Study on the Fine Characterization of Spatial Distribution and Predictive Modeling of Remediation of Site Pollution
Abstract
:1. Introduction
2. Materials and Methods
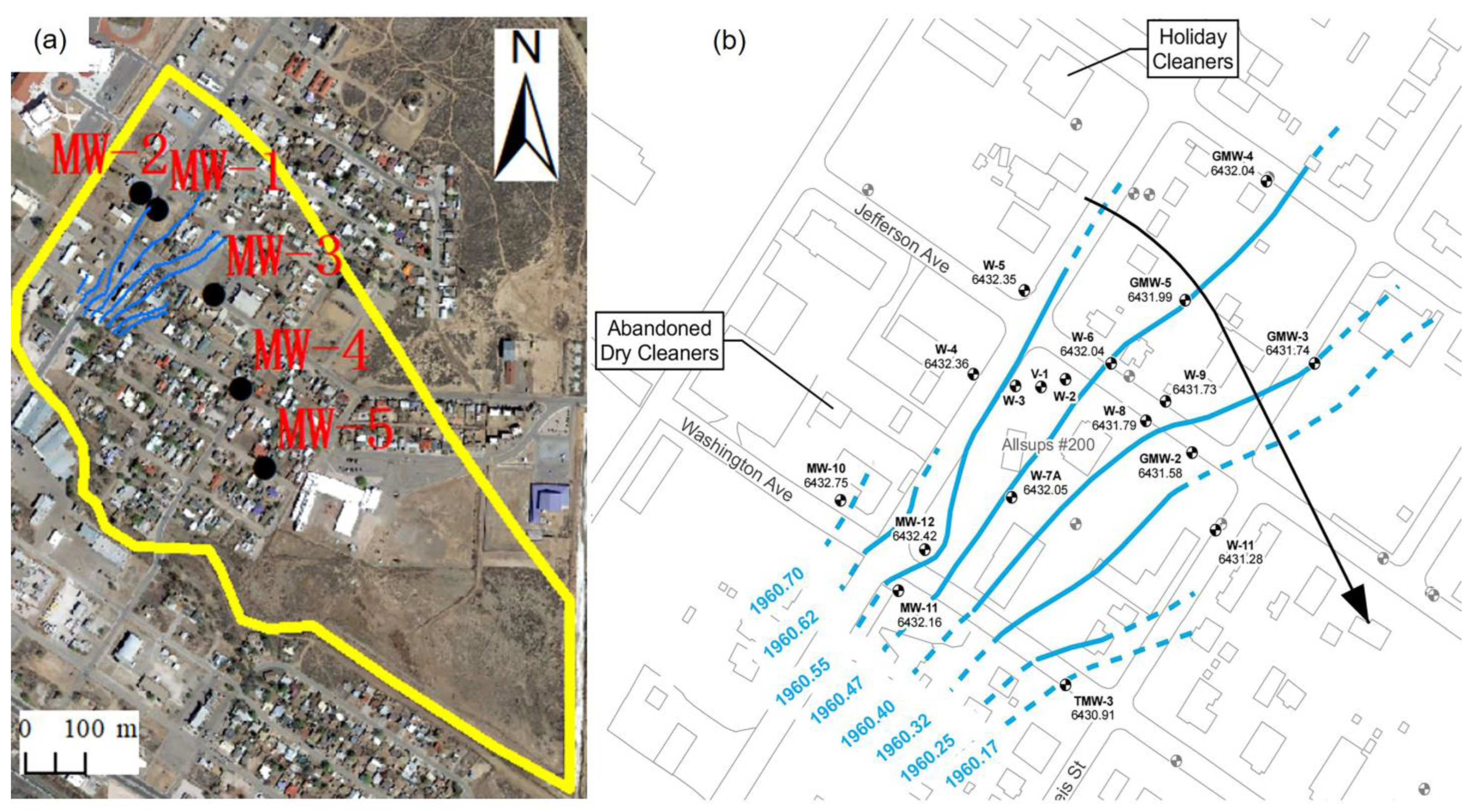
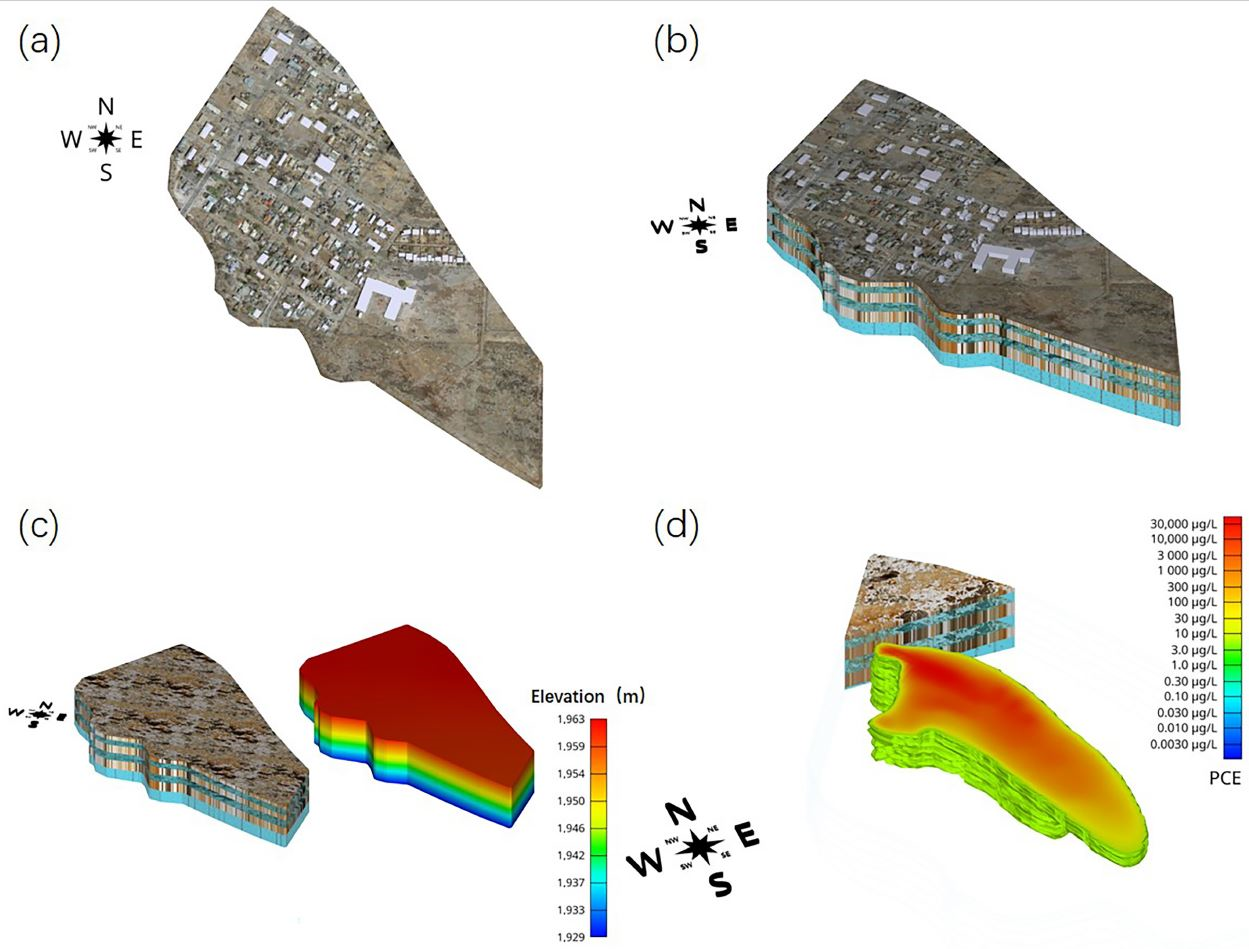
2.1. Study Area
2.2. Modeling
2.2.1. Conceptual Hydrogeological Modeling
2.2.2. Mathematical Modeling of Groundwater Pollution Processes
- (1)
- Mathematical Modeling of Groundwater Flow [37]
- (2)
- Mathematical modeling of solute transport [37]
- (3)
- Chemical reaction formulas:
2.2.3. Model Parameterization
- (1)
- Spatial Grid Distribution
- (2)
- Time Discretization
- (3)
- Permeability Coefficient
- (4)
- Boundary Conditions
- (5)
- Sources and Sinks
2.2.4. Optimization of Model Parameters
3. Results and Discussion
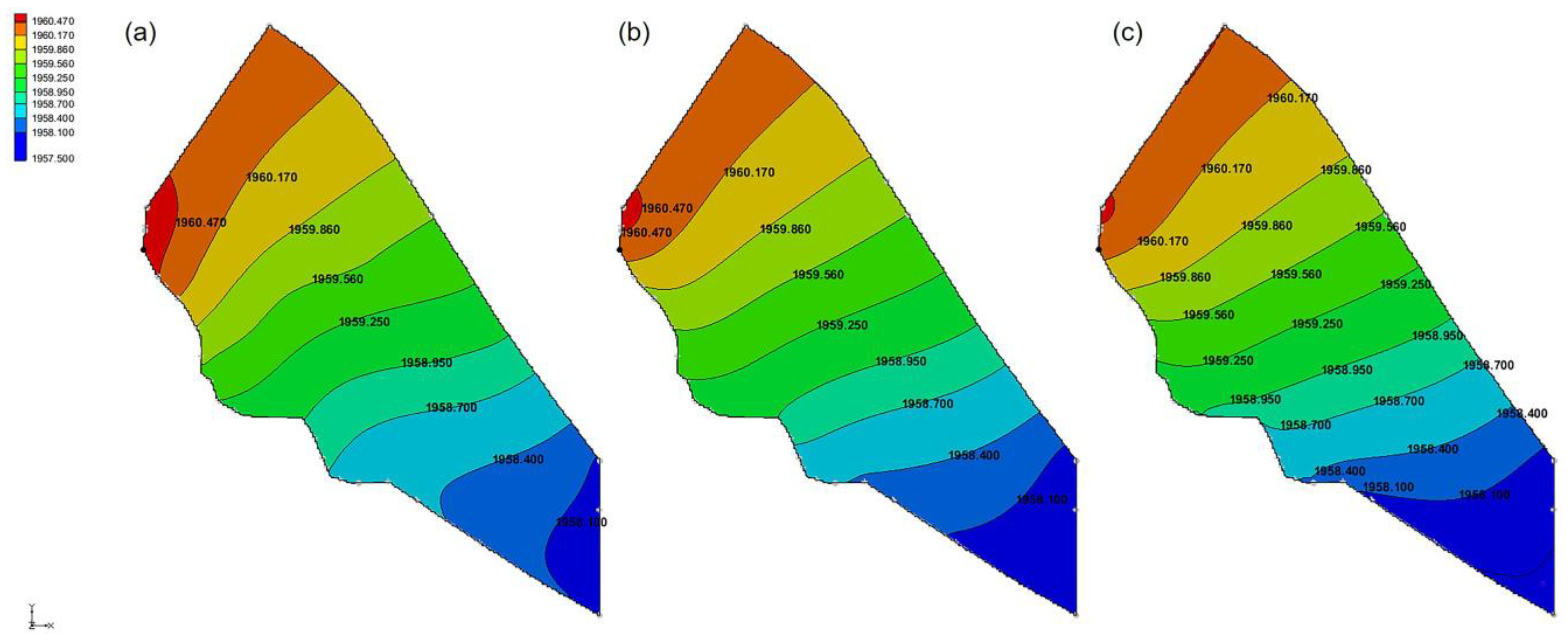
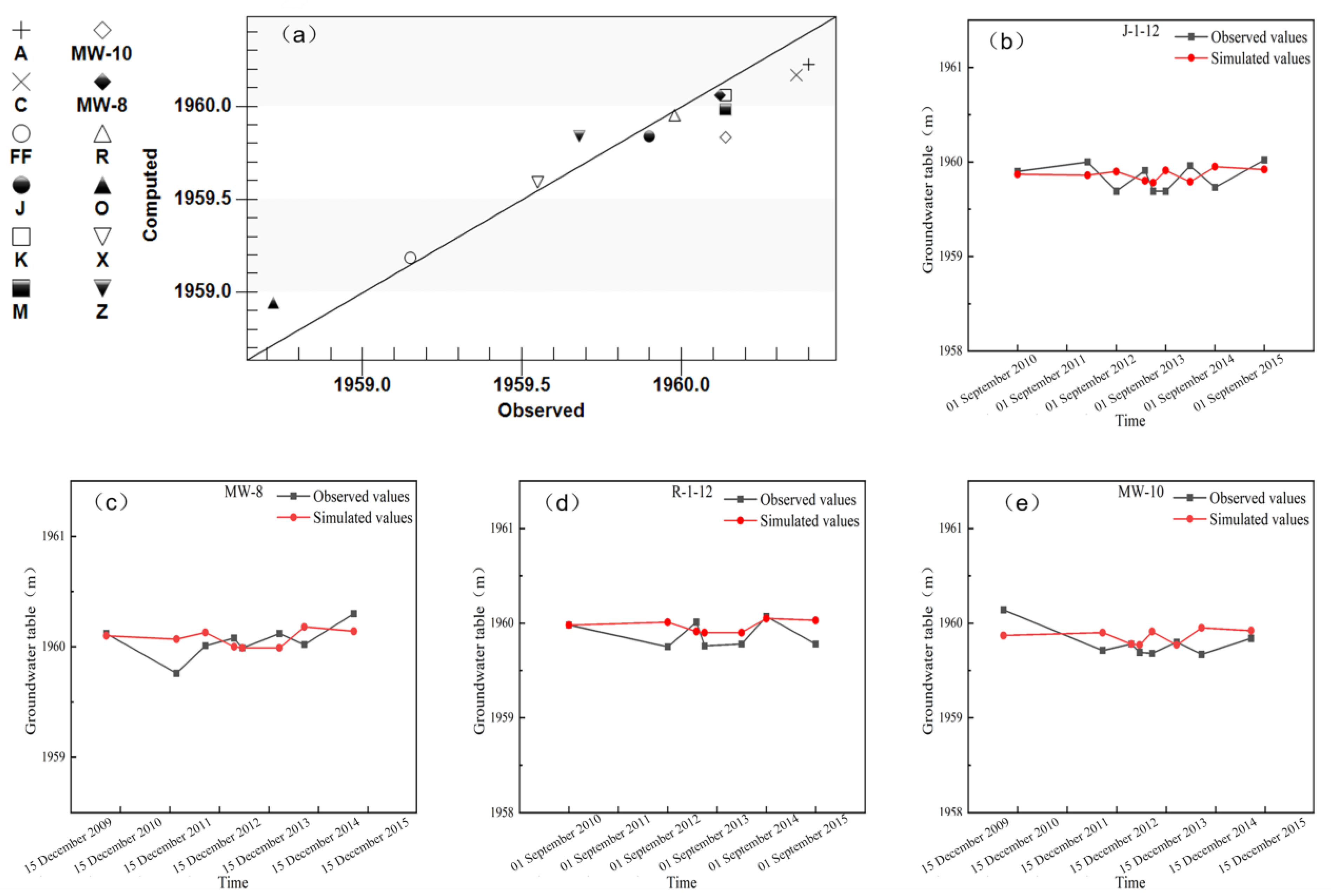
3.1. Calibration of Water Level Simulation Results
3.2. PCE Initial Concentration Distribution
3.3. Calibration of Solute Transport Simulation Results
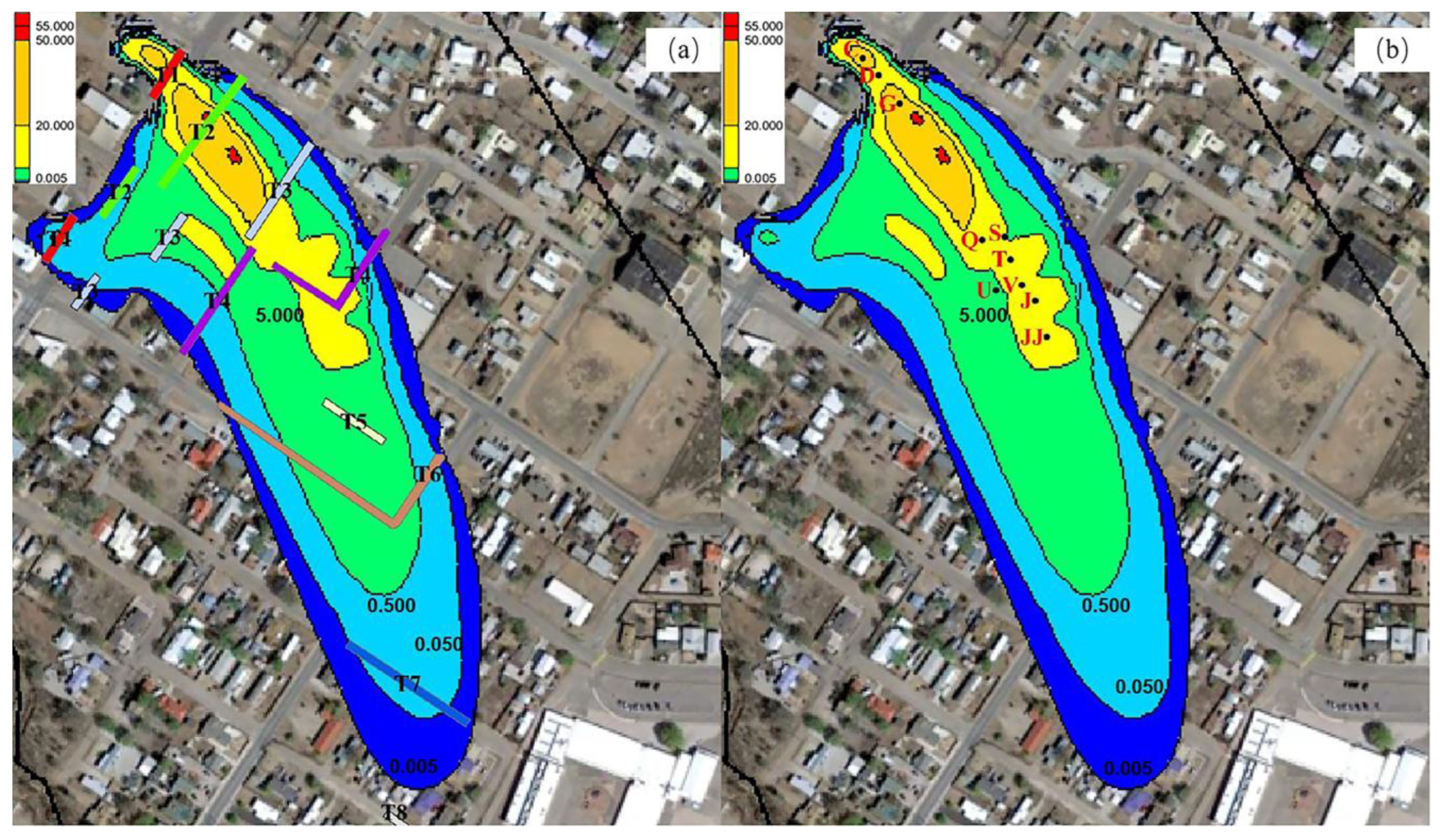
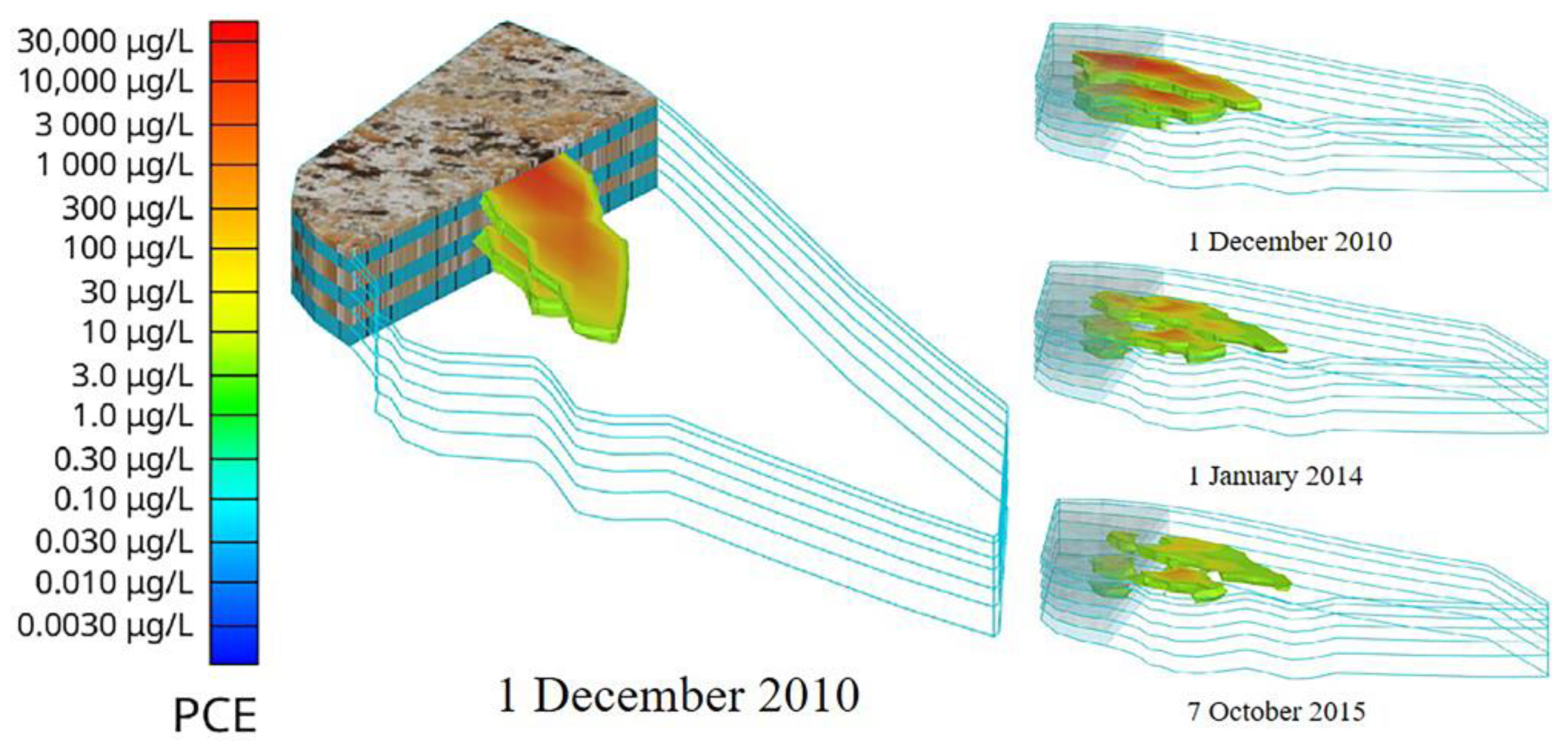
3.4. Contamination Plume Migration During Remediation
- (1)
- Insufficient sampling data. Due to the annual unit of the sampling data in the site information, the data volume is too small, which has consumed considerable time in model calibration to align simulated values with monitored values. It is recommended to enhance data collection efforts to obtain a sufficient quantity and quality of sampling data, ensuring data accuracy and completeness.
- (2)
- Simplified generalization of geological strata. Within the study area, there are interbedded layers of clay, silt, and sand. Rock formations with similar water-bearing and permeability characteristics have been merged and generalized, resulting in generalized aquifer and aquiclude groups. It is unclear whether this generalization has impacted the simulation results. It is suggested to utilize modern technological means such as remote sensing technology and geophysical exploration to acquire more comprehensive geological stratum information.
- (3)
- Determination of various parameters. For example, the set reaction rate parameters are obtained from site data, and the reaction rate constants at some points have been manually adjusted during the parameter tuning process. It is recommended that relevant parameters should be measured in a laboratory setting. If laboratory conditions are not feasible, consideration can be given to using existing data from the literature or standard parameters provided by professional institutions, but appropriate verification and calibration are necessary to ensure the accuracy and applicability of the parameters.
3.5. EVS Visualization Display
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.-B.; Huang, Z.; Zhao, J.-L.; He, L.-Y.; Liu, Y.-S.; Hu, L.-X.; Shi, Y.-J.; Ying, G.-G. Contamination Characteristics and Ecological Risk of Antibiotics in Contaminated Sites of Typical Pharmaceutical Factories in China. Huan Jing Ke Xue = Huanjing Kexue 2024, 45, 1004–1014. [Google Scholar] [PubMed]
- Li, W.; Liu, G.-P.; Liu, J.; Lu, L.-H.; Qiao, W.-J.; Yu, X.; Zhang, X.-Y.; Jiang, J.-D. Characterization of Reductive Dechlorination of Chlorinated Ethylenes by Anaerobic Consortium. Huan Jing Ke Xue = Huanjing Kexue 2024, 45, 1080–1089. [Google Scholar] [PubMed]
- Li, R.-Y.; Zeng, Y.-Y.; Zhou, J.-L.; Sun, Y.; Yan, Z.-Y. Prediction Model of Groundwater Sulphate Based on Combined Multi-source Spatio-temporal Data. Huan Jing Ke Xue = Huanjing Kexue 2024, 45, 3153–3164. [Google Scholar] [PubMed]
- Walaszek, M.; Cary, L.; Billon, G.; Blessing, M.; Bouvet-Swialkowski, A.; George, M.; Criquet, J.; Mossmann, J.R. Dynamics of chlorinated aliphatic hydrocarbons in the Chalk aquifer of northern France. Sci. Total Environ. 2021, 757, 143742. [Google Scholar] [CrossRef] [PubMed]
- Polasko, A.L.; Zulli, A.; Gedalanga, P.B.; Pornwongthong, P.; Mahendra, S. A Mixed Microbial Community for the Biodegradation of Chlorinated Ethenes and 1,4-Dioxane. Environ. Sci. Technol. Lett. 2019, 6, 49–54. [Google Scholar] [CrossRef]
- Frascari, D.; Zanaroli, G.; Danko, A.S. In situ aerobic cometabolism of chlorinated solvents: A review. J. Hazard. Mater. 2015, 283, 382–399. [Google Scholar] [CrossRef]
- Yang, X.; Du, J.; Jia, C.; Yang, T.; Shao, S. Groundwater pollution risk, health effects and sustainable management of halocarbons in typical industrial parks. Environ. Res. 2024, 250, 118422. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R.; Felkner-Poźniakowska, B. Assessment of the chlorinated hydrocarbons residues contamination in edible mushrooms from the North-Eastern part of Poland. Food Chem. Toxicol. 2012, 50, 4125–4129. [Google Scholar] [CrossRef]
- Gao, Y.W.; Zheng, F.; Shi, X.Q.; Sun, Y.Y.; Xu, H.X.; Wu, J.C. Laboratory Investigation of DNAPL Migration Behavior and Distribution at Varying Flow Velocities Based on Light Transmission Method. Huan Jing Ke Xue = Huanjing Kexue 2015, 36, 2532–2539. [Google Scholar]
- Lo, H.C.; Tabe, K.; Iskander, M.; Yoon, S.H. A Transparent Water-Based Polymer for Simulating Multiphase Flow. Geotech. Test. J. 2010, 33, 1–13. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Li, N.; Wang, Q.; Liao, C. Bioelectrochemical system for dehalogenation: A review. Environ. Pollut. 2022, 293, 118519. [Google Scholar] [CrossRef] [PubMed]
- Hiortdahl, K.M.; Borden, R.C. Enhanced Reductive Dechlorination of Tetrachloroethene Dense Nonaqueous Phase Liquid with EVO and Mg(OH)2. Environ. Sci. Technol. 2014, 48, 624–631. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, X.; Qiao, W.; Puentes Jácome, L.A.; Edwards, E.A.; He, Y.; Xu, J. Nanoscale zero-valent iron reduction coupled with anaerobic dechlorination to degrade hexachlorocyclohexane isomers in historically contaminated soil. J. Hazard. Mater. 2020, 400, 123298. [Google Scholar] [CrossRef]
- Pavelková, A.; Stejskal, V.; Vološčuková, O.; Nosek, J. Cost-Effective Remediation Using Microscale Zvi: Comparison of Commercially Available Products. Ecol. Chem. Eng. S 2020, 27, 211–224. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Liu, Y.; Lv, M.; Wang, Z.; Wen, L.L.; Li, A. In situ reductive dehalogenation of groundwater driven by innovative organic carbon source materials: Insights into the organohalide-respiratory electron transport chain. J. Hazard. Mater. 2023, 452, 131243. [Google Scholar] [CrossRef] [PubMed]
- Blázquez-Pallí, N.; Rosell, M.; Varias, J.; Bosch, M.; Soler, A.; Vicent, T.; Marco-Urrea, E. Multi-method assessment of the intrinsic biodegradation potential of an aquifer contaminated with chlorinated ethenes at an industrial area in Barcelona (Spain). Environ. Pollut. 2019, 244, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Leng, W.; Wang, J.; Zhi, L.; Wang, S.; Li, J.; Guo, P.; Wei, W.; Song, Y. Bioremediation technologies for cleaning up chlorinated-hydrocarbon contaminated sitesa review. Earth Sci. Front. 2024, 31, 157–172. [Google Scholar]
- Sun, M.; Dong, J.; Zhang, M.; Sun, C. Enhanced reductive dechlorination of chlorinated hydrocarbons in groundwater by emulsified zero-valentiron. China Environ. Sci. 2022, 42, 2690–2696. [Google Scholar]
- Ai, S.; Zhao, T.; Zhang, L.; Xu, J.; He, J.; Liu, X.; Guo, Y.; Li, X. TCE biodegradation in reactor with two openings with immobilized cells. Chin. J. Appl. Environ. Biol. 2017, 23, 900–906. [Google Scholar]
- Nijenhuis, I.; Kuntze, K. Anaerobic microbial dehalogenation of organohalides—State of the art and remediation strategies. Curr. Opin. Biotechnol. 2016, 38, 33–38. [Google Scholar] [CrossRef]
- Goyal, D.; Haritash, A.K.; Singh, S.K. A comprehensive review of groundwater vulnerability assessment using index-based, modelling, and coupling methods. J. Environ. Manag. 2021, 296, 113161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-c.; Fei, Y.-h.; Li, Y.-s.; Bao, X.-l.; Zhang, P.-w. Pollution source identification methods and remediation technologies of groundwater: A review. China Geol. 2024, 7, 125–137. [Google Scholar] [CrossRef]
- Deng, H.; Zhou, S.; He, Y.; Lan, Z.; Zou, Y.; Mao, X. Efficient Calibration of Groundwater Contaminant Transport Models Using Bayesian Optimization. Toxics 2023, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, Y.J.; Liu, J.Y.; Lu, S.G.; Feng, L.; Chen, X. The effects of aquifer heterogeneity on the 3D numerical simulation of soil and groundwater contamination at a chlor-alkali site in China. Environ. Earth Sci. 2018, 77, 15. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Xie, T.; Wen, N.; Yao, J. A Comprehensive Evaluation Model of Ammonia Pollution Trends in a Groundwater Source Area along a River in Residential Areas. Water 2021, 13, 1924. [Google Scholar] [CrossRef]
- Hussein, E.E.; Fouad, M.; Gad, M.I. Prediction of the pollutants movements from the polluted industrial zone in 10th of Ramadan city to the Quaternary aquifer. Appl. Water Sci. 2019, 9, 20. [Google Scholar] [CrossRef]
- Aly, A.I.M.; Hussien, R.A.; Nassar, N. Radioactive dispersion in groundwater resulting from postulated accident at a proposed nuclear power plant, northwestern coast of Egypt. Groundw. Sustain. Dev. 2020, 10, 100326. [Google Scholar] [CrossRef]
- Rad, P.R.; Fazlali, A. Optimization of permeable reactive barrier dimensions and location in groundwater remediation contaminated by landfill pollution. J. Water Process Eng. 2020, 35, 101196. [Google Scholar] [CrossRef]
- Wondzell, S.M.; LaNier, J.; Haggerty, R. Evaluation of alternative groundwater flow models for simulating hyporheic exchange in a small mountain stream. J. Hydrol. 2009, 364, 142–151. [Google Scholar] [CrossRef]
- Hirata, R.; Cagnon, F.; Bernice, A.; Maldaner, C.H.; Galvão, P.; Marques, C.; Terada, R.; Varnier, C.; Ryan, M.C.; Bertolo, R. Nitrate Contamination in Brazilian Urban Aquifers: A Tenacious Problem. Water 2020, 12, 2709. [Google Scholar] [CrossRef]
- Abd-Elaty, I.; Saleh, O.K.; Ghanayem, H.M.; Grischek, T.; Zelenakova, M. Assessment of hydrological, geohydraulic and operational conditions at a riverbank filtration site at Embaba, Cairo using flow and transport modeling. J. Hydrol. Reg. Stud. 2021, 37, 100900. [Google Scholar] [CrossRef]
- Musacchio, A.; Mas-Pla, J.; Soana, E.; Re, V.; Sacchi, E. Governance and groundwater modelling: Hints to boost the implementation of the EU Nitrate Directive. The Lombardy Plain case, N Italy. Sci. Total Environ. 2021, 782, 146800. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, M.; Safavi, H.R.; Taheriyoun, M.; Rezaei, F. Multi-objective optimization for optimal extraction of groundwater from a nitrate-contaminated aquifer considering economic-environmental issues: A case study. J. Contam. Hydrol. 2021, 241, 103806. [Google Scholar] [CrossRef] [PubMed]
- Bridget Odochi, U.; Iheanyichukwu, O.A.; Okechukwu, O.S.; Chukwuemeka, I.C.; Juliana, O.I.; Ifeanyi, O.S.; Nkiru, N.C.; Osita, I.P.; Emmanuel, A.E. Hydrogeological assessment and contaminant transport modelling of Enyimba landfill site in Aba, Nigeria. Water Pract. Technol. 2024, 19, 2108–2124. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Cui, W.; Cao, G.; Yao, J. Simulating arsenic discharge flux at a relic smelting site in Guangxi Zhuang Autonomous Region, China. Environ. Sci. Pollut. Res. 2024, 31, 12094–12111. [Google Scholar] [CrossRef]
- Al-Hadhrami, A.; Sana, A.; Etri, T.; Al-Mamun, A.; Nikoo, M.R.; Al-Rawas, G. Modeling of seawater intrusion into Salalah coastal plain aquifer, sultanate of Oman. Groundw. Sustain. Dev. 2024, 24, 101076. [Google Scholar] [CrossRef]
- Xue, Y.Q.; Xie, C.H. Numerical Simulationfor Groundwater; Science Press: Beijing, China, 2007. [Google Scholar]
- Cheung, C.W.; Porter, J.F.; McKay, G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Gao, S.; Wang, L.; Long, T.; Cao, S.; Chen, Q. Research Progress on Migration Characteristics and Investigation Technologies of DNAPLs Contaminated Sites. J. Ecol. Rural Environ. 2018, 34, 289–299. [Google Scholar]
- Parker, B.L.; Cherry, J.; Chapman, S.; Guilbeault, M. Review and analysis of chlorinated solvent dense nonaqueous phase liquid distributions in five sandy aquifers. Vadose Zone J. 2003, 2, 116–137. [Google Scholar] [CrossRef]
- Wymore, R.A.; Akladiss, N. Integrated DNAPL site characterization and tools selection. In Proceedings of the 6th International Contaminated Site Remediation Conference, Melbourne, VIC, Australia, 13–16 September 2015. [Google Scholar]
- Zhang, W.; Shi, X.; Wu, J.; Wu, J. Impacts of the Spatial Variation of Permeability on the Transport of Dense Non-aqueous Phase Liquids in Porous Media. Geol. J. China Univ. 2013, 19, 677–682. [Google Scholar]
- Khachikian, C.; Harmon, T.C. Nonaqueous phase liquid dissolution in porous media: Current state of knowledge and research needs. Transp. Porous Media 2000, 38, 3–28. [Google Scholar] [CrossRef]
- Sale, T.; Newell, C. Decision Guide: A Guide for Selecting Remedies for Subsurface Releases of Chlorinated Solvent Sites; ESTCP Project ER-0530; Environmental Security Technology Certification Program: Washington, DC, USA, 2011. [Google Scholar]





| Direction | Boundary Description |
|---|---|
| Northwest | Generalized to the recharge boundary using the parallel line of the groundwater contour. |
| East | Generalized to the zero-flow boundary using the vertical line of the groundwater contour. |
| South | Generalized to the boundary of the north-south river right bank and known head boundary. |
| West | Generalized to the boundary of the diagonally oriented river left bank and known head boundary. |
| Floor Number | Kxx, Kyy (m/d) | Kzz (m/d) | Specific Yield | Coefficient of Storage | αL | TRPT * | TRVT * | Effective Porosity |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.01 | 0.1 | - | 0.1 | 0.1 | 0.01 | 0.28 |
| 2 | 8.08 | 1 | - | 1.0 × 10−5 | 1.0 | 0.1 | 0.01 | 0.28 |
| 3 | 0.1 | 0.01 | - | 1.0 × 10−6 | 0.1 | 0.1 | 0.01 | 0.28 |
| 4 | 1.5 | 0.2 | - | 1.0 × 10−5 | 1.0 | 0.1 | 0.01 | 0.28 |
| 5 | 0.1 | 0.01 | - | 1.0 × 10−6 | 0.1 | 0.1 | 0.01 | 0.28 |
| 6 | 0.6 | 0.06 | - | 1.0 × 10−5 | 1.0 | 0.1 | 0.01 | 0.28 |
| Shallow Aquifer | Middle Aquifer | Deep Aquifer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boundary | Sources and Sinks | Boundary | Sources and Sinks | Boundary | Sources and Sinks | ||||||||
| Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | ||
| Flow upper face | 26.30 | Storage | 57.41 | 29.78 | 1.09 | ||||||||
| Flow lower face | 67.11 | Constant head | 63.94 | 47.26 | 1.35 | 40.13 | 7.32 | 28.80 | 1.37 | 31.10 | |||
| Flow left face | Head dep bounds | ||||||||||||
| Flow right face | Wells | 12.83 | 2.87 | 1.04 | |||||||||
| Flow front face | Recharge | 11.47 | |||||||||||
| Flow back face | |||||||||||||
| Total inflow | 114.54 | Total outflow | 114.37 | Total inflow | 68.95 | Total outflow | 68.93 | Total inflow | 32.19 | Total outflow | 32.19 | ||
| Shallow Aquifer | Middle Aquifer | Deep Aquifer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boundary | Sources and Sinks | Boundary | Sources and Sinks | Boundary | Sources and Sinks | ||||||||
| Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | Inflow | Outflow | ||
| Flow upper face | 28.51 | Storage | 58.06 | 30.01 | 1.09 | ||||||||
| Flow lower face | 67.79 | Constant head | 63.05 | 48.36 | 1.35 | 40.40 | 7.26 | 29.14 | 1.35 | 31.31 | |||
| Flow left face | Head dep bounds | ||||||||||||
| Flow right face | Wells | 12.83 | 2.87 | 1.04 | |||||||||
| Flow front face | Recharge | 11.76 | |||||||||||
| Flow back face | |||||||||||||
| Total inflow | 116.15 | Total outflow | 116.15 | Total inflow | 69.55 | Total outflow | 69.55 | Total inflow | 32.40 | Total outflow | 32.40 | ||
| Remediation Process | Time Period | Remediation Measures | Corresponding Areas | Model Number | Injection Well Remediation Rate (/d) |
|---|---|---|---|---|---|
| 1 December 2011~ 1 June 2012 | In situ thermal desorption | Holiday Cleaners and thermal desorption region near T1 and T2 | 0.0274 | ||
| Completed the initial full site ERD treatment in four injection events | 1 December 2010~ 1 March 2011 | ERD (injection of emulsified oil EVO) | Cross-section of T8 | PM1.1 * | 0.0137 |
| 1 March 2011~ 1 May 2011 | ERD (injection of EVO) | T3 well section | PM2.1 | 0.0137 | |
| 1 March 2012~ 1 May 2012 | ERD (injection of EVO) | Remainder of T3, T1, and T2 sections, SPC, and wells T4 to T7 | PM3.1 | 0.0137 | |
| 1 September 2012~ 1 November 2012 | ERD, EHC reagent (a repair reagent with an integrated carbon matrix and zero-valent iron source) | T1 and T2 and the remainder of the Holiday Cleaners wells | PM4.1 | 0.1096 | |
| Second site-wide ERD treatment | 31 December 2013~ 1 March 2014 | ERD, KB-1 bacterial agent (a biofortified culture) | Entire target area | PM5.1 | 0.1096 |
| Date | Grid Near G | Grid Near V | Grid Near J | Grid Near JJ |
|---|---|---|---|---|
| 1 June 2011 | 38 | 28 | - | - |
| 1 December 2011 | 38 | 28 | - | 6.5 |
| 1 June 2012 | 38 | 28 | 13 | 13 |
| 1 December 2012 | 48 | 15 | 12 | 9 |
| 1 June 2013 | 20 | 1.7 | 15 | 1.2 |
| 1 January 2014 | - | 1.5 | 2.9 | 4.5 |
| 1 June 2014 | - | - | - | 1.6 |
| 1 June 2015 | - | 0.2 | - | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wei, C. Study on the Fine Characterization of Spatial Distribution and Predictive Modeling of Remediation of Site Pollution. Water 2024, 16, 3154. https://doi.org/10.3390/w16213154
Yang J, Wei C. Study on the Fine Characterization of Spatial Distribution and Predictive Modeling of Remediation of Site Pollution. Water. 2024; 16(21):3154. https://doi.org/10.3390/w16213154
Chicago/Turabian StyleYang, Jun, and Caijie Wei. 2024. "Study on the Fine Characterization of Spatial Distribution and Predictive Modeling of Remediation of Site Pollution" Water 16, no. 21: 3154. https://doi.org/10.3390/w16213154
APA StyleYang, J., & Wei, C. (2024). Study on the Fine Characterization of Spatial Distribution and Predictive Modeling of Remediation of Site Pollution. Water, 16(21), 3154. https://doi.org/10.3390/w16213154





