Succession Characteristics and Influencing Factors of Phytoplankton Communities in Qionghai Lake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.3. Data Analysis
2.3.1. Diversity Index and Dominance Analysis
2.3.2. Trophic Level of the Water Body
2.3.3. Statistical Analysis
3. Results and Analysis
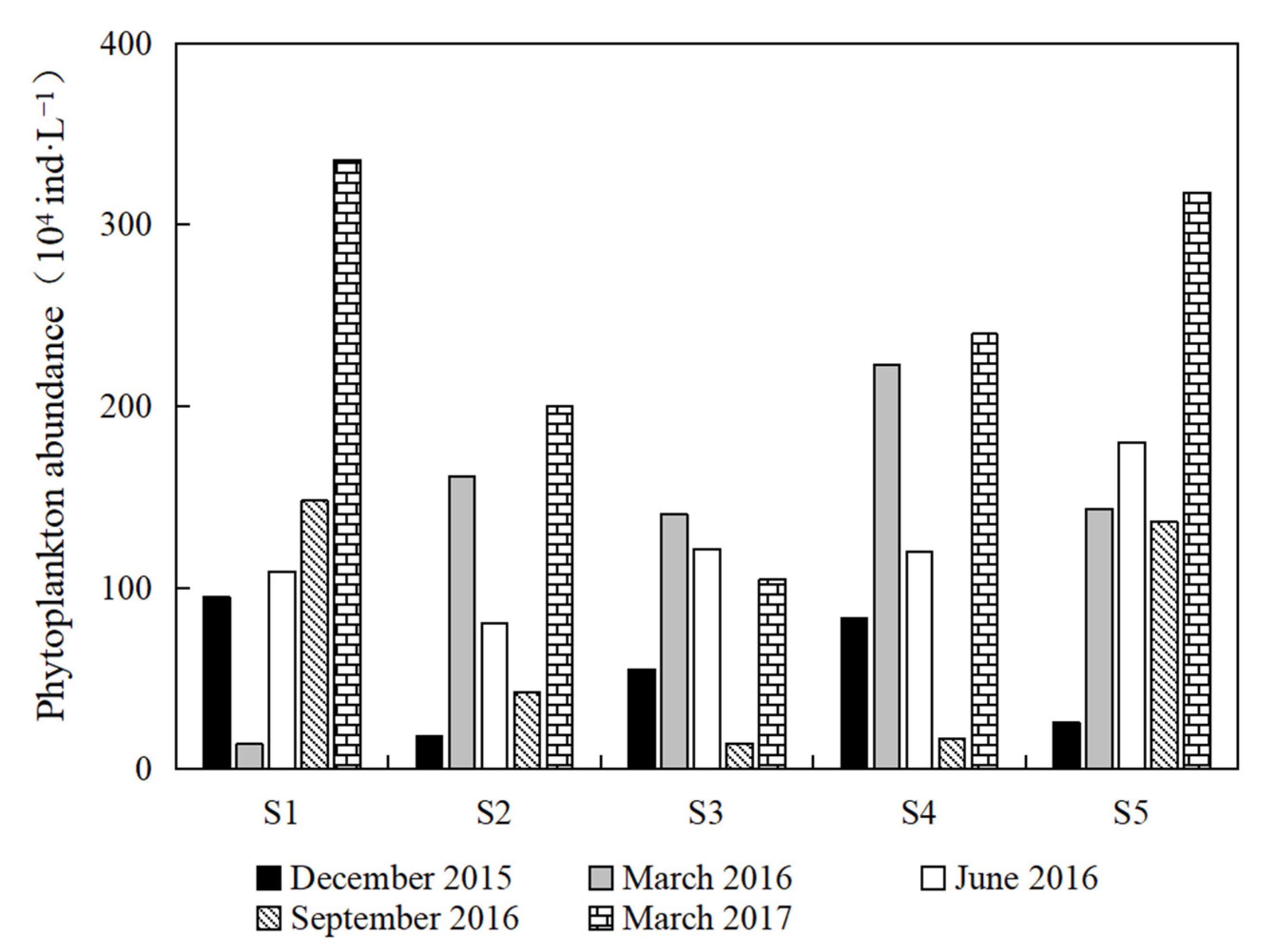
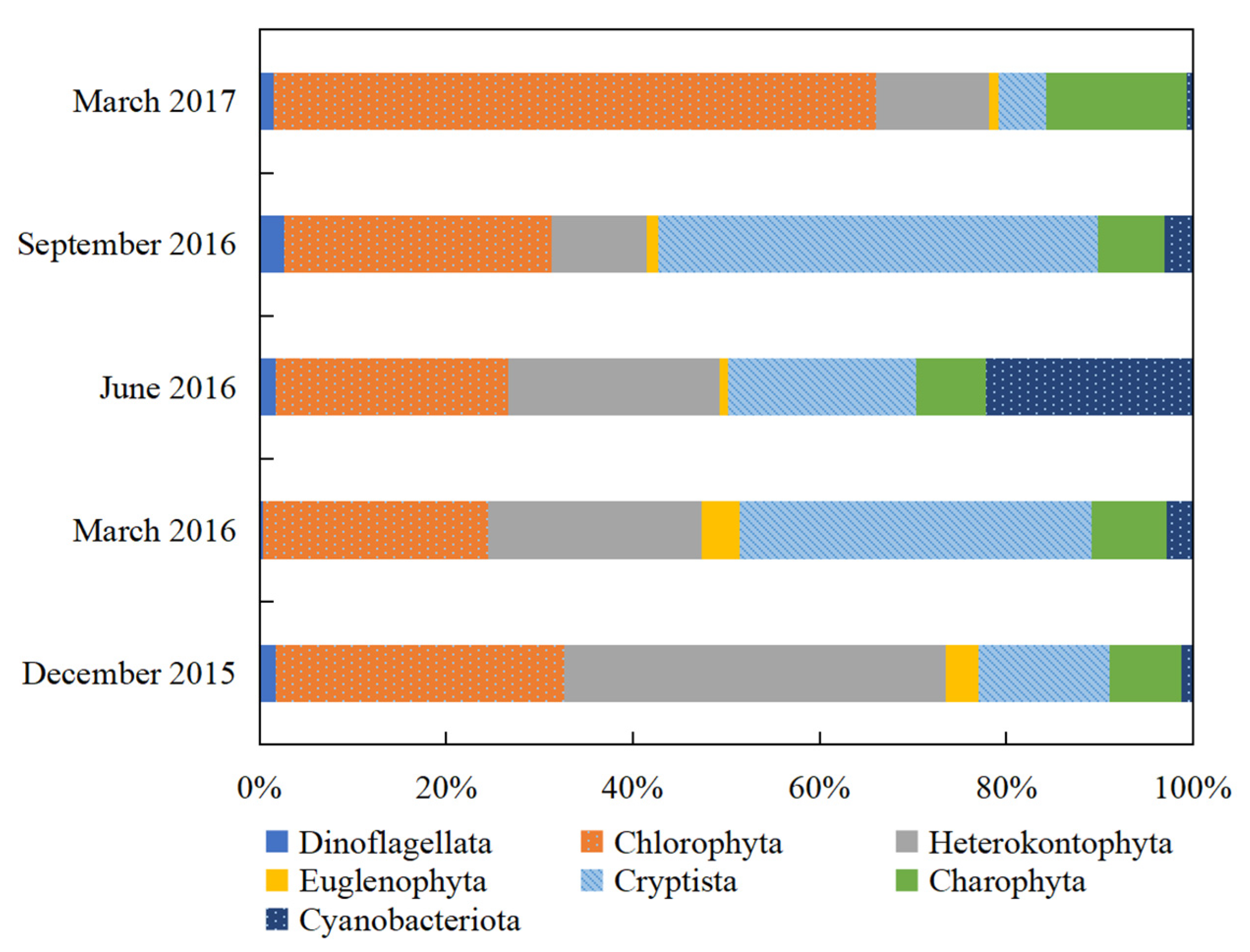
3.1. Composition
3.2. Diversity
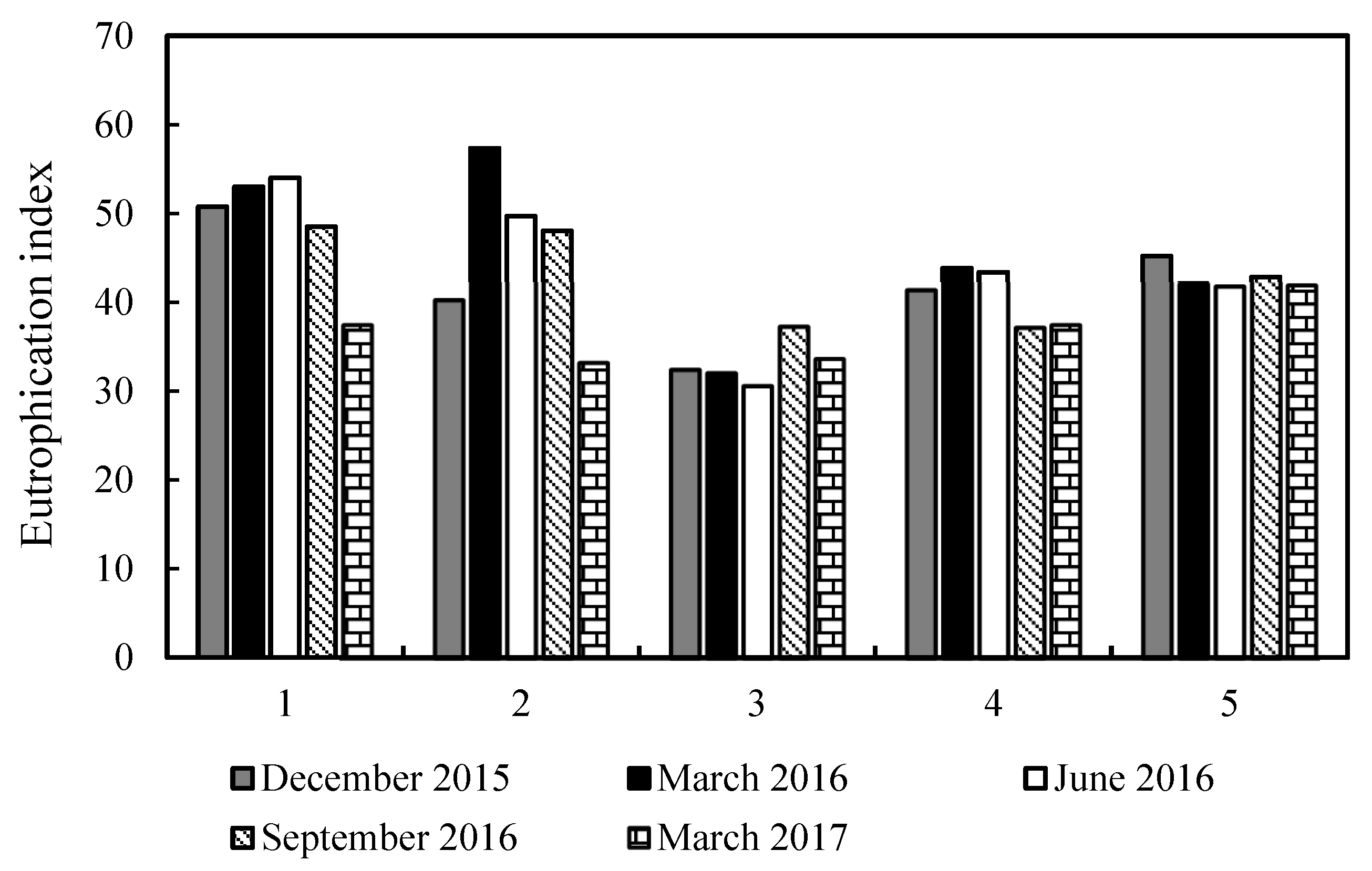
3.3. Trophic Level of the Water Body
3.4. Correlations between Phytoplankton and Environmental Factors
4. Discussions
4.1. Community Characteristics
4.2. Trophic Level of the Water Body
4.3. Correlation between Phytoplankton and Environmental Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, B.H.; Herlihy, A.T.; Kaufmann, P.R.; DeCelles, S.J.; Borgh, M.A.V. Assessment of streams of the eastern United States using a periphyton index of biotic integrity. Ecol. Indic. 2003, 2, 325–338. [Google Scholar] [CrossRef]
- Miller, S.J.; Wardrop, D.H.; Mahaney, W.M.; Brooks, R.P. A plant-based index of biological integrity (IBI) for headwater wetlands in central Pennsylvania. Ecol. Indic. 2006, 6, 290–312. [Google Scholar] [CrossRef]
- Padisák, J.; Borics, G.; Grigorszky, I.; Soróczki-Pintér, É. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: The assemblage index. Hydrobiologia 2006, 553, 1–14. [Google Scholar] [CrossRef]
- Zalack, J.T.; Smucker, N.J.; Vis, M.L. Development of a diatom index of biotic integrity for acid mine drainage impacted streams. Ecol. Indic. 2010, 10, 287–295. [Google Scholar] [CrossRef]
- Wu, N.C.; Schmalz, B.; Fohrer, N. Development and testing of a phytoplankton index of biotic integrity (P-IBI) for a German lowland river. Ecol. Indic. 2012, 13, 158–167. [Google Scholar] [CrossRef]
- Znachor, P.; Nedom, J.; Hejzlar, J.; Seďa, J.; Komárková, J.; Kolář, V.; Mrkvička, T.; Boukal, D.S. Changing environmental conditions underpin long-term patterns of phytoplankton in a freshwater reservoir. Sci. Total Environ. 2020, 710, 135626. [Google Scholar] [CrossRef]
- Chao, C.X.; Lv, T.; Wang, L.G.; Li, Y.; Han, C.; Yu, W.C.; Yan, Z.W.; Ma, X.W.; Zhao, H.C.; Zuo, Z.J.; et al. The spatiotemporal characteristics of water quality and phytoplankton community in a shallow eutrophic lake: Implications for submerged vegetation restoration. Sci. Total Environ. 2022, 821, 153460. [Google Scholar] [CrossRef]
- Havens, K.E.; Ji, G.; Beaver, J.R.; Fulton, R.S.; Teacher, C.E. Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change. Hydrobiologia 2019, 829, 43–59. [Google Scholar] [CrossRef]
- Brookes, J.D.; Carey, C.C. Resilience to blooms. Science 2011, 334, 46–47. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Räike, A.; Pietil¨ainen, O.P.; Rekolainen, S.; Kauppila, P.; Pitkänen, H.; Niemi, J.; Raateland, A.; Vuorenmaa, J. Trends of phosphorus, nitrogen and chlorophyll a concentrations in Finnish rivers and lakes in 1975–2000. Sci. Total Environ. 2003, 310, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, M.; Shi, X.L.; Kong, F.X.; Ma, R.H.; Yu, Y. Nutrient reduction magnifies the impact of extreme weather on cyanobacterial bloom formation in large shallow Lake Taihu (China). Water Res. 2016, 302, 302–310. [Google Scholar] [CrossRef]
- Muhid, P.; Davis, T.W.; Bunn, S.E.; Burford, M.A. Effects of inorganic nutrients in recycled water on freshwater phytoplankton biomass and composition. Water Res. 2013, 47, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Pei, H.Y.; Hu, W.R.; Tian, C.; Hao, D.P.; Wei, J.L.; Feng, Y.W. Spatiotemporal distribution pattern of cyanobacteria community and its relationship with the environmental factors in Hongze Lake, China. Environ. Monit. Assess. 2014, 186, 6919–6933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Wang, Y.; Yang, L.L.; Yuan, L.W.; Peng, D.C. Relationship between phytoplankton and environmental factors inlandscape water supplemented with reclaimed water. Ecol. Indic. 2015, 58, 113–121. [Google Scholar] [CrossRef]
- Shan, K.; Song, L.R.; Chen, W.; Li, L.; Liu, M.L.; Wu, Y.L.; Jia, Y.L.; Zhou, Q.C.; Peng, L. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef]
- Navas-Parejoa, J.C.C.; Corzoa, A.; Papaspyroua, S. Seasonal cycles of phytoplankton biomass and primary production in a tropical temporarily open-closed estuarine lagoon—The effect of an extreme climatic event. Sci. Total Environ. 2020, 723, 138014. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Li, Y.; Lin, Q.W.; He, C.; Huang, S.Z.; Li, H.; Zhang, X.Y.; Liu, B.Y.; Ge, F.J.; et al. The changing characteristics of phytoplankton community and biomass in subtropical shallow lakes: Coupling effects of land use patterns and lake morphology. Water Res. 2021, 200, 117235. [Google Scholar] [CrossRef]
- Tian, C.; Pei, H.Y.; Hu, W.R.; Xie, J. Phytoplankton variation and its relationship with the environmental factors in Nansi Lake, China. Environ. Monit. Assess. 2013, 185, 295–310. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Z.; Zheng, C.; Lu, K.H.; Ding, D.W.; Zhu, J.Y. Temporal variations in a phytoplankton community in a subtropical reservoir: An interplay of extrinsic and intrinsic community effects. Sci. Total Environ. 2017, 612, 720–727. [Google Scholar] [CrossRef]
- Stockwell, J.D.; Doubek, J.P.; Adrian, R.; Anneville, O.; Carey, C.C.; Carvalho, L.; Domis, L.N.D.S.; Dur, G.; Frassl, M.A.; Grossart, H.P.; et al. Storm impacts on phytoplankton community dynamics in lakes. Global Change Biol. 2020, 26, 2756–2784. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.G.; Gu, X.; Cao, Y.; Luo, J.H.; Zheng, Q.F.; Chen, H.H.; Jeppesen, E. Pelagic energy flow supports the food web of a shallow lake following a dramatic regime shift driven by water level changes. Sci. Total Environ. 2021, 756, 143642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Hu, M.Q.; Shi, K.; Zhang, M.; Han, T.; Lai, l.; Zhan, P.F. Sensitivity of phytoplankton to climatic factors in a large shallow lake revealed by column-integrated algal biomass from long-term satellite observations. Water Res. 2021, 207, 117786. [Google Scholar] [CrossRef] [PubMed]
- Noori, R.; Ansari, E.; Bhattarai, R.; Tang, Q.H.; Aradpour, S.; Maghrebi, M.; Haghighi, A.T.; Bengtsson, L.; Kløve, B. Complex dynamics of water quality mixing in a warm mono-mictic reservoir. Sci. Total Environ. 2021, 777, 146097. [Google Scholar] [CrossRef] [PubMed]
- Noori, R.; Ansari, E.; Jeong, Y.W.; Aradpour, S.; Maghrebi, M.; Hosseinzadeh, M.; Bateni, S.M. Hyper-Nutrient Enrichment Status in the Sabalan Lake, Iran. Water 2021, 13, 2874. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; He, P. A preliminary investigatory report of the algae in Qinghai Lake of Sichuan. J. Southwest China Norm. Univ. Nat. Sci. Ed. 1995, 2, 187–194. [Google Scholar]
- Yao, W.Z.; Zhou, Y.J.; Feng, J.G. A preliminary study on the plankton in Qionghai Lake. J. Fish. China 1996, 2, 183–187. [Google Scholar]
- Yao, W.Z.; Zhou, Y.J.; Feng, J.G. A study on the evaluation of water pollution and eutrophication of the Qionghai Lake by means of phytoplankton. J. Southwest Agric. Univ. 1996, 2, 170–173. [Google Scholar]
- Dong, Y.X. Research and Development of Algae in the Nine Plateau Lakes in Yunnan. Environ. Sci. Surv. 2014, 33, 1–8. [Google Scholar]
- Du, X. Research on Community Structure and Ecological Evaluation of Planktonic Algae and Epiphytic Algae in Caohai, Guizhou; Guizhou Normal University: Guiyang, China, 2021. [Google Scholar]
- State Environmental Protection Administration. Methods for Monitoring and Analysis of Water and Wastewater, 4th ed.; Chinese Environmental Press: Beijing, China, 2002. [Google Scholar]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of CHINA: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Sun, J.; Liu, D.Y. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Aksnes, D.L.; Wassmann, P. Modeling the significance of zooplankton grazing for export production. Limnol. Oceanogr. 1993, 38, 978–985. [Google Scholar] [CrossRef]
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Wang, M.C.; Liu, X.Q. Evaluate method and classification standard on lake eutrophication. Environ. Monit. China 2002, 18, 47–49. [Google Scholar]
- Ji, Z.Y.; Liu, S.J. Phytoplankton Community Structure, Related Influencing Factors and the Evaluation of Water Quality in the Fuxian Lake. Environ. Monit. China 2019, 35, 67–77. [Google Scholar]
- Pang, Q.J.; LI, B.Y. Assessment of eutrophication of Dongping Lake water body. Water Resour. Prot. 2003, 19, 42–44. [Google Scholar]
- Matthews, M.W. Eutrophication and cyanobacterial blooms in South African inland waters: 10 years of MERIS observations. Remote Sens. Environ. 2014, 155, 161–177. [Google Scholar] [CrossRef]
- Tang, W.H.; Rao, Y.P.; Peng, X. Investigation and Analysis of the Current Situation of Water Quality in Qionghai Lake, Xichang. Chongqing Environ. Sci. 1993, 4, 54–57. [Google Scholar]
- Liang, J.; Zhang, S.D.; Wang, H.B. Preliminary Study of Water Eutrophication in Qionghai Lake. Sichuan Environ. 2009, 28, 33–36. [Google Scholar]
- Zhu, J.P.; Liu, H.; Zhao, B. Evaluation of Water Eutrophication in Qionghai Lake of Xichang in 2010. Ningxia J. Agric. For. Sci. Technol. 2011, 52, 45–46. [Google Scholar]
- Wang, C.; Yuan, T.; Yu, J.X.; Xu, M.; Liu, Y. Spatial-temporal Fluctuations of Water Quality and Nutritional Status Partitioning of Caohai Lake in Guizhou. J. Yangtze River Sci. Res. Inst. 2019, 36, 14–19. [Google Scholar]
- Li, C.Y.; Yang, Z.L. Evaluation of Phytoplankton Community and Nutritional Status in Yangzonghai. Pearl River 2013, 34, 20–23. [Google Scholar]
- Alam, M.G.M.; Jahan, N.; Thalib, L.; Wei, B.; Maekawa, T. Effects of environmental factors on the seasonally change of phytoplankton populations in a closed freshwater pond. Environ. Int. 2001, 27, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Phosphorus enrichment and carbon depletion contribute to high Microcystis biomass and microcystin concentrations in Ugandan lakes. Limnol. Oceanogr. 2013, 58, 1075–1088. [Google Scholar] [CrossRef]
- Abirhire, N.R.L.; North, R.; Hunter, K.; Vandergucht, D.M.; Sereda, J.; Hudson, J.J. Environmental factors influencing phyto-plankton communities in Lake Diefenbaker, Saskatchewan, Canada. J. Great Lakes Res. 2015, 41, 118–128. [Google Scholar] [CrossRef]
- Varol, M. Phytoplankton functional groups in a monomictic reservoir: Seasonal succession, ecological preferences, and re-lationships with environmental variables. Environ. Sci. Pollut. Res. 2019, 26, 20439–20453. [Google Scholar] [CrossRef]
- Cui, G.Y.; Wang, B.L.; Xiao, J.; Qiu, X.L.; Liu, C.Q.; Li, X.D. Water column stability driving the succession of phytoplankton functional groups in karst hydroelectric reservoirs. J. Hydrol. 2021, 592, 125607. [Google Scholar] [CrossRef]
- Arhonditsis, G.B.; Winder, M.; Brett, M.T.; Schindler, D.E. Patterns and mechanisms of phytoplankton variability in Lake Washington (USA). Water Res. 2004, 38, 4013–4027. [Google Scholar] [CrossRef] [PubMed]
- Cloern, J.E. Turbidity as a control on phytoplankton biomass and productivity in estuaries. Cont. Shelf Res. 1987, 7, 1367–1381. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, J.; Huang, X.P.; Zhang, X.; Jiang, Z.J.; Ye, F.; Liang, X.M. Relationship between nutrients and plankton biomass in the turbidity maximum zone of the Pearl River Estuary. J. Environ. Sci. 2017, 57, 72–84. [Google Scholar] [CrossRef]
- Wilkinson, C.R.; Fay, P. Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature 1979, 279, 527–529. [Google Scholar] [CrossRef]
- Mitsui, A.; Kumazawa, S.; Takahashi, A.; Ikemoto, H.; Cao, S.; Arai, T. Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. Nature 1986, 323, 720–722. [Google Scholar] [CrossRef]
- Bohme, H. Regulation of nitrogen fixation in heterocyst-forming cyanobacteria. Trends Plant Sci. 1998, 3, 346–351. [Google Scholar] [CrossRef]
- Moore, L.R.; Post, A.F.; Rocap, G.; Chisholm, S.W. Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus and Synechococcus. Limnol. Oceanogr. 2002, 47, 989–996. [Google Scholar] [CrossRef]
- Haande, S.; Rohrlack, T.; Semyalo, R.P.; Brettum, P.; Edvardsen, B.; Lyche-Solheim, A.; Sørensen, K.; Larsson, P. Phytoplankton dynamics and cyanobacterial dominance in Murchison Bay of Lake Victoria (Uganda) in relation to environmental conditions. Limnologica 2011, 41, 20–29. [Google Scholar] [CrossRef]
- Liu, X.; Qian, K.M.; Chen, Y.W.; Gao, J.F. A comparison of factors influencing the summer phytoplankton biomass in China’s three largest freshwater lakes: Poyang, Dongting, and Taihu. Hydrobiologia 2017, 792, 283–302. [Google Scholar] [CrossRef]
- Cao, J.; Hou, Z.Y.; Li, Z.K.; Chu, Z.S.; Yang, P.P.; Zheng, B.H. Succession of phytoplankton functional groups and their driving factors in a subtropical plateau lake. Sci. Total Environ. 2018, 631–632, 1127–1137. [Google Scholar] [CrossRef]





| Site | Name and Location | Latitude | Longitude | Water Depth (m) | Sampled Water Layer (m) |
|---|---|---|---|---|---|
| S1 | Tangjia Bay (TJB) | 100°20′17.6″ N | 27.50′13.l″ E | 5–6 | 0.5, 2.5, 5 |
| S2 | Qinglun Bay (QLB) | 100°20′33.1″ N | 27.48′11.7″ E | 14.7–15 | 0.5, 3.5, 9 |
| S3 | Center of Qionghai Lake (CQL) | 102°18′54.2″ N | 27.48′59″ E | 17.5–18.7 | 0.5, 3.5, 9 |
| S4 | Hainan Town (HNT) | 102°17′46.6″ N | 27°48′35.3″ E | 11.7–12.5 | 0.5, 3.5, 9 |
| S5 | Border of Wetland Park Phase 2 (BWP) | 102°16′41.5″ N | 27°51′17.9″ E | 2.5–4 | 0.5, 2.5 |
| Phylum | Genus | Species | Phylum | Genus | Species | ||
|---|---|---|---|---|---|---|---|
| 1 | Chlorophyta | Actinastrum | A. sp. | 99 | Nitzschia | N. lanceolata W. Smith | |
| 2 | Ankistrodesmus | A. sp. | 100 | N. acicularis (Kützing) W.Smith | |||
| 3 | A. falcatus var. mirabilis (West and G.S.West) G.S.West | 101 | Pantocsekiella | P. ocellata (Pantocsek) K.T.Kiss and Ács | |||
| 4 | A. spiralis (W.B.Turner) Lemmermann | 102 | Pinnularia | P. sp. | |||
| 5 | A. angustus (C.Bernard) Oettli | 103 | Planktoniella | P. sp. | |||
| 6 | A. convolutus Corda | 104 | Surirella | S. elegans Ehrenberg | |||
| 7 | A. falcatus (Corda) Ralfs | 105 | Synedra | S. sp. | |||
| 8 | A. fusiformis Corda | 106 | S. famelica Kützing | ||||
| 9 | Carteria | C. multifilis (Fresenius) O.Dill | 107 | Chrysococcus | C. diaphanus Skuja | ||
| 10 | Chlamydomonas | C. globosa J.W.Snow | 108 | Dinobryon | D. sp. | ||
| 11 | C. ovalis Korshikov | 109 | D. sertularia Ehrenberg | ||||
| 12 | C. pertusa Chodat | 110 | D. sociale Ehrenberg | ||||
| 13 | C. reinhardtii P.A.Dangeard | 111 | D. divergens O.E.Imhof | ||||
| 14 | C. conferta Korshikov | 112 | Synura | S. uvella Ehrenberg | |||
| 15 | Chlorella | C. vulgaris Beijierinck | 113 | S. echinulata Korshikov | |||
| 16 | C. ellipsoidea Gerneck | 114 | Tribonema | T. vulgare Pascher | |||
| 17 | Chlorococcum | C. sp. | 115 | T. minus (Wille) Hazen | |||
| 18 | Closteridium | C. sp. | 116 | Cyanobacteriota | Anabaena | A. sp. | |
| 19 | Coelastrum | C. sphaericum Nägeli | 117 | A. affinis Lemmermann | |||
| 20 | C. reticulatum (P.A.Dangeard) Senn | 118 | Asterocapsa | A. sp. | |||
| 21 | C. proboscideum Bohlin | 119 | Chroococcus | C. sp. | |||
| 22 | Crucigenia | C. tetrapedia (Kirchner) Kuntze | 120 | Dactylococcopsis | D. irregularis G.M.Smith | ||
| 23 | C. quadrata Morren | 121 | D. acicularis Lemmermann | ||||
| 24 | C. apiculata (Lemmermann) Schmidle | 122 | D. sp. | ||||
| 25 | C. fenestrate (Schmidle) Schmidle | 123 | Gloeocapsa | G. limnetica (Lemmermann) Hollerbach | |||
| 26 | Dictyosphaeria | D. sp. | 124 | G. minima (Keissler) Hollerbach | |||
| 27 | Dictyosphaerium | D. pulchellum H.C.Wood | 125 | G. turgida (Kützing) Hollerbach | |||
| 28 | Eudorina | E. elegans Ehrenberg | 126 | Leptolyngbya | L. sp. | ||
| 127 | L. tenuis (Gomont) Anagnostidis and Komárek | ||||||
| 29 | Franceia | F. tuberculata G.M.Smith | 128 | Merismopedia | M. sp. | ||
| 30 | Golenkinia | G. radiata Chodat | 129 | M. sinica S.-H.Ley | |||
| 31 | Gonium | G. pectorale O.F.Müller | 130 | M. minima G.Beck | |||
| 32 | Nephrocytium | N. agardhianum Nägeli | 131 | M. punctata Meyen | |||
| 33 | N. lunatum West | 132 | M. elegans A.Braun ex Kützing | ||||
| 34 | Oocystis | O. lacustris Chodat | 133 | M. aeruginea Brébisson | |||
| 35 | O. naegelii A.Braun | 134 | Microcystis | M. sp. | |||
| 36 | O. solitaria Wittrock | 135 | Nephrococcus | N. confertus Y.-Y.Li | |||
| 37 | O. parva West and G.S.West | 136 | Nostoc | N. sp. | |||
| 38 | Palmella | P. mucosa Kützing | 137 | Oscillatoria | O. sp. | ||
| 39 | Pandorina | P. morum (O.F.Müller) Bory | 138 | O. acuminata Gomont | |||
| 40 | P. charkowiensis Korschikov | 139 | O. princeps Vaucher ex Gomont | ||||
| 41 | Pediastrum | P. simplex var. duodenarium (Bailey) Rabenhorst | 140 | Phormidium | P. sp. | ||
| 42 | P. biradiatum (Meyen) E.Hegewald | 141 | P. corium Gomont | ||||
| 43 | P. clathratum (Schröder) Lemmermann | 142 | P. mucosum N.L.Gardner | ||||
| 44 | P. sp. | 143 | Trichodesmium | T. erythraeum Ehrenberg ex Gomont | |||
| 45 | P. sturmii Reinsch | 144 | Charophyta | Closterium | C. intermedium Ralfs | ||
| 46 | P. tetras (Ehrenberg) Ralfs | 145 | C. parvulum Nägeli | ||||
| 47 | P. tetras var. excisum (A.Braun) Hansgirg | 146 | C. cynthia De Notaris | ||||
| 48 | P. biradiatum var. longicornutum Gutwinski | 147 | C. limneticum Lemmermann | ||||
| 49 | P. duplex var. gracillimum West and G.S.West | 148 | C. venus Kützing ex Ralfs | ||||
| 50 | P. duplex Meyen | 149 | C. sp. | ||||
| 51 | Planktosphaeria | P. gelatinosa G.M.Smith | 150 | C. kuetzingii Brébisson | |||
| 52 | Pleodorina | P. californica W.R.Shaw | 151 | Cosmarium | C. impressulum Elfving | ||
| 53 | Quadrigula | Q. chodatii (Tanner-Füllemann) G.M.Smith | 152 | C. obtusatum Schmidle | |||
| 54 | Scenedesmus | S. quadricauda Chodat | 153 | C. formosulum Hoff | |||
| 55 | S. dimorphus (Turpin) Kützing | 154 | C. cucumis Corda ex Ralfs | ||||
| 56 | S. sp. | 155 | C. granatum Brébisson ex Ralfs | ||||
| 57 | S. obliquus var. acuminatus (Lagerheim) Chodat | 156 | C. quadratum Ralfs ex Ralfs | ||||
| 58 | S. bijugatus Kützing | 157 | C. reniforme (Ralfs) W.Archer | ||||
| 59 | S. bijugus (Turpin) Lagerheim | 158 | C. circulare (Hassall ex Ralfs) Kützing | ||||
| 60 | S. bijugus var. alternans (Reinsch) Borge | 159 | C. botrytis Joshua | ||||
| 61 | S. ovalternus Chodat | 160 | Staurastrum | S. cuspidatum Brébisson | |||
| 62 | S. acuminatus (Lagerheim) Chodat | 161 | S. tetracerum Ralfs ex Ralfs | ||||
| 63 | S. armatus (Chodat) Chodat | 162 | S. polymorphum Brébisson | ||||
| 64 | S. arcuatus (Lemmermann) Lemmermann | 163 | S. gracile Ralfs ex Ralfs | ||||
| 65 | Selenastrum | S. gracile Reinsch | 164 | S. retusum W.B.Turner | |||
| 66 | S. bibraianum Reinsch | 165 | S. paradoxum Meyen ex Ralfs | ||||
| 67 | Sphaerocystis | S. schroeteri Chodat | 166 | S. erasum Brébisson | |||
| 68 | Tetrachlorella | T. alternans (G.M.Smith) Korshikov | 167 | S. inflexum Brébisson | |||
| 69 | Tetraëdron | T. sp. | 168 | S. oxyacanthum W.Archer | |||
| 70 | T. tumidulum (Reinsch) Hansgirg | 169 | Euglenophyta | Euglena | E. sp. | ||
| 71 | T. enorme (Ralfs) Hansgirg | 170 | E. acutissima Lemmermann | ||||
| 72 | T. bifurcatum (Wille) Lagerheim | 171 | E. oxyuris Schmarda | ||||
| 73 | T. minimum (A.Braun) Hansgirg | 172 | E. caudata E.F.W.Hübner | ||||
| 74 | T. caudatum (Corda) Hansgirg | 173 | E. variabilis G.A.Klebs | ||||
| 75 | Tetraspora | T. sp. | 174 | E. geniculata Dujardin | |||
| 76 | Treubaria | T. crassicornuta Z.-Y.Hu | 175 | E. gracilis G.A.Klebs | |||
| 77 | Heterokontophyta | Achnanthes | A. exigua Grunow | 176 | E. viridis (O.F.Müller) Ehrenberg | ||
| 78 | Asterionella | A. sp. | 177 | E. tristella S.P.Chu | |||
| 79 | A. formosa Hassall | 178 | E. pisciformis Klebs | ||||
| 80 | Attheya | A. sp. | 179 | Lepocinclis | L. steinii Lemmermann | ||
| 81 | A. zachariasii Brun | 180 | Phacus | P. acuminatus A.Stokes | |||
| 82 | Cyclotella | C. sp. | 181 | P. sp. | |||
| 83 | Cymbella | C. sp. | 182 | P. caudatus Hübner | |||
| 84 | C. cistula (Ehrenberg) O.Kirchner | 183 | Trachelomonas | T. euchlora (Ehrenberg) Lemmermann | |||
| 85 | C. tumidula Grunow | 184 | Cryptista | Chilomonas | C. paramaecium Ehrenberg | ||
| 86 | C. ventricosa Kützing | 185 | Cryptomonas | C. sp. | |||
| 87 | C. naviculiformis Auerswald ex Heiberg | 186 | C. marssonii Skuja | ||||
| 88 | Ditylum | D. sp. | 187 | C. erosa Ehrenberg | |||
| 89 | Fragilaria | F. capucina Desmazières | 188 | C. ovata Ehrenberg | |||
| 90 | Gomphonema | G. sp. | 189 | C. obovate Skuja | |||
| 91 | Gyrosigma | G. attenuatum (Kützing) Rabenhorst | 190 | Komma | K. sp. | ||
| 92 | Melosira | M. sp. | 191 | Komma caudata (L.Geitler) D.R.A.Hill | |||
| 93 | M. granulate (Ehrenberg) Ralfs | 192 | Dinoflagellata | Ceratium | C. hirundinella (O.F.Müller) Dujardin | ||
| 94 | M. varians C.Agardh | 193 | Glenodinium | G. sp. | |||
| 95 | Navicula | N. sp. | 194 | G. cinctum Ehrenberg | |||
| 96 | N. cryptocephala Kützing | 195 | Peridinium | P. sp. | |||
| 97 | N. minuscula Grunow | 196 | P. minutum Kofoid | ||||
| 98 | N. viridula (Kützing) Ehrenberg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Yan, G.; Wang, X.; Dong, Y.; Zheng, B. Succession Characteristics and Influencing Factors of Phytoplankton Communities in Qionghai Lake. Water 2024, 16, 229. https://doi.org/10.3390/w16020229
Yin X, Yan G, Wang X, Dong Y, Zheng B. Succession Characteristics and Influencing Factors of Phytoplankton Communities in Qionghai Lake. Water. 2024; 16(2):229. https://doi.org/10.3390/w16020229
Chicago/Turabian StyleYin, Xueyan, Guanghan Yan, Xing Wang, Yanzhen Dong, and Binghui Zheng. 2024. "Succession Characteristics and Influencing Factors of Phytoplankton Communities in Qionghai Lake" Water 16, no. 2: 229. https://doi.org/10.3390/w16020229




