Copper and Temperature Interaction Induced Gill and Liver Lesions and Behaviour Alterations in Mozambique Tilapia (Oreochromis mossambicus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Exposure Conditions
2.3. Histological Slide Preparation
2.4. Gills’ Lesions Scoring
2.5. Liver Histopathology Scoring
2.6. Condition Factor and Hepatosomatic Index
2.7. Behavioural Analysis
2.8. Statistical Analysis
3. Results
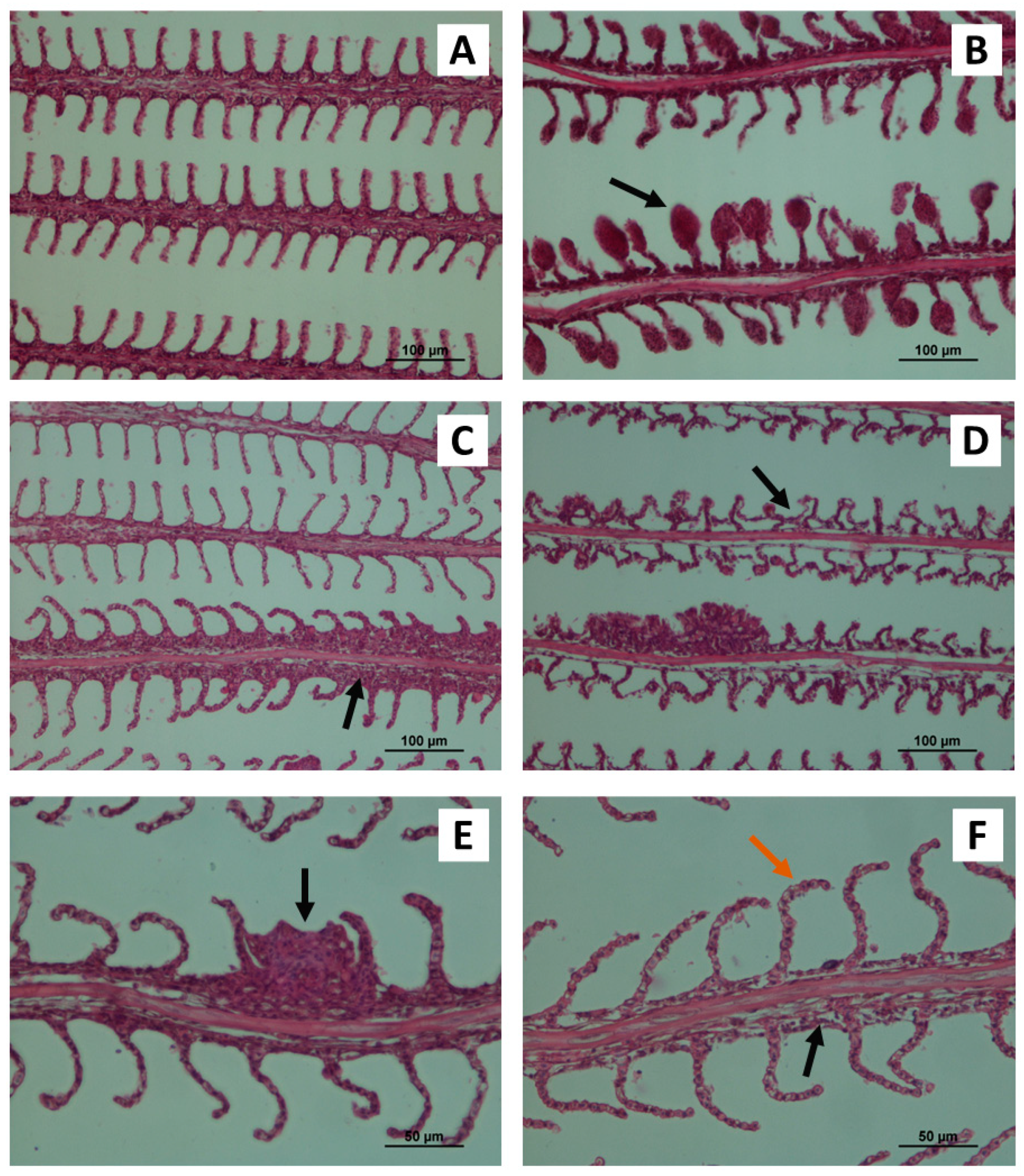
3.1. Gill Histopathology
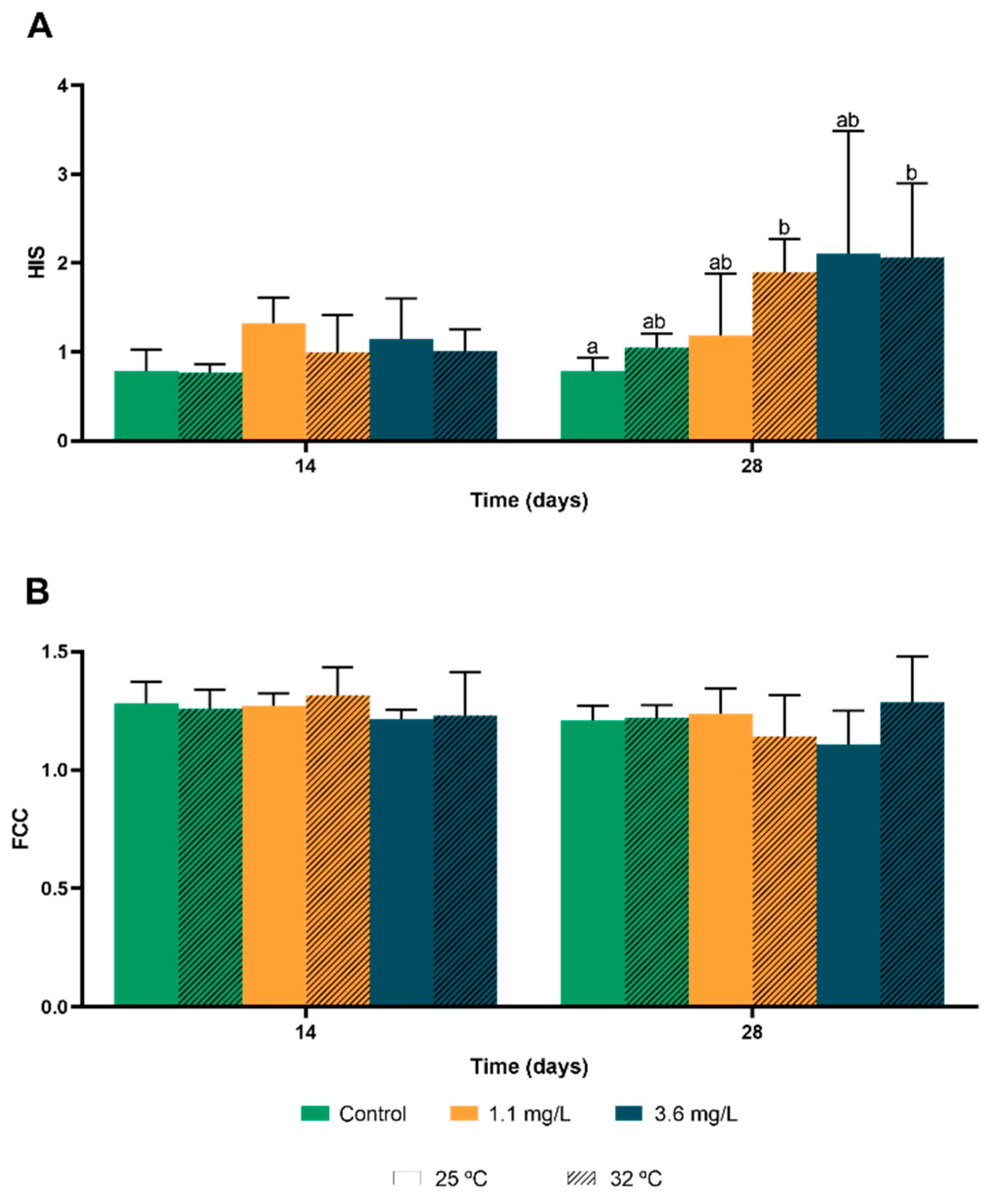
3.2. Hepatosomatic Index and Condition Factor
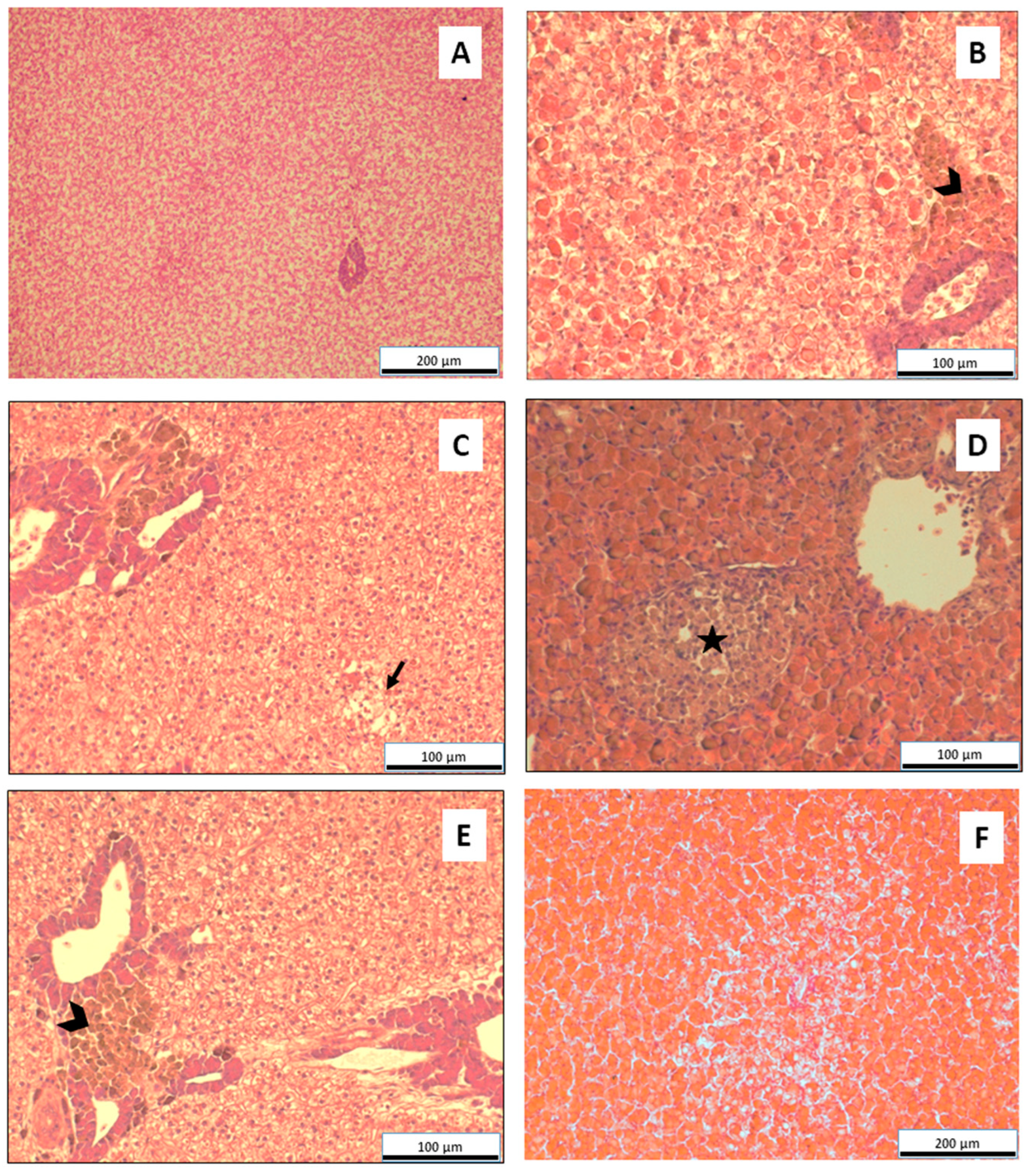
3.3. Liver Histopathology
3.4. Behavioural Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Priya, A.K.; Muruganandam, M.; Sivarethinamohan, R.; Sivarethinamohan, S.; Gaddam, M.K.R.; Velusamy, P.; Gomathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of climate change and anthropogenic activities on aquatic ecosystem—A review. Environ. Res. 2023, 238, 117233. [Google Scholar] [CrossRef]
- Prakash, S. Impact of Climate change on Aquatic Ecosystem and its Biodiversity: An overview. Int. J. Biol. Innov. 2021, 3, 312–317. [Google Scholar] [CrossRef]
- Alava, J.J.; Cheung, W.W.; Ross, P.S.; Sumaila, U.R. Climate change–contaminant interactions in marine food webs: Toward a conceptual framework. Glob. Chang. Biol. 2017, 23, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Brack, W.; Van den Brink, P.J.; Deutschmann, B.; Hollert, H.; Posthuma, L.; Segner, H.; Seiler, T.-B.; Teodorovic, I.; Focks, A. Assessing the ecological impact of chemical pollution on aquatic ecosystems requires the systematic exploration and evaluation of four lines of evidence. Environ. Sci. Eur. 2019, 31, 98. [Google Scholar] [CrossRef]
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; de Palma, M.; Donko, A.; Experton, C. Use of copper-based fungicides in organic agriculture in twelve European countries. Agronomy 2022, 12, 673. [Google Scholar] [CrossRef]
- Tavares-Dias, M. Toxic, physiological, histomorphological, growth performance and antiparasitic effects of copper sulphate in fish aquaculture. Aquaculture 2021, 535, 736350. [Google Scholar] [CrossRef]
- Nunes, B.; Caldeira, C.; Pereira, J.L.; Gonçalves, F.; Correia, A.T. Perturbations in ROS-related processes of the fish Gambusia holbrooki after acute and chronic exposures to the metals copper and cadmium. Environ. Sci. Pollut. Res. 2015, 22, 3756–3765. [Google Scholar] [CrossRef]
- Nunes, B.; Capela, R.C.; Sérgio, T.; Caldeira, C.; Gonçalves, F.; Correia, A.T. Effects of chronic exposure to lead, copper, zinc, and cadmium on biomarkers of the European eel, Anguilla anguilla. Environ. Sci. Pollut. Res. 2014, 21, 5689–5700. [Google Scholar] [CrossRef]
- Nouh, W.G.; Selim, A.G. Toxopathological studies on the effect of formalin and copper sulphate in tilapia as a commonly used disinfectant in aquaculture. J. Appl. Environ. Biol. Sci. 2013, 3, 7–20. [Google Scholar]
- Senior, W.; de La Cruz, R.; Troccoli, L. Copper: Essential and noxious to aquatic organisms. In Coastal and Deep Ocean Pollution; CRC Press: Boca Raton, FL, USA, 2020; pp. 107–152. [Google Scholar]
- Rader, K.J.; Carbonaro, R.F.; van Hullebusch, E.D.; Baken, S.; Delbeke, K. The fate of copper added to surface water: Field, laboratory, and modeling studies. Environ. Toxicol. Chem. 2019, 38, 1386–1399. [Google Scholar] [CrossRef]
- Akilan, C.; Hefter, G.; Rohman, N.; Buchner, R. Ion association and hydration in aqueous solutions of copper (II) sulfate from 5 to 65 C by dielectric spectroscopy. J. Phys. Chem. B 2006, 110, 14961–14970. [Google Scholar] [CrossRef]
- Vasconcelos, R.T.; Copatti, C.E.; Albinati, A.C.; Campeche, D.F.B.; Bonfá, H.; Melo, J. Waterborne copper sulfate toxicity in Nile tilapia (Oreochromis niloticus) juveniles affect survival, growth, and physiology. J. Appl. Aquac. 2024, 36, 129–150. [Google Scholar] [CrossRef]
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. In Reviews of Environmental Contamination and Toxicology Volume 213; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–26. [Google Scholar]
- El-Sherif, M.S.; El-Feky, A.M.I. Performance of Nile tilapia (Oreochromis niloticus) fingerlings. II. Influence of different water temperatures. Int. J. Agric. Biol 2009, 11, 1814–9596. [Google Scholar]
- Lee, Y.-W.; Oh, Y.H.; Lee, S.H.; Kim, D.; Joung, D. Assessment of water quality in a coastal region of sea dike construction in Korea and the impact of low dissolved oxygen concentrations on pH changes. J. Mar. Sci. Eng. 2023, 11, 1247. [Google Scholar] [CrossRef]
- Debels, P.; Figueroa, R.; Urrutia, R.; Barra, R.; Niell, X. Evaluation of water quality in the Chillán River (Central Chile) using physicochemical parameters and a modified water quality index. Environ. Monit. Assess. 2005, 110, 301–322. [Google Scholar] [CrossRef]
- Nunes, B.; Brandão, F.; Sérgio, T.; Rodrigues, S.; Gonçalves, F.; Correia, A.T. Effects of environmentally relevant concentrations of metallic compounds on the flatfish Scophthalmus maximus: Biomarkers of neurotoxicity, oxidative stress and metabolism. Environ. Sci. Pollut. Res. 2014, 21, 7501–7511. [Google Scholar] [CrossRef]
- Chinnadurai, K.; Prema, P.; Veeramanikandan, V.; Kumar, K.R.; Nguyen, V.-H.; Marraiki, N.; Zaghloul, N.S.S.; Balaji, P. Toxicity evaluation and oxidative stress response of fumaronitrile, a persistent organic pollutant (POP) of industrial wastewater on tilapia fish (Oreochromis mossambicus). Environ. Res. 2022, 204, 112030. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cordero, F.J.; Delgadillo, T.; Sanchez-Zazueta, E.; Cai, J. Tilapia Aquaculture in Mexico-Assessment with a Focus on Social and Economic Performance; Food & Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Rashed, M. Cadmium and lead levels in fish (Tilapia nilotica) tissues as biological indicator for lake water pollution. Environ. Monit. Assess. 2001, 68, 75–89. [Google Scholar] [CrossRef]
- Monteiro, S.M.; dos Santos, N.M.; Calejo, M.; Fontainhas-Fernandes, A.; Sousa, M. Copper toxicity in gills of the teleost fish, Oreochromis niloticus: Effects in apoptosis induction and cell proliferation. Aquat. Toxicol. 2009, 94, 219–228. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Badran, S.R.; Marie, M.A. Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus, using hematological, bioaccumulation and histological biomarkers. Fish Physiol. Biochem. 2016, 42, 1225–1236. [Google Scholar] [CrossRef]
- Ali, A.; Al-Ogaily, S.; Al-Asgah, N.; Gropp, J. Effect of sublethal concentrations of copper on the growth performance of Oreochromis niloticus. J. Appl. Ichthyol. 2003, 19, 183–188. [Google Scholar] [CrossRef]
- Mutlu, E.; Aydın, S.; Kutlu, B. Alterations of growth performance and blood chemistry in nile tilapia (Oreochromis niloticus) affected by copper sulfate in long-term exposure. Turk. J. Fish. Aquat. Sci. 2015, 15, 481–488. [Google Scholar] [CrossRef]
- Ezeonyejiaku, C.D.; Obiakor, M.O.; Ezenwelu, C.O. Toxicity of copper sulphate and behavioral locomotor response of tilapia (Oreochromis niloticus) and catfish (Clarias gariepinus) species. Online J. Anim. Feed Res. (OJAFR) 2011, 1, 130–134. [Google Scholar]
- Pandit, N.; Nakamura, M. Effect of high temperature on survival, growth and feed conversion ratio of Nile tilapia, Oreochromis niloticus. Our Nat. 2010, 8, 219–224. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- IPCC. Annex VI: Climatic Impact-driver and Extreme Indices. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 2205–2214. [Google Scholar]
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Di Luca, A.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; et al. Weather and Climate Extreme Events in a Changing Climate. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 1513–1766. [Google Scholar]
- Verburga, P.; Hecky, R.E. The physics of the warming of Lake Tanganyika by climate change. Limnol. Oceanogr. 2009, 54, 2418–2430. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- Dan-kishiya, A.S.; Solomon, J.R.; Alhaji, U.A. Influence of temperature on the respiratory rate of Nile Tilapia, Oreochromis niloticus (Pisces: Cichlidae) in the laboratory. Res. J. Costa Rican Distance Educ. Univ. 2016, 8, 27–30. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Daniel, D.; Nunes, B.; Pinto, E.; Ferreira, I.M.; Correia, A.T. Assessment of paracetamol toxic effects under varying seawater pH conditions on the marine polychaete Hediste diversicolor using biochemical endpoints. Biology 2022, 11, 581. [Google Scholar] [CrossRef]
- Dionisio, R.; Daniel, D.; Arenas, F.; Campos, J.C.; Costa, P.C.; Nunes, B.; Correia, A.T. Effects of pH on salicylic acid toxicity in terms of biomarkers determined in the marine gastropod Gibbula umbilicalis. Mar. Environ. Res. 2020, 158, 104995. [Google Scholar] [CrossRef]
- Barbosa, C.C.; Calijuri, M.d.C.; dos Santos, A.C.A.; Ladwig, R.; de Oliveira, L.F.A.; Buarque, A.C.S. Future projections of water level and thermal regime changes of a multipurpose subtropical reservoir (Sao Paulo, Brazil). Sci. Total Environ. 2021, 770, 144741. [Google Scholar] [CrossRef]
- Singh, D.; Nath, K.; Sharma, Y.; Trivedi, S. Hepatotoxic effect of Cu (II) in freshwater fish, Channa punctatus: A histopathological study. Res. Environ. Life Sci. 2008, 1, 13–16. [Google Scholar]
- Arellano, J.M.; Storch, V.; Sarasquete, C. Histological changes and copper accumulation in liver and gills of the Senegales sole, Solea senegalensis. Ecotoxicol. Environ. Saf. 1999, 44, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Fernandes, A.; Ferreira-Cardoso, J.V.; Garcia-Santos, S.; Monteiro, S.M.; Carrola, J.; Matos, P.; Fontaínhas-Fernandes, A. Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesqui. Veterinária Bras. 2007, 27, 103–109. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. Histopathology of lambda-cyhalothrin on tissues (gill, kidney, liver and intestine) of Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2007, 24, 286–291. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Rocha, E.; Fontaínhas-Fernandes, A.; Sousa, M. Quantitative histopathology of Oreochromis niloticus gills after copper exposure. J. Fish Biol. 2008, 73, 1376–1392. [Google Scholar] [CrossRef]
- Flores-Lopes, F.; Thomaz, A. Histopathologic alterations observed in fish gills as a tool in environmental monitoring. Braz. J. Biol. 2011, 71, 179–188. [Google Scholar] [CrossRef]
- Carrola, J.S. Histopatologia Hepática como Biomarcador da Poluição em Sistemas Aquáticos—Bacias Hidrográficas dos Rios Douro e Ave. Master’s Thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal, 2003. [Google Scholar]
- Lima-Junior, S.E.; Cardone, I.B.; Goitein, R. Determination of a method for calculation of Allometric Condition Factor of fish. Acta Sci. Biol. Health Sci. 2002, 24, 397–400. [Google Scholar]
- Morado, C.N.; Araújo, F.G.; Gomes, I.D. The use of biomarkers for assessing effects of pollutant stress on fish species from a tropical river in Southeastern Brazil. Acta Scientiarum. Biol. Sci. 2017, 39, 431–439. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Murray, L.; Rennie, M.D.; Enders, E.C.; Pleskach, K.; Martin, J.D. Effect of nanosilver on cortisol release and morphometrics in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2017, 36, 1606–1613. [Google Scholar] [CrossRef]
- Pinto, A.L.; Varandas, S.; Coimbra, A.M.; Carrola, J.; Fontaínhas-Fernandes, A. Mullet and gudgeon liver histopathology and macroinvertebrate indexes and metrics upstream and downstream from a wastewater treatment plant (Febros River—Portugal). Environ. Monit. Assess. 2010, 169, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- van Hullebusch, E.; Chatenet, P.; Deluchat, V.; Chazal, P.M.; Froissard, D.; Botineau, M.; Ghestem, A.; Baudu, M. Copper accumulation in a reservoir ecosystem following copper sulfate treatment (St. Germain Les Belles, France). Water Air Soil Pollut. 2003, 150, 3–22. [Google Scholar] [CrossRef]
- Smriti, A.A.; Lodhi, S.; Shukla, S. Copper toxicity in aquatic ecosystem: A Review. Int. J. Fish Aquat. Stud. 2023, 11, 134–138. [Google Scholar]
- Hoseini, S.M.; Hedayati, A.; Mirghaed, A.T.; Ghelichpour, M. Toxic effects of copper sulfate and copper nanoparticles on minerals, enzymes, thyroid hormones and protein fractions of plasma and histopathology in common carp Cyprinus carpio. Exp. Toxicol. Pathol. 2016, 68, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, V.K.; Gougoula, C.; Anestis, A.; Pörtner, H.O.; Michaelidis, B. Monitoring the biochemical and cellular responses of marine bivalves during thermal stress by using biomarkers. Mar. Environ. Res. 2012, 73, 70–77. [Google Scholar] [CrossRef]
- Carvalho, C.S.; Fernandes, M.N. Effect of temperature on copper toxicity and hematological responses in the neotropical fish Prochilodus scrofa at low and high pH. Aquaculture 2006, 251, 109–117. [Google Scholar] [CrossRef]
- Xiang, Q.-Q.; Yan, H.; Luo, X.-W.; Kang, Y.-H.; Hu, J.-M.; Chen, L.-Q. Integration of transcriptomics and metabolomics reveals damage and recovery mechanisms of fish gills in response to nanosilver exposure. Aquat. Toxicol. 2021, 237, 105895. [Google Scholar] [CrossRef]
- Jin, Z.; Xu, Y.; Zhou, H.; Cui, A.; Jiang, Y.; Wang, B.; Zhang, W. Effects of copper exposure and recovery in juvenile yellowtail kingfish (Seriola lalandi): Histological, physiological and molecular responses. Aquac. Rep. 2023, 31, 101669. [Google Scholar] [CrossRef]
- Garcia-Santos, S.; Fontaínhas-Fernandes, A.; Wilson, J.M. Cadmium tolerance in the Nile tilapia (Oreochromis niloticus) following acute exposure: Assessment of some ionoregulatory parameters. Environ. Toxicol. Int. J. 2006, 21, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Eom, J. The osmorespiratory compromise in the fish gill. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 254, 110895. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Houben, N.; Pauly, D. On being the wrong size, or the role of body mass in fish kills and hypoxia exposure. Environ. Biol. Fishes 2023, 106, 1651–1667. [Google Scholar] [CrossRef]
- Gashkina, N.A.; Moiseenko, T.I.; Shuman, L.A.; Koroleva, I.M. Biological responses of whitefish (Coregonus lavaretus L.) to reduced toxic impact: Metal accumulation, haematological, immunological, and histopathological alterations. Ecotoxicol. Environ. Saf. 2022, 239, 113659. [Google Scholar] [CrossRef]
- Fernandes, M.N.; Mazon, A.F. Environmental pollution and fish gill morphology. In Fish Adaptations; Science Publishers: Enfield, CT, USA, 2003; pp. 203–231. [Google Scholar]
- Fernandes, M.; Paulino, M.; Sakuragui, M.; Ramos, C.; Pereira, C.D.S.; Sadauskas-Henrique, H. Organochlorines and metals induce changes in the mitochondria-rich cells of fish gills: An integrative field study involving chemical, biochemical and morphological analyses. Aquat. Toxicol. 2013, 126, 180–190. [Google Scholar] [CrossRef]
- Paruruckumani, P.; Maharajan, A.; Ganapiriya, V.; Narayanaswamy, Y.; Jeyasekar, R.R. Surface ultrastructural changes in the gill and liver tissue of asian sea bass Lates calcarifer (Bloch) exposed to copper. Biol. Trace Elem. Res. 2015, 168, 500–507. [Google Scholar] [CrossRef]
- Carrola, J.; Fontaínhas-Fernandes, A.; Matos, P.; Rocha, E. Liver Histopathology in Brown Trout (Salmo trutta f. fario) from the Tinhela River, Subjected to Mine Drainage from the Abandoned Jales Mine (Portugal). Bull. Environ. Contam. Toxicol. 2009, 83, 35–41. [Google Scholar] [CrossRef]
- Camara, E.M.; Caramaschi, E.P.; Petry, A.C. Fator de condição: Bases conceituais, aplicações e perspectivas de uso em pesquisas ecológicas com peixes. Oecol. Aust. 2011, 15, 249–274. [Google Scholar] [CrossRef]
- Fontaínhas-Fernandes, A. The use of biomarkers in aquatic toxicology studies. Rev. Port. Zootec. 2005, 12, 67–86. [Google Scholar]
- Abdel-Moneim, A.M.; Al-Kahtani, M.A.; Elmenshawy, O.M. Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 2012, 88, 1028–1035. [Google Scholar] [CrossRef]
- Camargo, M.M.P.; Martinez, C.B.R. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Al-Bairuty, G.A.; Shaw, B.J.; Handy, R.D.; Henry, T.B. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2013, 126, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.C.; Wheeler, J.R. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquat. Toxicol. 2018, 197, 60–78. [Google Scholar] [CrossRef]
- Wolf, J.C.; Wolfe, M.J. A Brief Overview of Nonneoplastic Hepatic Toxicity in Fish. Toxicol. Pathol. 2005, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]
- van Dyk, J.C.; Pieterse, G.M.; van Vuren, J.H.J. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol. Environ. Saf. 2007, 66, 432–440. [Google Scholar] [CrossRef]
- Nussey, G.; Van Vuren, J.H.J.; Du Preez, H.H. Acute toxicity tests of copper on juvenile Mozambique tilapia, Oreochromis mossambicus (Cichlidae), at different temperatures. S. Afr. J. Wildl. Res.—24-Mon. Delayed Open Access 1996, 26, 47–55. [Google Scholar]
- Perschbacher, P.W. Temperature effects on acute copper toxicity to juvenile channel catfish Ictalurus punctatus. Aquaculture 2005, 243, 225–228. [Google Scholar] [CrossRef]
- Al-Tamimi, A.H.; Al-Azzawi, A.J. The acute and chronic toxicity of copper on the behavioral responses and hematological parameters of freshwater fish, common carp (Cyprinus carpio L.). Iraqi J. Sci. 2015, 56, 2835–2845. [Google Scholar]
- Siddiqui, A.A.; Noori Arifa, N.A. Toxicity of heavy metal copper and its effect on the behaviour of freshwater Indian catfish, Clarias batrachus (Linn.). Curr. Biot. 2011, 4, 405–411. [Google Scholar]
- Sloman, K.A.; Baker, D.W.; Wood, C.M.; McDonald, G. Social interactions affect physiological consequences of sublethal copper exposure in rainbow trout, Oncorhynchus mykiss. Environ. Toxicol. Chem. Int. J. 2002, 21, 1255–1263. [Google Scholar] [CrossRef]
- King, M.; Sardella, B. The effects of acclimation temperature, salinity, and behavior on the thermal tolerance of Mozambique tilapia (Oreochromis mossambicus). J. Exp. Zool. Part A Ecol. Integr. Physiol. 2017, 327, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chai, Z.Y.; Yen, P.-L.; How, C.M.; Yu, C.-W.; Chang, C.-H.; Liao, V.H.-C. The bioavailability and potential ecological risk of copper and zinc in river sediment are affected by seasonal variation and spatial distribution. Aquat. Toxicol. 2020, 227, 105604. [Google Scholar] [CrossRef] [PubMed]
| CuSO4 Nominal Exposure Concentrations (mg/L) | Measured Cu Concentrations in Water (mg/L ± SD) |
|---|---|
| 0 | 0.006 ± 0.002 |
| 1.1 | 1.768 ± 0.242 |
| 3.6 | 4.786 ± 0.604 |
| Time (days) | 14 | 28 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 0 | 1.1 | 3.6 | 0 | 1.1 | 3.6 | p-Value | ||||||
| Temperature ( °C) | 25 | 32 | 25 | 32 | 25 | 32 | 25 | 32 | 25 | 32 | 25 | 32 | |
| Aneurysms | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.02 ± 0.04 a | 0.60 ± 0.49 b | 0.16 ± 0.34 a | 0.02 ± 0.03 a | 0.01 ± 0.02 a | 0.25 ± 0.10 ab | 0.11 ± 0.09 a | 0.59 ± 0.12 b | 0.20 ± 0.06 a | <0.0001 |
| Vasodilatation | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.07 ± 0.10 | 0.02 ± 0.04 | 0.03 ± 0.06 | 0.00 ± 0.00 | 0.04 ± 0.06 | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.02 ± 0.05 | 0.05 ± 0.07 | 0.09 ± 0.21 | 0.7360 |
| Lamellar epithelium lifting | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.02 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.12 | 0.4064 |
| Filament epithelium proliferation | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.08 ± 0.17 | 0.3962 |
| Lamellar fusion | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.05 | 0.02 ± 0.01 | 0.2444 |
| Oedema | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.04 | 0.00 ± 0.00 | 0.04 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.00 | 0.05 ± 0.07 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.5632 |
| Bending of gill lamellae | 0.00 ± 0.00 a | 0.03 ± 0.05 ab | 0.10 ± 0.11 ab | 0.17 ± 0.11 ab | 0.08 ± 0.06 ab | 0.00 ± 0.00 a | 0.02 ± 0.02 a | 0.01 ± 0.01 a | 0.10 ± 0.11 ab | 0.19 ± 0.09 ab | 0.10 ± 0.07 ab | 0.24 ± 0.03 b | <0.0001 |
| Time (days) | 14 | 28 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 0 | 1.1 | 3.6 | 0 | 1.1 | 3.6 | p-Value | ||||||
| Temperature (°C) | 25 | 32 | 25 | 32 | 25 | 32 | 25 | 32 | 25 | 32 | 25 | 32 | |
| Macrophage aggregates | 1.17 ± 0.89 | 1.08 ± 0.42 | 1.12 ± 0.15 | 1.20 ± 0.55 | 0.76 ± 0.30 | 0.85 ± 0.78 | 1.56 ± 0.63 | 1.24 ± 0.61 | 1.08 ± 0.28 | 0.74 ± 0.44 | 0.76 ± 0.31 | 0.92 ± 0.39 | 0.7545 |
| Vacuolization | 0.00 ± 0.00 a | 0.76 ± 0.88 ab | 2.20 ± 1.73 b | 0.46 ± 0.68 ab | 0.88 ± 1.24 ab | 0.00 ± 0.00 a | 0.40 ± 0.65 ab | 0.08 ± 0.10 a | 0.88 ± 1.36 ab | 0.00 ± 0.00 a | 0.78 ± 0.87 ab | 0.00 ± 0.00 a | 0.0124 |
| Hyalinization | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.04 ± 0.29 acd | 2.24 ± 0.83 cde | 1.32 ± 1.64 acd | 4.00 ± 0.60 be | 0.64 ± 0.43 ac | 0.60 ± 0.71 ac | 1.38 ± 1.17 acd | 4.42 ± 0.62b | 2.42 ± 1.03 de | 4.62 ± 0.58 b | <0.0001 |
| Granulomas | 0.00 ± 0.00 | 0.02 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.3754 |
| Necrosis | 0.14 ± 0.12 | 0.60 ± 0.52 | 0.40 ± 0.19 | 0.24 ± 0.19 | 0.34 ± 0.09 | 0.14 ± 0.17 | 0.26 ± 0.17 | 0.24 ± 0.29 | 0.18 ± 0.08 | 0.16 ± 0.25 | 0.28 ± 0.15 | 0.18 ± 0.19 | 0.5350 |
| Temperature | Concentration | Period of Observation | ||
|---|---|---|---|---|
| P0 | P1 | P2 | ||
| 25 °C | 0 mg/L | Normal | Normal | Normal |
| 25 °C | 1.1 mg/L | Normal | FI | SB |
| 25 °C | 3.6 mg/L | HC | FI | SB |
| 32 °C | 0 mg/L | Normal | Normal | FI |
| 32 °C | 1.1 mg/L | Normal | FI | GS |
| 32 °C | 3.6 mg/L | Normal | GS | GS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, O.; Pinto, M.Q.; Tavares, D.; Ferreira-Cardoso, J.V.; Correia, A.T.; Carrola, J.S. Copper and Temperature Interaction Induced Gill and Liver Lesions and Behaviour Alterations in Mozambique Tilapia (Oreochromis mossambicus). Water 2024, 16, 2499. https://doi.org/10.3390/w16172499
Ribeiro O, Pinto MQ, Tavares D, Ferreira-Cardoso JV, Correia AT, Carrola JS. Copper and Temperature Interaction Induced Gill and Liver Lesions and Behaviour Alterations in Mozambique Tilapia (Oreochromis mossambicus). Water. 2024; 16(17):2499. https://doi.org/10.3390/w16172499
Chicago/Turabian StyleRibeiro, Ondina, Mónica Quelhas Pinto, Diana Tavares, Jorge Ventura Ferreira-Cardoso, Alberto Teodorico Correia, and João Soares Carrola. 2024. "Copper and Temperature Interaction Induced Gill and Liver Lesions and Behaviour Alterations in Mozambique Tilapia (Oreochromis mossambicus)" Water 16, no. 17: 2499. https://doi.org/10.3390/w16172499
APA StyleRibeiro, O., Pinto, M. Q., Tavares, D., Ferreira-Cardoso, J. V., Correia, A. T., & Carrola, J. S. (2024). Copper and Temperature Interaction Induced Gill and Liver Lesions and Behaviour Alterations in Mozambique Tilapia (Oreochromis mossambicus). Water, 16(17), 2499. https://doi.org/10.3390/w16172499







