy Metals: A Public Health Concern. In Heavy Metals—Their Environmental Impacts and Mitigation; Khaled Nazal, M., Zhao, H., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Mahey, S.; ·Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef]
- Al-Maliky, E.A.; Gzar, H.A.; Al-Azawy, M.G. Determination of Point of Zero Charge (PZC) of Concrete Particles Adsorbents. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1184, p. 012004. [Google Scholar]
- Sočo, E.; Papciak, D.; Michel, M.M.; Pająk, D.; Domoń, A.; Kupiec, B. Characterization of the Physical, Chemical, and Adsorption Properties of Coal-Fly-Ash–Hydroxyapatite Composites. Minerals 2021, 11, 774. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Bruanuer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–316. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Removal of Cadmium(II) and Zinc(II) Metal Ions from Binary Aqueous Solution by Rice Husk Ash. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 172–184. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Savastru, E.; Bulgariu, D.; Zamfir, C.-I.; Bulgariu, L. Application of Saccharomyces Cerevisiae in the Biosorption of Co(II), Zn(II) and Cu(II) Ions from Aqueous Media. Water 2022, 14, 976. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of Methylene Blue from Aqueous Solution by Sewage Sludge-Derived Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics and Mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Nasanjargal, S.; Munkhpurev, B.-A.; Kano, N.; Kim, H.-J.; Ganchimeg, Y. The Removal of Chromium(VI) from Aqueous Solution by Amine-Functionalized Zeolite: Kinetics, Thermodynamics, and Equilibrium Study. J. Environ. Protect. 2021, 12, 654–675. [Google Scholar] [CrossRef]
- Gunorubon, A.J.; Chukwunonso, N. Adsorption of Heavy Metals: Mechanisms, Kinetics, Equilibrium and Thermodynamics Studies of Fe3+ Ion Removal from Aqueous Solutions Using Periwinkle Shell Activated Carbon. Adv. Chem. Eng. Sci. 2018, 8, 775–805. [Google Scholar] [CrossRef]
- Rojas, J.; Suarez, D.; Moreno, A.; Silva-Agredo, J.; Torres-Palma, R.A. Kinetics, Isotherms and Thermodynamic Modeling of Liquid Phase Adsorption of Crystal Violet Dye onto Shrimp-Waste in Its Raw, Pyrolyzed Material and Activated Charcoals. Appl. Sci. 2019, 9, 5337. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral Characterization Using Scanning Electron Microscopy (SEM): A Review of the Fundamentals, Advancements, and Research Directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Dery, B.; Zaixiang, L. Scanning Electron Microscopy (SEM) as an Effective Tool for Determining the Morphology and Mechanism of Action of Functional Ingredients. Food Rev. Int. 2023, 39, 2007–2026. [Google Scholar] [CrossRef]
- Sun, C.; Lux, S.; Müller, E.; Meffert, M.; Gerthsen, D. Versatile application of a modern scanning electron microscope for materials characterization. J. Mater. Sci. 2020, 55, 13824–13835. [Google Scholar] [CrossRef]
- Duygu, D.Y.; Udoh, A.U.; Ozer, T.B.; Akbulut, A.; Erkaya, I.A.; Yildiz, K.; Guler, D. Fourier Transform Infrared (FTIR) Spectroscopy for Identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar]
- Tattibayeva, Z.; Tazhibayeva, S.; Kujawski, W.; Zayadan, B.; Musabekov, K. Peculiarities of adsorption of Cr(VI) ions on the surface of Chlorella vulgaris ZBS1 algae cells. Helion 2022, 8, e10468. [Google Scholar] [CrossRef]
- Ramana, D.K.V.; Jamuna, K.; Satyanarayana, B.; Venkateswarlu, B.; Rao, M.M.; Seshaiah, K. Removal of Heavy Metals from Aqueous Solutions Using Activated Carbon Prepared from Cicer Arietinum. Toxicol. Environ. Chem. 2010, 92, 1447–1460. [Google Scholar] [CrossRef]
- Areco, M.M.; Hanela, S.; Duran, J.; Dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by Dead Biomasses of Green Alga Ulva Lactuca and the Development of a Sustainable Matrix for Adsorption Implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Freire, S.J.; Santos, L.V.S.; Palladino, F.; Jacob, R.S. Biosorption of Copper Ions from Aqueous Solution Using Chlorella Pyrenoidosa: Optimization, Equilibrium and Kinetics Studies. Microchem. J. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Do Nascimento, J.M.; De Oliveira, J.D.; Rizzo, A.C.L.; Leite, S.G.F. Biosorption Cu (II) by the Yeast Saccharomyces Cerevisiae. Biotechnol. Rep. 2019, 21, e00315. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Application of the Biochar Derived from Orange Peel for Effective Biosorption of Copper and Cadmium in Batch Studies: Isotherm Models and Kinetic Studies. Arab. J. Geosci. 2019, 12, 46. [Google Scholar] [CrossRef]
- Bouzidi, A.; Djedid, M.; Ad, C.; Benalia, M.; Hafez, B.; Elmsellem, H. Biosorption of Co (II) Ions from Aqueous Solutions Using Selected Local Luffa Cylindrica: Adsorption and Characterization Studies. Mor. J. Chem. 2021, 9, 156–167. [Google Scholar]
- Vilvanathan, S.; Shanthakumar, S. Modeling of Fixed-Bed Column Studies for Removal of Cobalt Ions from Aqueous Solution Using Chrysanthemum Indicum. Res. Chem. Intermed. 2017, 43, 229–243. [Google Scholar] [CrossRef]
- Tofan, L.; Teodosiu, C.; Paduraru, C.; Wenkert, R. Cobalt (II) Removal from Aqueous Solutions by Natural Hemp Fibers: Batch and Fixed-Bed Column Studies. Appl. Surf. Sci. 2013, 285, 33–39. [Google Scholar] [CrossRef]
- Galedar Biosorption of ternary cadium, niciel and cobalt ions from aqueous solution onto saccharomyces cerevisiae cells: Batch and column studies. Am. J. Biochem. Biotechnol. 2013, 9, 47–60. [CrossRef]
- Hymavathi, D.; Prabhakar, G. Studies on the Removal of Cobalt(II) from Aqueous Solutions by Adsorption with Ficus Benghalensis Leaf Powder through Response Surface Methodology. Chem. Eng. Commun. 2017, 204, 1401–1411. [Google Scholar] [CrossRef]




| Isotherm | Abbreviations |
|---|---|
| Freundlich Nonlinear form: Linear form: | qe—the amount of adsorbate adsorbed [mg∙g−1], KF—Freundlich adsorption constant [mg1−1/n∙L1/n∙g−1], Ce—the concentration of adsorbate remaining in the solution equilibrium [mg∙L−1], n—an empirical parameter related to adsorption intensity (in Freundlich), qmax—maximum adsorption capacity [mg∙g−1], KL—Langmuir adsorption constant [L∙mg−1], C0—initial concentration of the substance [mg∙L−1], KBET—adsorption equilibrium constant [L−1∙mg], ε—Dubinin–Radushkevich adsorption constant [mol2∙J−2], E—free energy [kJ∙mol−1]. |
| Langmuir Nonlinear form: Linear form: | |
| Brunauer, Emmett, and Teller (BET) | |
| Dubinin–Radushkevich (R-D) Nonlinear form: ; E = Linear form: |
| Isotherm | Equations |
|---|---|
| Jain–Snoeyink | Where, |
| Extended Langmuir |
| Kinetic Model | Pseudo-First-Order (PFO) | Pseudo-Second-Order (PSO) | Intra-Particle Diffusion |
|---|---|---|---|
| Equation | dqt/dt = k1(qe − qt) ln(qe − qt)= −k1t + ln qe | dqt/dt = k2(qe − qt)2 t/qt = 1/k2qe2 + t/qe | qt = k’it1/2 + b |
| Isotherm | Parameter | Cu(II) | Co(II) |
|---|---|---|---|
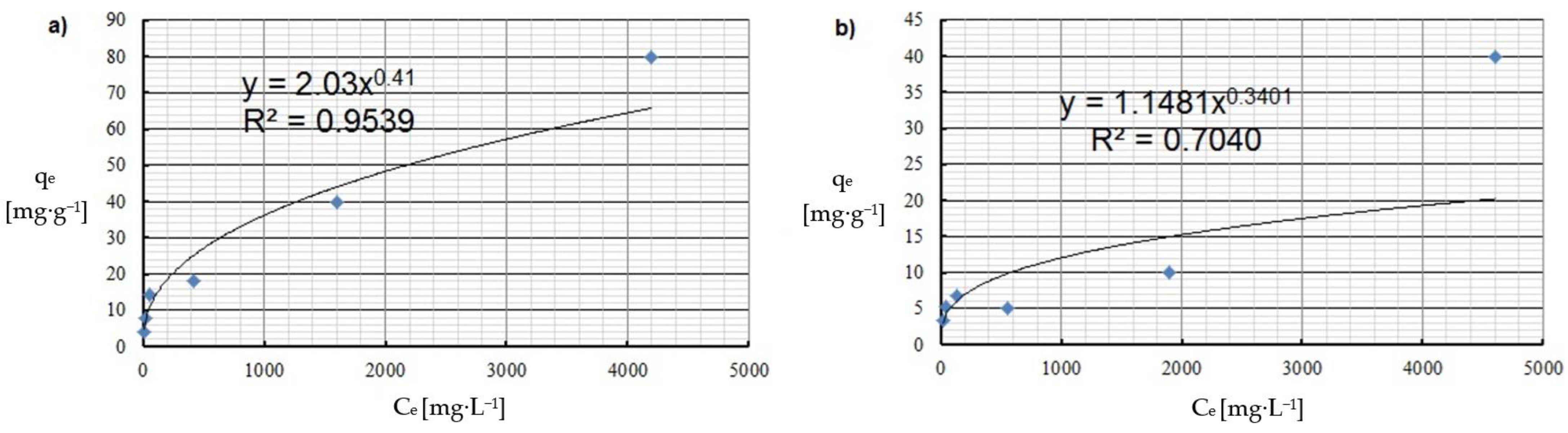
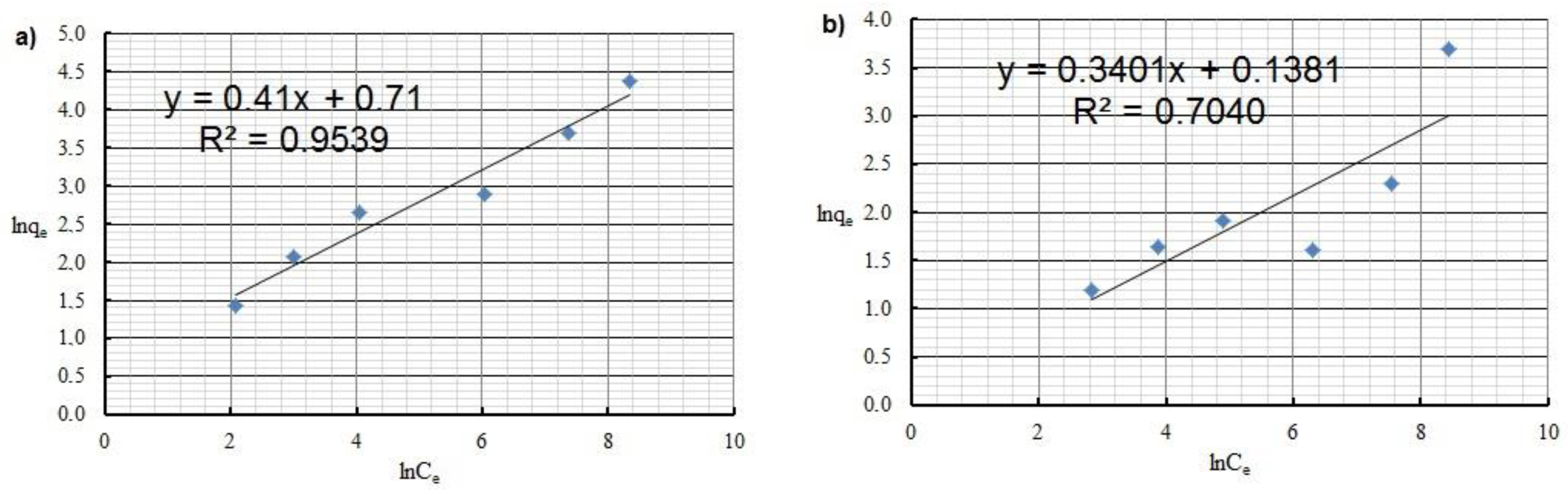
| Freundlich | KF [mg1−1/n·L1/n·g−1] | 2.034 | 1.148 |
| n | 2.4 | 2.9 | |
| R2 | 0.954 | 0.704 | |
| χ2/DoF | 0.42 | 5.2 | |
| Langmuir | KL [L·mg−1] | 0.055 | 0.376 |
| qmax [mg·g−1 ] | 30.3 | 9.0 | |
| R2 | 0.971 | 0.670 | |
| χ2/DoF | 4.6 | 9.5 | |
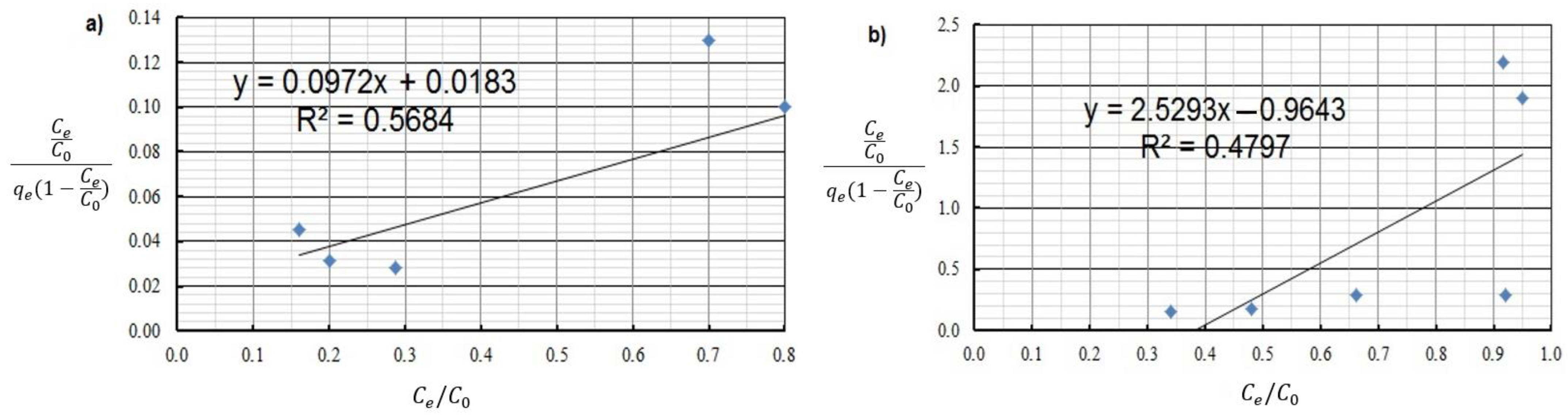
| Brunauer, Emmett, and Teller | KBET [L·mg−1] | 1.00 | 3.44 |
| qmax [mg·g−1 ] | 0.018 | 0.280 | |
| R2 | 0.568 | 0.480 | |
| χ2/DoF | 8.7 | 9.2 | |
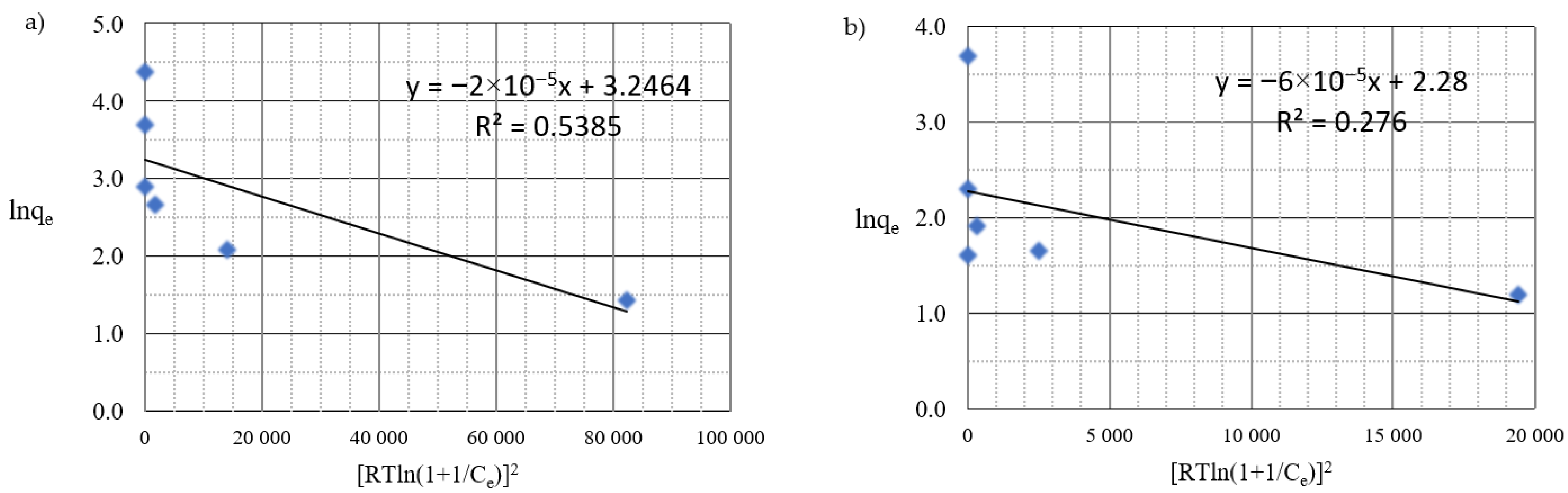
| Dubinin–Radushkevich | E [kJ·mol−1] | 0.14 | 0.09 |
| qmax [mg·g−1] | 25.7 | 9.78 | |
| R2 | 0.539 | 0.276 |
| Isotherm | Parameter | Cu(II) | Co(II) |
|---|---|---|---|
| Jain-Snoeyinka | KLi [L·mg−1] | 6.1·10−2 | 5.8·10−3 |
| q∞i [mg·g−1] | 23.5 | 16.7 | |
| R2 | 0.8531 | 0.7520 | |
| Extended Langmuir | KLi [L·mg−1] | 7.5·10−2 | 3.8·10−2 |
| q∞i [mg·g−1] | 20.8 | 19.6 | |
| R2 | 0.7233 | 0.5745 |
| Kinetic Model | Parameter | Cu(II) Ions | Co(II) Ions |
|---|---|---|---|
| Pseudo-first-order (PFO) | R2 k1 [min−1] | 0.8272 6·10−4 | 0.2344 0.014 |
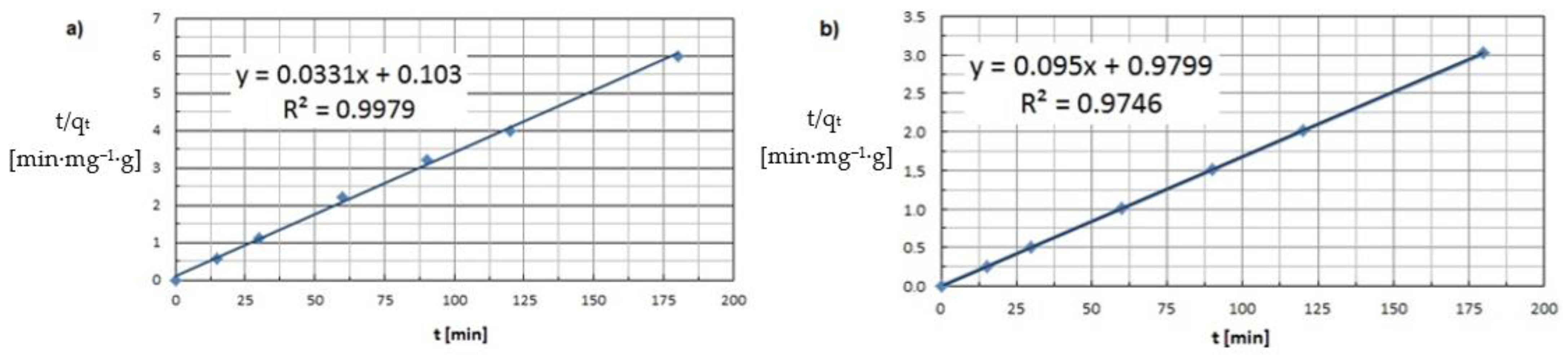
| Pseudo-second-order (PSO) | R2 k2 [g·mg−1·min−1] | 0.9979 2.7·10−3 | 0.9746 2.8·10−4 |
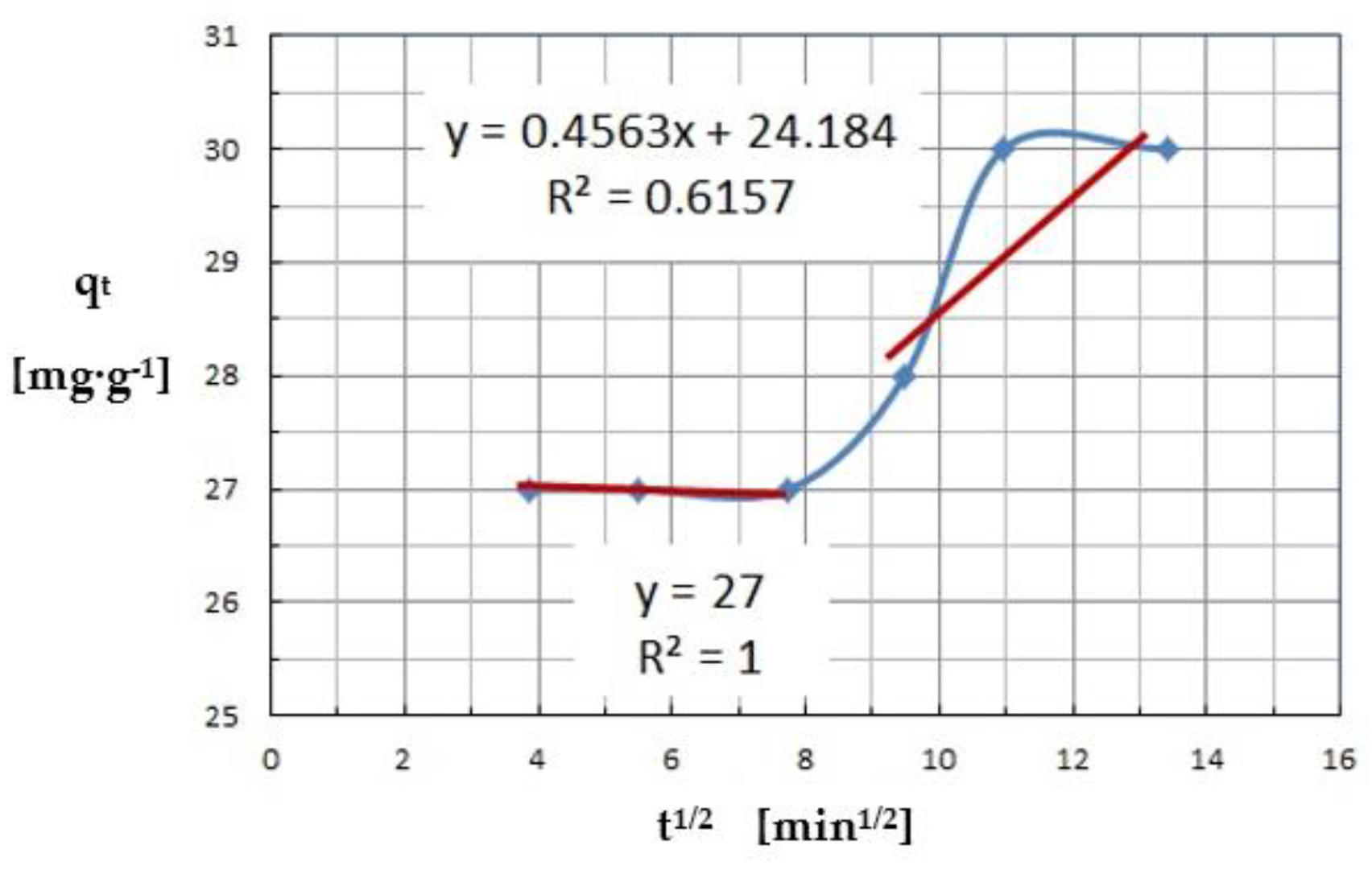
| Intra-particle diffusion | R2 k’1 [mg·g−1·min−1/2] b1 [mg·g−1] | 0.6157 0.4563 24.18 | - |
| R2 k’2 [mg·g−1·min−1/2] b2 [mg·g−1] | 1.000 - 27.000 | - |
| Adsorbent | qmax [mg g−1] | References | |
|---|---|---|---|
| Chlorella vulgaris (this work) | Cu and Co | 30.3 and 9.0 | - |
| Stems and seed hulls of Cicer arietinum | Cu | 18 | [43] |
| Ulva lactuca (in suspension and fixed in agar) | 32.80 and 10.01 | [44] | |
| Chlorella pyrenoidosa | 11.88 | [45] | |
| Saccharomyces cerevisiae | 4.73 | [46] | |
| Orange peel-derived biochar | 72.99 | [47] | |
| Luffa cylindrica | Co | 2.53 | [48] |
| Chrysanthemum indicum flower (raw and biochar) | 4.84 and 28.34 | [49] | |
| Natural hemp fibers | 13.58 | [50] | |
| Cells of Saccharomyces cerevisiae | 0.68 | [51] | |
| Ficus benghalensis L. | 5.65 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sočo, E.; Papciak, D.; Domoń, A.; Pająk, D. Modern Treatment Using Powdered Chlorella vulgaris for Adsorption of Heavy Metals from Freshwater. Water 2024, 16, 2388. https://doi.org/10.3390/w16172388
Sočo E, Papciak D, Domoń A, Pająk D. Modern Treatment Using Powdered Chlorella vulgaris for Adsorption of Heavy Metals from Freshwater. Water. 2024; 16(17):2388. https://doi.org/10.3390/w16172388
Chicago/Turabian StyleSočo, Eleonora, Dorota Papciak, Andżelika Domoń, and Dariusz Pająk. 2024. "Modern Treatment Using Powdered Chlorella vulgaris for Adsorption of Heavy Metals from Freshwater" Water 16, no. 17: 2388. https://doi.org/10.3390/w16172388
APA StyleSočo, E., Papciak, D., Domoń, A., & Pająk, D. (2024). Modern Treatment Using Powdered Chlorella vulgaris for Adsorption of Heavy Metals from Freshwater. Water, 16(17), 2388. https://doi.org/10.3390/w16172388










