Biodiversity and Soil Reinforcement Effect of Vegetation Buffer Zones: A Case Study of the Tongnan Section of the Fujiang River Basin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Quadrat Survey Sampling and Biodiversity Analysis
2.3. Determination of Root Mechanics and Morphological Characteristics
2.4. Measuring Soil Anti-Scouribility
2.5. Large Box Shear Test
3. Result
3.1. Species Composition and Biodiversity Analysis of Vegetation Buffer Zones
3.2. Mechanical and Morphological Characteristics of Root
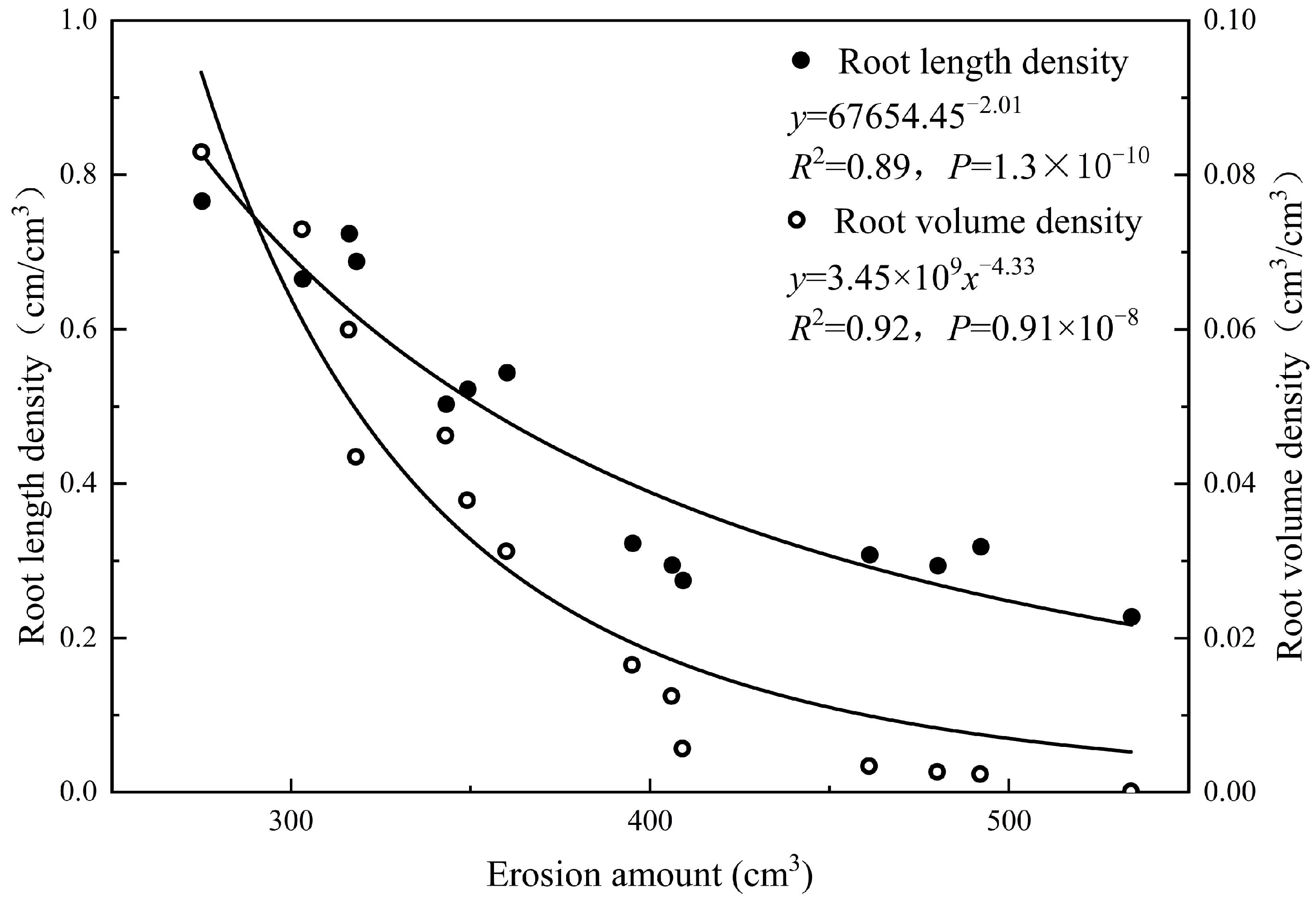
3.3. Hydraulic Effects of Roots on Scour
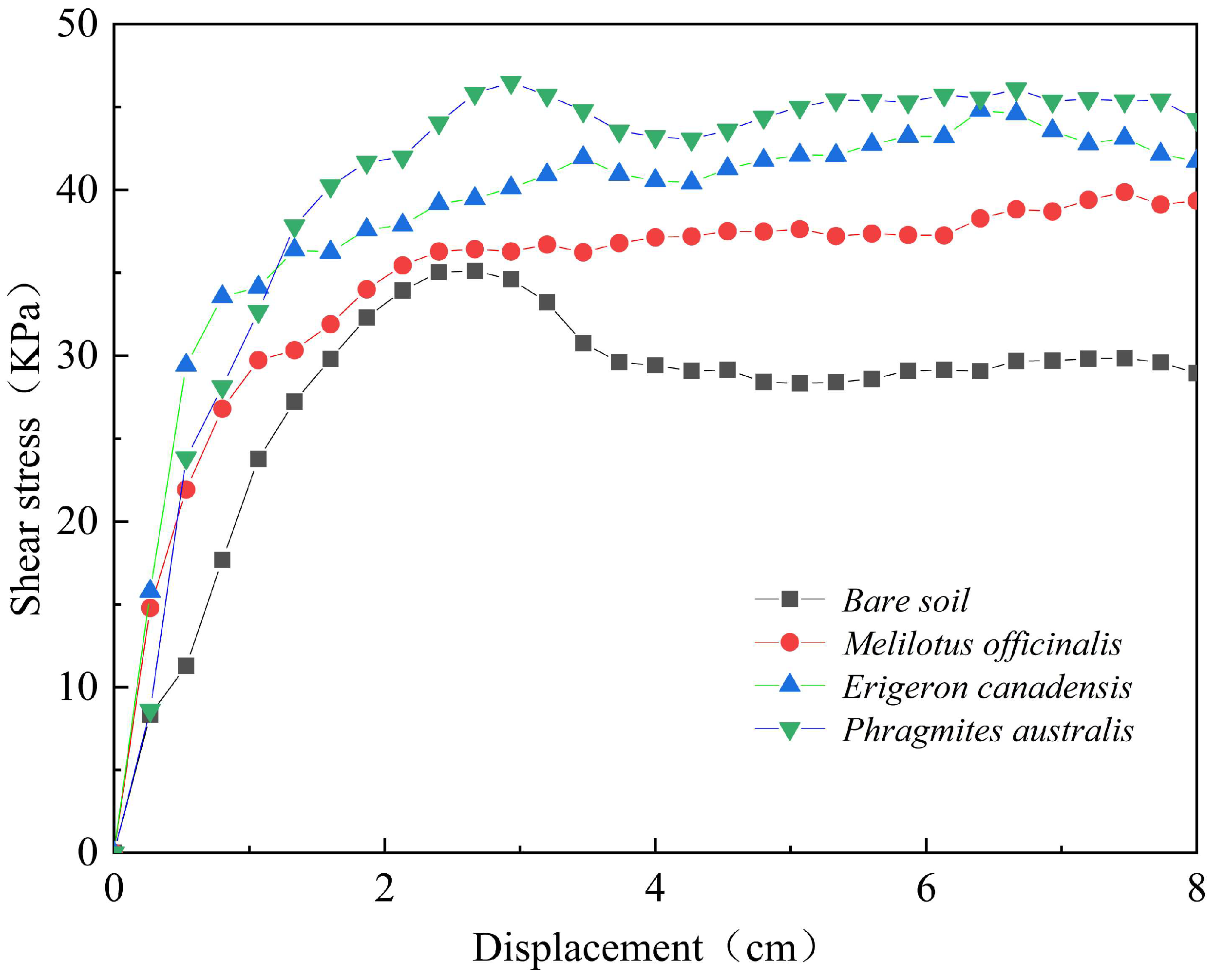
3.4. Shear Characteristics of Root–Soil Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naiman, R.J.; Decamps, H. The ecology of interface: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Guo, E.; Sun, R.; Chen, L. Main ecological service functions in riparian vegetation buffer zone: Research progress and prospects. Chin. J. Ecol. 2011, 30, 1830–1837. [Google Scholar]
- United States. Environmental Protection Agency. Office of Water. Guidance specifying management measures for sources of nonpoint pollution in coastal waters: Issued under the authority of Section 6217(g) of the Coastal Zone Act Reauthorization Amendments of 1990. Afr. J. Reprod. Health 2007, 11, 182–196. [Google Scholar]
- Nilsson, C.; Berggrea, K. Alterations of riparian ecosystems caused by river regulation. Bioscience 2000, 50, 783–793. [Google Scholar] [CrossRef]
- Narumalan, S.; Zhou, Y.; Jensen, J.R. Application of remote sensing and geographic information systems to the delineation and analysis of riparian buffer zones. Aquat. Bot. 1997, 58, 393–409. [Google Scholar] [CrossRef]
- Phillips, J.D. An evaluation of the factors determining the effectiveness of water quality buffer zones. J. Hydrol. 1989, 107, 133–145. [Google Scholar] [CrossRef]
- Dillaha, T.A.; Reneau, R.B.; Mostaghimi, S.; Lee, D. Vegetative Filter Strips for Agricultural Nonpoint Source Pollution Control. Trans. Am. Soc. Agric. Eng. 1989, 32, 513–519. [Google Scholar] [CrossRef]
- Sabater, S.; Butturini, A.; Clement, J.C.; Burt, T.; Dowrick, D.; Hefting, M.; Matre, V.; Pinay, G.; Postolache, C.; Rzepecki, M. Nitrogen removal by riparian buffers along a European climatic gradient: Patterns and factors of variation. Ecosystems 2003, 6, 20–30. [Google Scholar] [CrossRef]
- Hilary, B.; Chris, B.; North, B.E.; Maria, A.Z.A.; Andrew, W. Riparian buffer length is more influential than width on river water quality: A case study in southern Costa Rica. J. Environ. Manag. 2021, 286, 112132. [Google Scholar]
- Aguiar, T.R.; Rasera, K.; Parron, L.M.; Brito, A.G.; Ferreira, M.T. Nutrient removal effectiveness by riparian buffer zones in rural temperate watersheds: The impact of no-till crops practices. Agric. Water Manag. 2015, 149, 74–80. [Google Scholar] [CrossRef]
- China Wetland Vegetation Committee. Wetland Vegetation in China; Science Press: Beijing, China, 1999. [Google Scholar]
- Yang, F.; Wang, Y.; Chan, Z. Review of environmental conditions in the water level fluctuation zone: Perspectives on riparian vegetation engineering in the Three Gorges Reservoir. Aquat. Ecosyst. Health Manag. 2015, 18, 240–249. [Google Scholar] [CrossRef]
- Gu, L.; Gao, J.; Wang, Y.; Qian, B.; Wang, Y. Soil bioengineering-a new Technique to Generate Riverbank Restoration. Adv. Mater. Res. 2012, 518–523, 1795–1799. [Google Scholar] [CrossRef]
- Rey, F. Effectiveness of vegetation barriers for marly sediment trapping. Earth Surf. Process. Landf. 2010, 29, 1161–1169. [Google Scholar] [CrossRef]
- Mankin, K.R.; Ngandu, D.M.; Barden, C.J.; Hutchinson, S.L.; Geyer, W.A. Grassshrub riparian buffer removal of sediment, phosphorus, and nitrogen from simulated runoff. JAWRA J. Am. Water Resour. Assoc. 2010, 43, 1108–1116. [Google Scholar] [CrossRef]
- Norris, J.E.; Stokes, A.; Mickovski, S.B.; Cammeraat, E.; Achim, A. Slope Stability and Erosion Control: Ecotechnological Solutions; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Ji, X.; Xia, G.; Zhang, H. Effects of Amorpha Fruticosa Root System on Slope Soil and Water Conservation. Hubei For. Sci. Technol. 2016, 45, 16–19. [Google Scholar]
- Xu, W. Anti-Scourability of the Herbaceous Root-Soil System in the Riparian Zone of the Three Gorges Reservoir, China; Chongqing Normal University: Chongqing, China, 2019. [Google Scholar]
- Cheng, H.; Yu, Y.; Tang, X.; Zhou, Z.; Zhao, Y. Effect of the Root Systems of Herbaceous Plant on Soil Anti-scourbility in the Riparian Area of Lower Reaches of Puyang River. Res. Soil Water Conserv. 2017, 24, 60–63+69. [Google Scholar]
- Shen, Z.; Li, G.; Liu, C.; Yu, D.; Hu, X.; Li, X. Study on the Root Mechanical Properties and Shear Strength of Root-Soil Composites for Four Plant Species in the Yellow River Source Region. Soil Water Conserv. China 2021, 7, 49–52. [Google Scholar]
- Hao, G.; Liu, X.; Zhang, Q.; Xiang, L.; Yu, B. Optimum Selection of Soil-Reinforced Herbaceous Plants Considering Plant Growth and Distribution Characteristics. J. Soil Sci. Plant Nutr. 2022, 22, 1743–1757. [Google Scholar] [CrossRef]
- Li, H.; Qin, H.; Zhang, H.; Li, J.; Liu, Y. Characteristics of Plant Communities in Different Herbaceous Layers of Plantation Forests in Pingshuo Coal Mine Reclamation Area. J. Shanxi Univ. (Nat. Sci. Ed.) 2023, 1–11. Available online: http://kns.cnki.net/kcms/detail/14.1105.n.20231016.0945.003.html (accessed on 1 October 2024).
- Li, Y.; Wang, Y.; Wang, Y.; Ma, C. Effects of Vitex negundo roo t properties on soil resistance caused by pull-out forces at different positions around the stem. Catena 2017, 158, 148–160. [Google Scholar] [CrossRef]
- Fan, C.C. A displacement-based model for estimating the shear resistance of root-permeated soils. Plant Soil 2012, 355, 103–119. [Google Scholar] [CrossRef]
- Preti, F.; Giadrossich, F. Root reinforcement and slope bioengineering stabilization by Spanish Broom (Spartium junceum L.). Hydrol. Earth Syst. Sci. Discuss 2009, 6, 1713–1726. [Google Scholar] [CrossRef]
- Hanson, G.J.; Simon, A. Erodibility of cohesive streambeds in the loess area of the midwestern USA. Hydrol. Process. 2001, 15, 23–38. [Google Scholar] [CrossRef]
- Pollen-Bankhend, N.; Simon, A. Hydrologic and hydraulic effects of riparian root networks on streambank stability: Is mechanical root-reinforcement the whole story? Geomorphology 2010, 116, 353–362. [Google Scholar] [CrossRef]
- Ekanayake, J.C.; Phillips, C.J. A method for stability analysis of vegetated hillslopes: An energy approach. Can. Geotech. J. 1999, 36, 1172–1184. [Google Scholar] [CrossRef]
- Wu, J.; Lv, J. Effects of land use change on the biodiversity. Ecol. Environ. 2008, 17, 1276–1281. [Google Scholar]
- Falcucci, A.; Maiorano, L.; Boitani, L. Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landsc. Ecol. 2007, 22, 617–631. [Google Scholar] [CrossRef]
- Marques, A.; Martins, I.S.; Kastner, T.; Plutzar, C.; Theurl, M.C.; Eisenmenger, N.; Huijbregts, M.A.J.; Wood, R.; Stadler, K.; Bruckner, M.; et al. Increasing impacts of land use on biodiversity and carbon sequestration driven by population and economic growth. Nat. Ecol. Evol. 2019, 3, 628–637. [Google Scholar]
- Fan, C.C.; Tsai, M.H. Spatial distribution of plant root forces in root-permeated soils subject to shear. Soil Tillage Res. 2016, 156, 1–15. [Google Scholar] [CrossRef]
- Saifuddin, M.; Osman, N. Evaluation of hydro-mechanical properties and root architecture of plants for soil reinforcement. Curr. Sci. A Fortn. J. Res. 2014, 107, 845–852. [Google Scholar]
- Adhikari, A.R.; Gautam, M.R.; Yu, Z.; Imada, S.; Acharya, K. Estimation of root cohesion for desert shrub species in the Lower Colorado riparian ecosystem and its potential for streambank stabilization. Ecol. Eng. 2013, 51, 33–44. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; Baerdemaeker, J.D.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Wang, Y.; Zhang, H.; Li, Y.; Liu, Y. An analysis on soil physical enhancement effects of root system of pinus tabulae formis and acer truncatum based on two models. Bull. Soil Water Conserv. 2015, 35, 277–282. [Google Scholar]
- Liu, G. Study on soil anti-scourability and its mechanism of grassland on Loess Plateau. J. Soil Eros. Soil Water Conserv. 1998, 4, 94–97. [Google Scholar]
- Hudek, C.; Stanchi, S.; D’Amico, M.; Freppaz, M. Quantifying the contribution of the root system of alpine vegetation in the soil aggregate stability of moraine. Int. Soil Water Conserv. Res. 2017, 5, 36–42. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J. The importance of plant root characteristics in controlling concentrated flow erosion rates. Earth Surf. Process Landf. 2003, 28, 371–384. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Gyssels, G.; Knapen, A. Effects of grass roots on the erodibility of topsoils during concentrated flow. Geomorphology 2006, 76, 54–67. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, S.; Lu, Z.; Zhang, T. Effect of Plant Roots on Penetrability and Anti-Scouri-bility of Red Soil Derived from Granite. Pedosphere 2000, 10, 183–188. [Google Scholar]
- Angela, G. Plants as river system engineers. Earth Surf. Process. Landf. 2014, 39, 4–25. [Google Scholar]
- Ding, P. The impact of root density on soil erosion and erosiveness under simulated rainfall. Hydro Sci. Cold Zone Eng. 2022, 5, 40–44. [Google Scholar]
- Zhang, Z. Effect of Salix Psammophyla Roots on Soil Anti-Erosion and Its Mechanisms in the Water-Wind Erosion Crisscross Region on the Loess Plateau; Northwest A&F University: Xianyang, China, 2018. [Google Scholar]
- Li, Z.; Ouyang, M.; Xiao, H.; Wang, J.; Li, T.; Liu, S. Improvement of slope soil consolidation capacity of plant root system based on regulation of root architecture. Rock Soil Mech. 2021, 42, 3271–3280+3290. [Google Scholar]
- Zhang, Y.; Li, C.; Han, S.J.; Chen, X. Root architecture of main tree species and the effects on soil reinforcement in typical black soil region. Chin. J. Appl. Ecol. 2021, 32, 1726–1734. [Google Scholar]
- Forster, M.; Ugarte, C.; Lamande, M.; Faucon, M.P. Root traits of crop species contributing to soil shear strength. Geoderma 2022, 409, 115642. [Google Scholar] [CrossRef]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Pret, F.; Or, D. Mechanical interactions between neighbouring roots during pullout tests. Plant Soil 2013, 367, 391–406. [Google Scholar] [CrossRef]
- Leung, F.T.Y.; Yan, W.M.; Hau, B.C.H.; Tham, L.G. Root systems of native shrubs and trees in Hong Kong and their effects on enhancing slope stability. Catena 2015, 125, 102–110. [Google Scholar] [CrossRef]
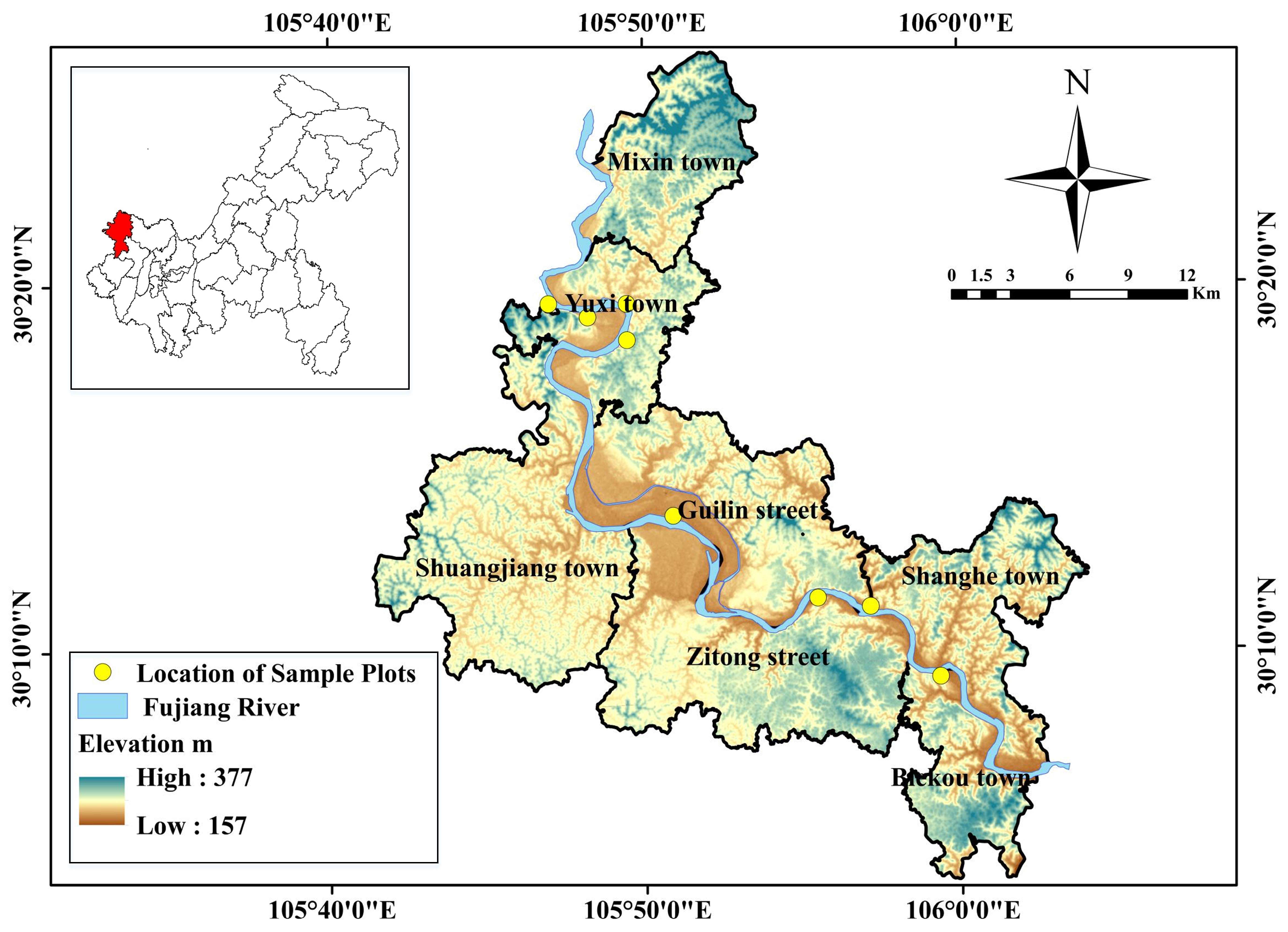

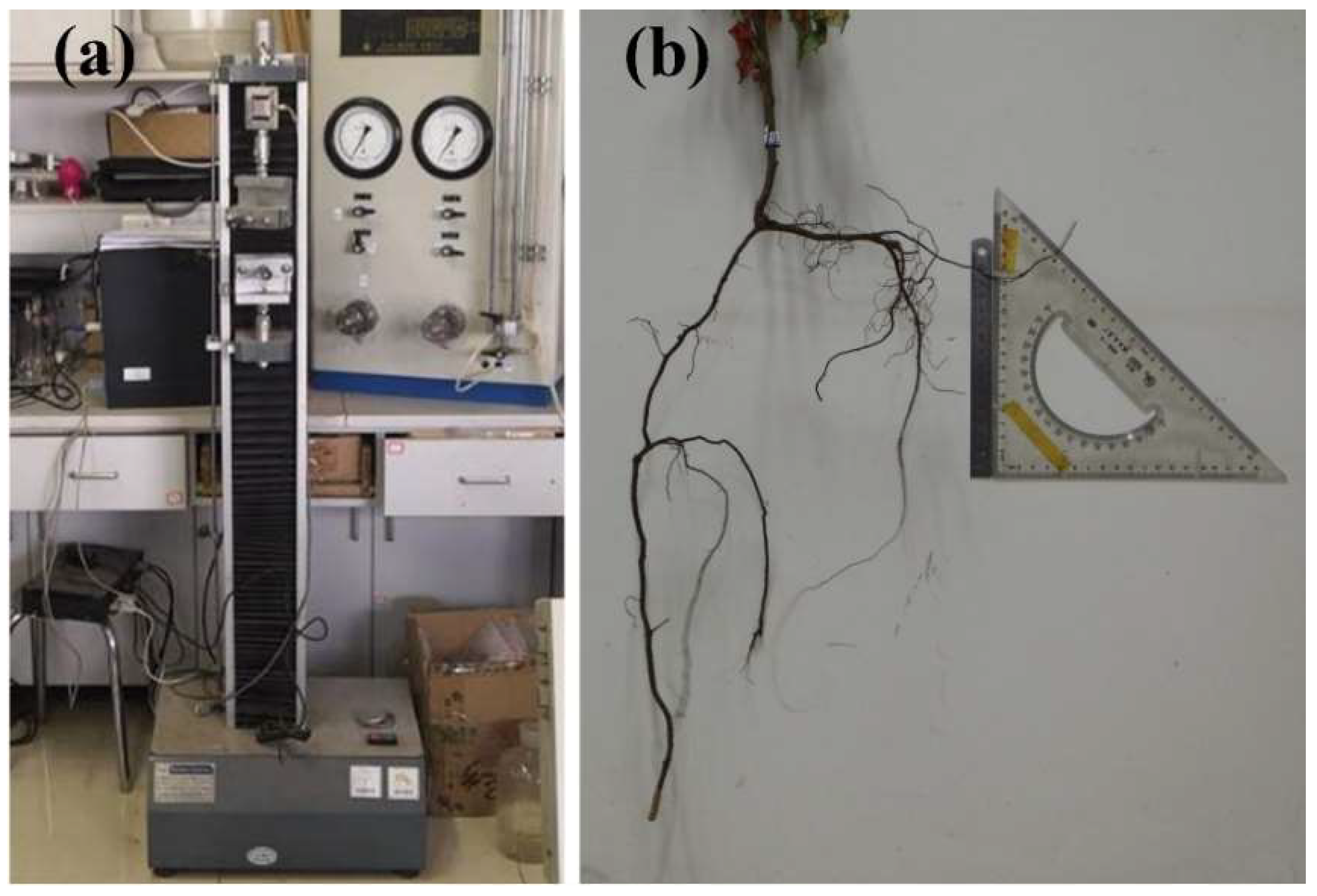




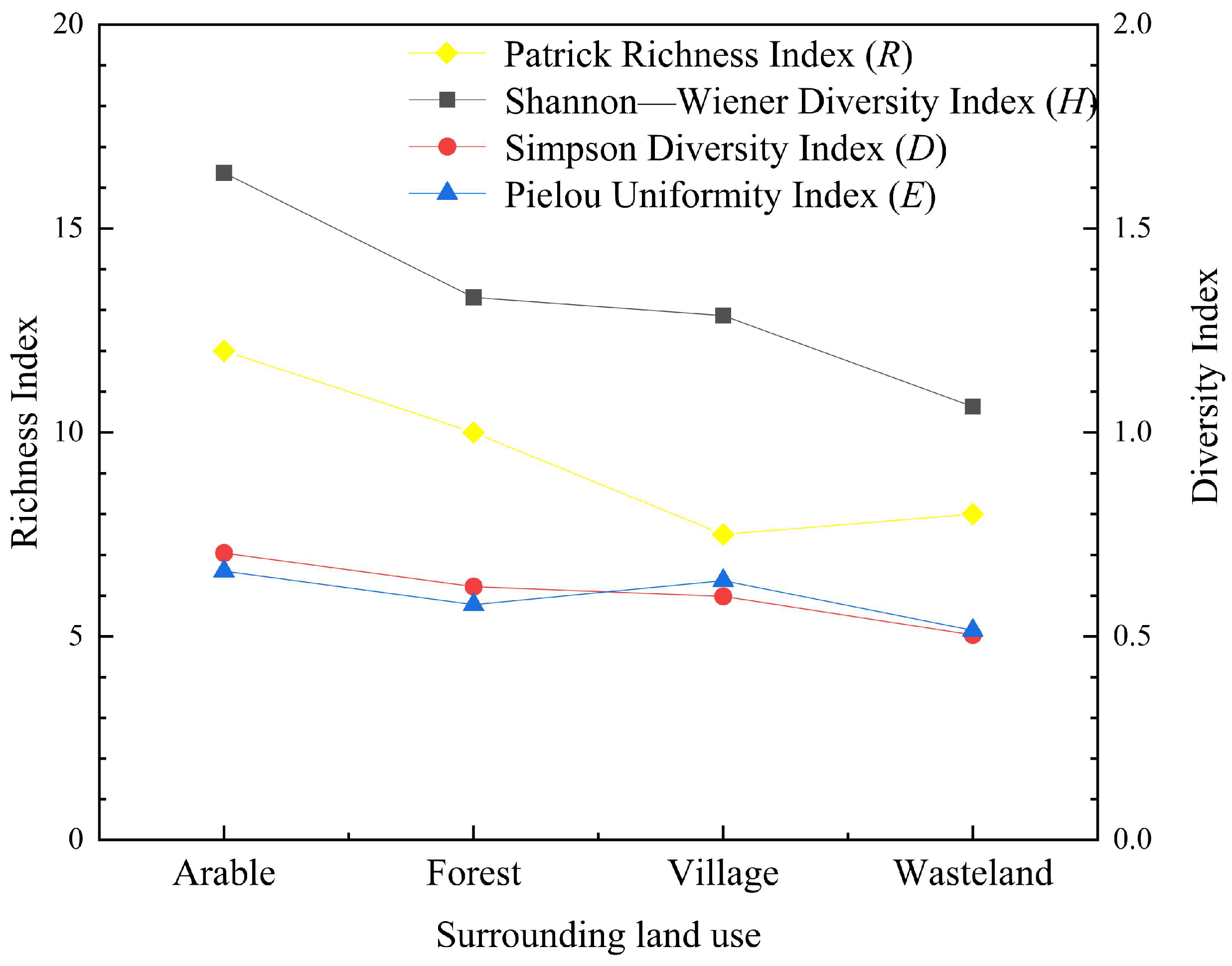


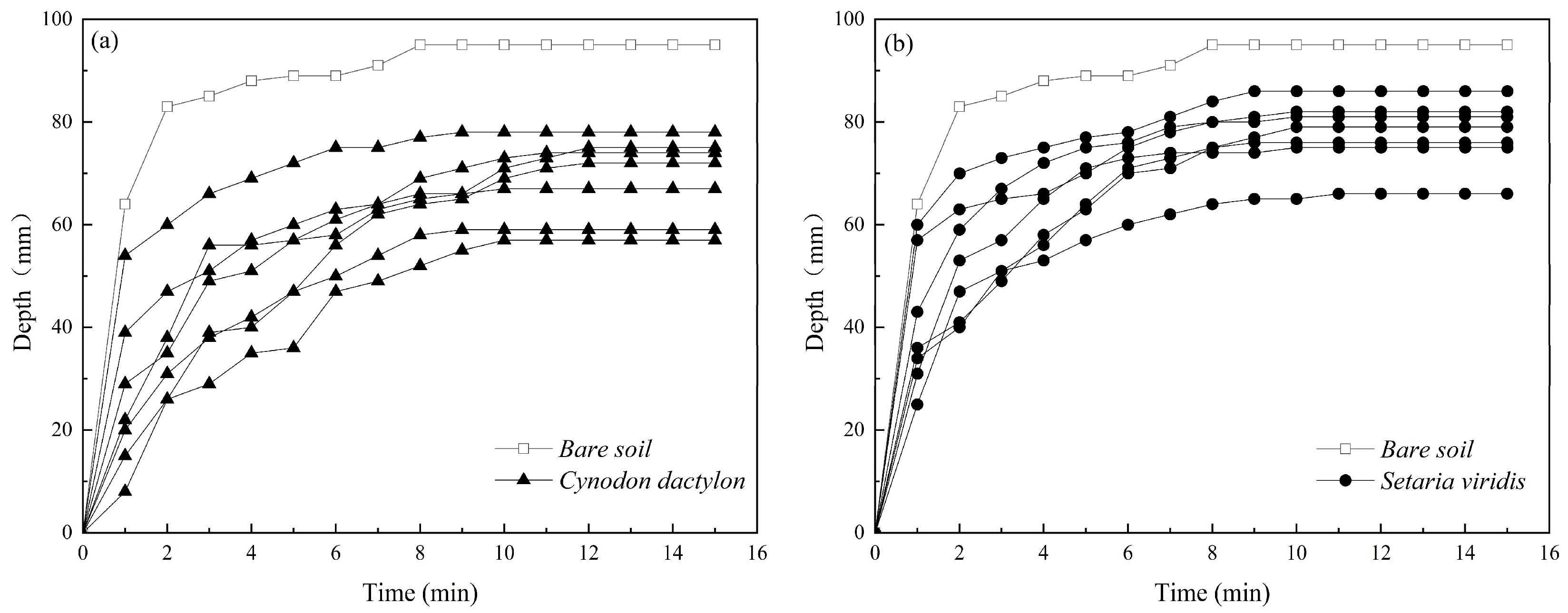
| No. | Name of Sample Plots | Geographical Position | Elevation (m) | Site Type | Soil Type | Surrounding Land Use |
|---|---|---|---|---|---|---|
| 1 | Lujiaba | 105.941, 30.155 | 231.27 | Soft slope | Loam | Arable |
| 2 | Yuxingba | 105.899, 30.187 | 231.60 | Soft slope | Loam | Wasteland |
| 3 | Zhangjiawan | 105.871, 30.190 | 231.10 | Soft slope | Loam | Village |
| 4 | Zhongba | 105.798, 30.228 | 238.87 | Soft slope | Sandy | Arable |
| 5 | Caojiagou | 105.774, 30.314 | 261.85 | Soft slope | Loam | Forest |
| 6 | Yuxi Town | 105.774, 30.324 | 247.81 | Soft slope | Sandy | Village |
| 7 | Yinglong Street | 105.751, 30.321 | 250.44 | Soft slope | Sandy | Wasteland |
| 8 | Hujiawan | 105.734, 30.326 | 254.48 | Soft slope | Sandy | Forest |
| No. | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| 1 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Erigeron | Erigeron canadensis L. |
| 2 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Erigeron | Erigeron annuus (L.) Pers. |
| 3 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Artemisia | Artemisia selengensis Turcz. ex Besser |
| 4 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Artemisia | Artemisia annua L. |
| 5 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Artemisia | Artemisia indica Willd. |
| 6 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Artemisia | Artemisia argyi H. Lév. & Vaniot |
| 7 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Lactuca | Lactuca serriola L. |
| 8 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Symphyotrichum | Symphyotrichum subulatum (Michx.) G. L. Nesom |
| 9 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Xanthium | Xanthium strumarium L. |
| 10 | Angiospermae | Pteridophyta | Asterales | Asteraceae | Carpesium | Carpesium abrotanoides L. |
| 11 | Angiospermae | Pteridophyta | Poales | Poaceae | Cynodon | Setaria viridis (L.) P. Beauv. |
| 12 | Angiospermae | Pteridophyta | Poales | Poaceae | Cynodon | Cynodon dactylon (L.) Persoon |
| 13 | Angiospermae | Pteridophyta | Poales | Poaceae | Phragmites | Phragmites australis (Cav.) Trin. ex Steud |
| 14 | Angiospermae | Pteridophyta | Poales | Typhaceae | Typha | Typha orientalis C. Presl |
| 15 | Angiospermae | Pteridophyta | Caryophyllales | Amaranthaceae | Chenopodium | Chenopodium album L. |
| 16 | Angiospermae | Pteridophyta | Caryophyllales | Amaranthaceae | Dysphania | Dysphania ambrosioides (L.) Mosyakin & Clemants |
| 17 | Angiospermae | Pteridophyta | Caryophyllales | Amaranthaceae | Alternanthera | Alternanthera sessilis (L.) R. Br. ex DC. |
| 18 | Angiospermae | Pteridophyta | Caryophyllales | Polygonaceae | Rumex | Rumex crispus L. |
| 19 | Angiospermae | Pteridophyta | Lamiales | Lamiaceae | Clinopodium | Clinopodium chinense (Benth.) Kuntze |
| 20 | Angiospermae | Pteridophyta | Lamiales | Lamiaceae | Clinopodium | Clinopodium polycephalum (Vaniot) C. Y. Wu & S. J. Hsuan ex P. S. Hsu |
| 21 | Angiospermae | Pteridophyta | Lamiales | Lamiaceae | Leonurus | Leonurus japonicus Houtt. |
| 22 | Angiospermae | Pteridophyta | Fabales | Fabaceae | Melilotus | Melilotus officinalis (L.) Pall. |
| 23 | Angiospermae | Pteridophyta | Fabales | Fabaceae | Glycine | Glycine soja Siebold & Zucc. |
| 24 | Angiospermae | Pteridophyta | Rosales | Rosaceae | Duchesnea | Duchesnea indica (Andr.) Focke |
| 25 | Angiospermae | Pteridophyta | Rosales | Cannabaceae | Humulus | Humulus scandens (Lour.) Merr. |
| 26 | Angiospermae | Pteridophyta | Gentianales | Apocynaceae | Cynanchum | Cynanchum auriculatum Royle ex Wight |
| 27 | Angiospermae | Pteridophyta | Boraginales | Boraginaceae | Cynoglossum | Cynoglossum furcatum Wall. in Roxb. |
| 28 | Angiospermae | Pteridophyta | Boraginales | Brassicaceae | Raphanus | Raphanus raphanistrum L. |
| 29 | Angiospermae | Pteridophyta | Contortae | Loganiaceae | Buddleja | Buddleja asiatica Lour. |
| 30 | Angiospermae | Pteridophyta | Asparagales | Asparagaceae | Anemarrhena | Anemarrhena asphodeloides Bunge |
| 31 | Angiospermae | Pteridophyta | Myrtales | Onagraceae | Oenothera | Oenothera rosea L’Hér. ex Aiton |
| 32 | Angiospermae | Pteridophyta | Solanales | Solanaceae | Solanum | Solanum americanum Mill. |
| 33 | Angiospermae | Pteridophyta | Acorales | Araceae | Acorus | Acorus calamus L. |
| 34 | Pteridophyta | Filicopsida | Polypodiales | Pteridaceae | Pteris | Pteriscretica L. var.nervosa (Thunb.) Ching et S. H. Wu |
| 35 | Pteridophyta | Equisetopsida | Equisetales | Equisetaceae | Equisetum | Equisetum arvense L. |
| No. | Sample Plots | Main Species and Important Value |
|---|---|---|
| 1 | Lujiaba | Cynodon dactylon (54.8%), Raphanus raphanistrum (14.7%), Erigeron canadensis (9.9%), Melilotus officinalis (8.7%) |
| 2 | Yuxingba | Cynodon dactylon (42.9%), Phragmites australis (16.6%), Duchesnea indica (10.0%), Melilotus officinalis (6.2%) |
| 3 | Zhangjiawan | Cynodon dactylon (48.8%), Phragmites australis (15.6), Raphanus raphanistrum (14.2%), Erigeron Canadensis (13.4%) |
| 4 | Zhongba | Erigeron Canadensis (25.9%), Cynodon dactylon (25.7%), Melilotus officinalis (17.3%), Setaria viridis (16.1%) |
| 5 | Zengjiagou | Cynodon dactylon (51.3%), Erigeron Canadensis (25.1%), Glycine soja (12.5%), Raphanus raphanistrum (3.4%) |
| 6 | Yuxi Town | Melilotus officinalis (32.7%), Cynodon dactylon (20.0%), Leonurus japonicas (11.2%), Raphanus raphanistrum (3.8%) |
| 7 | Yinglong Street | Cynodon dactylon (38.3%), Erigeron Canadensis (16.2%), Dysphania ambrosioides (14.5%), Artemisia selengensis (9.0%) |
| 8 | Hujiawan | Cynodon dactylon (53.5%), Erigeron canadensis (12.3%), Melilotus officinalis (9.6%), Leonurus japonicas (9.3%) |
| Species | Function | R2 | n |
|---|---|---|---|
| Cynodon dactylon | y = 20.38x−0.75 | 0.78 | 60 |
| Setaria viridis | y = 44.73x−0.47 | 0.66 | 55 |
| Melilotus officinalis | y = 49.61x−0.63 | 0.71 | 53 |
| Erigeron canadensis | y = 23.00x−0.59 | 0.63 | 54 |
| Phragmites australis | y = 23.18x−0.91 | 0.74 | 62 |
| Species | Energy Consumption (J) | Root Biomass (cm3) | Root Area Ratio (%) |
|---|---|---|---|
| Bare soil | 323.51 | 0 | 0 |
| Melilotus officinalis | 400.36 | 1.43 | 6.67 × 10−3 |
| Erigeron canadensis | 450.37 | 2.25 | 3.91 × 10−3 |
| Phragmites australis | 472.66 | 1.41 | 4.71 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, D.; Chang, Z.; Tang, J.; Wang, Y.; Wang, Y.; Huang, S.; Li, T.; Qi, Z.; Hu, Y. Biodiversity and Soil Reinforcement Effect of Vegetation Buffer Zones: A Case Study of the Tongnan Section of the Fujiang River Basin. Water 2024, 16, 2847. https://doi.org/10.3390/w16192847
Wang X, Liu D, Chang Z, Tang J, Wang Y, Wang Y, Huang S, Li T, Qi Z, Hu Y. Biodiversity and Soil Reinforcement Effect of Vegetation Buffer Zones: A Case Study of the Tongnan Section of the Fujiang River Basin. Water. 2024; 16(19):2847. https://doi.org/10.3390/w16192847
Chicago/Turabian StyleWang, Xinhao, Dongsheng Liu, Zhihui Chang, Jiang Tang, Yunqi Wang, Yanlei Wang, Sheng Huang, Tong Li, Zihan Qi, and Yue Hu. 2024. "Biodiversity and Soil Reinforcement Effect of Vegetation Buffer Zones: A Case Study of the Tongnan Section of the Fujiang River Basin" Water 16, no. 19: 2847. https://doi.org/10.3390/w16192847






