Abstract
This work investigates how peatlands can be used as archives of past environmental conditions to record changes in atmospheric deposition of some (especially lithophile and chalcophile) elements and any water–rock–organic matter interactions followed by the sedimentation or leaching of others. We have provided a detailed description of both the distribution of various groups of elements and their chemical species in the entire depth of peat deposits. The study analyzes the features of the peat sediment formation and element distribution in the 0–310 cm depth core sample of peat deposits of the Ubinskoye peat bog located in the forest-steppe zone of Western Siberia. The study reveals a profound diagenetic transformation of water and peat chemical composition. Element speciation investigated using the modified Tessier sequential extraction procedure showed the vertical transition of an oxidative geochemical environment to a reducing one with the formation of geochemical barriers for variably valent elements. Computer modeling calculations of saturation indices of pore solutions in relation to a number of minerals allowed us to estimate the degree of equilibrium of the system and the direction of its transformation. Early diagenetic processes lead to the deposition of authigenic minerals. Therefore, barite forms on the redox line, while pyrite is found in the reducing environment. With depth, the content of Ca, Mg and Sr increases, which leads to the formation of authigenic carbonates.
1. Introduction
Peat bogs are of great interest for the study of the Earth’s surface geochemical background. Western Siberia is a unique region leading the world in terms of the extent of waterlogging, which is still observed today [1,2,3,4]. According to the data of these authors, the rate of vertical growth of peat varies from 0.39 to 2.62 mm/year. Studies of peat formation processes and sources of elements are the key points in constructing the concept of peat environment evolution. Trace element concentration data in the peat sediment profile can be used to assess environmental pollution as well as to track sources of substance entry [5,6,7,8,9,10]. Furthermore, knowledge of element speciation and physical–chemical processes existing at the water–solid interface is very important for predicting the accumulation of elements at geochemical barriers and shaping the pattern of diagenetic changes in sediments [11,12,13,14].
One of the main factors affecting the accumulation ability of peat is the presence of a huge amount of humic substances characterized by high absorption capacity [15,16,17,18,19,20,21]. Organic peat deposits must be characterized in terms of many parameters of the geological environment, such as water composition, pH and Eh conditions, botanical composition and microbiological processes [12]. Organic matter in peat deposits acts as a biogeochemical barrier that concentrates a large range of incoming elements, both precious metals and potentially toxic elements, and even rare earth elements. In general, it can be stated that, despite the abundant evidence of the concentration of chemical elements in peat deposits, the mechanisms of this process, the role of organic matter in it and questions concerning the species of elements in peat are still poorly understood.
Recently, comprehensive geochemical studies of complete Holocene peatland sections in the West Siberian have been undertaken by the authors of [11,13] in the forest-steppe zone and by the authors of [1] in the Southern Taiga Subzone. Active research in this area is carried out for the swamps of the forest and forest-steppe zones of Western Siberia, such as, for example, a series of works on the geochemistry of peat deposits [7,12,14,22,23,24,25,26,27,28,29,30] and the geochemistry of bog waters [5,15,16,17,18,31,32,33,34,35,36,37,38,39,40,41]. Most scientific research is related to the study of the chemical composition of swamp waters and peat deposits, the mineralogical composition of the ash part of the sediments and the hydrological regime. Insufficient attention is paid to the study of complete Holocene sections of deposits, including the distribution of chemical elements [42,43,44,45].
Bog waters are the subject of extensive research, including the study of water exchange and water balance of wetland landscapes, the chemical composition of bog waters, biogeochemistry and other aspects [46,47,48]. However, many problems remain unresolved, including the composition of organic compounds dominating in bog waters; the relationship between the composition of bog waters and associated groundwater; the identification of patterns of changes in the chemical composition of bogs; and the distribution and migration of chemical elements depending on the territory, type of bog and depth of the peat deposits [49,50,51,52,53,54,55,56,57]. The factors of transformation of the bog water chemical composition in the early diagenesis of peat have also been studied to a lesser extent.
Thus, the mechanisms of changes in the chemical composition of bog waters in the diagenesis of peatlands, both on a global scale and in Western Siberia, require further detailed studies, representing an urgent problem in the biogeochemical studies of wetland ecosystems and their transformation along the entire section of a peat deposit in theoretical and practical terms.
The goal of this study is to conduct a detailed examination of the hydrogeochemical features of the Ubinskoye oligotrophic peat bog of the forest-steppe zone located in the south of Western Siberia (N 55°18.211′, E 79°42.711′), aged 4.5–5.5 thousand years (Middle Holocene), with a focus on analyzing the diagenetic transformations in the chemical composition of water and peat. The location of the bog on the southern boundary of the bog distribution (Baraba lowland), as well as near settlements, contributes to its dependence on the impact of climate change and anthropogenic impact. We aim to assess how peatlands can serve as archives of past environmental conditions, recording changes in atmospheric deposition of elements and their subsequent interaction with water, rocks and organic matter, leading to the sedimentation or leaching of other elements. Special attention is given to studying the distribution of various groups of elements and their chemical species throughout the depth of peat deposits, as well as identifying vertical transitions from an oxidative geochemical environment to a reducing one and the formation of geochemical barriers for elements with variable valency.
The novelty of the work lies in the use of a computer modeling method to calculate the saturation of pore solutions in relation to sediment minerals with a subsequent assessment of the direction of transformation of the composition of peat deposits.
2. Materials and Methods
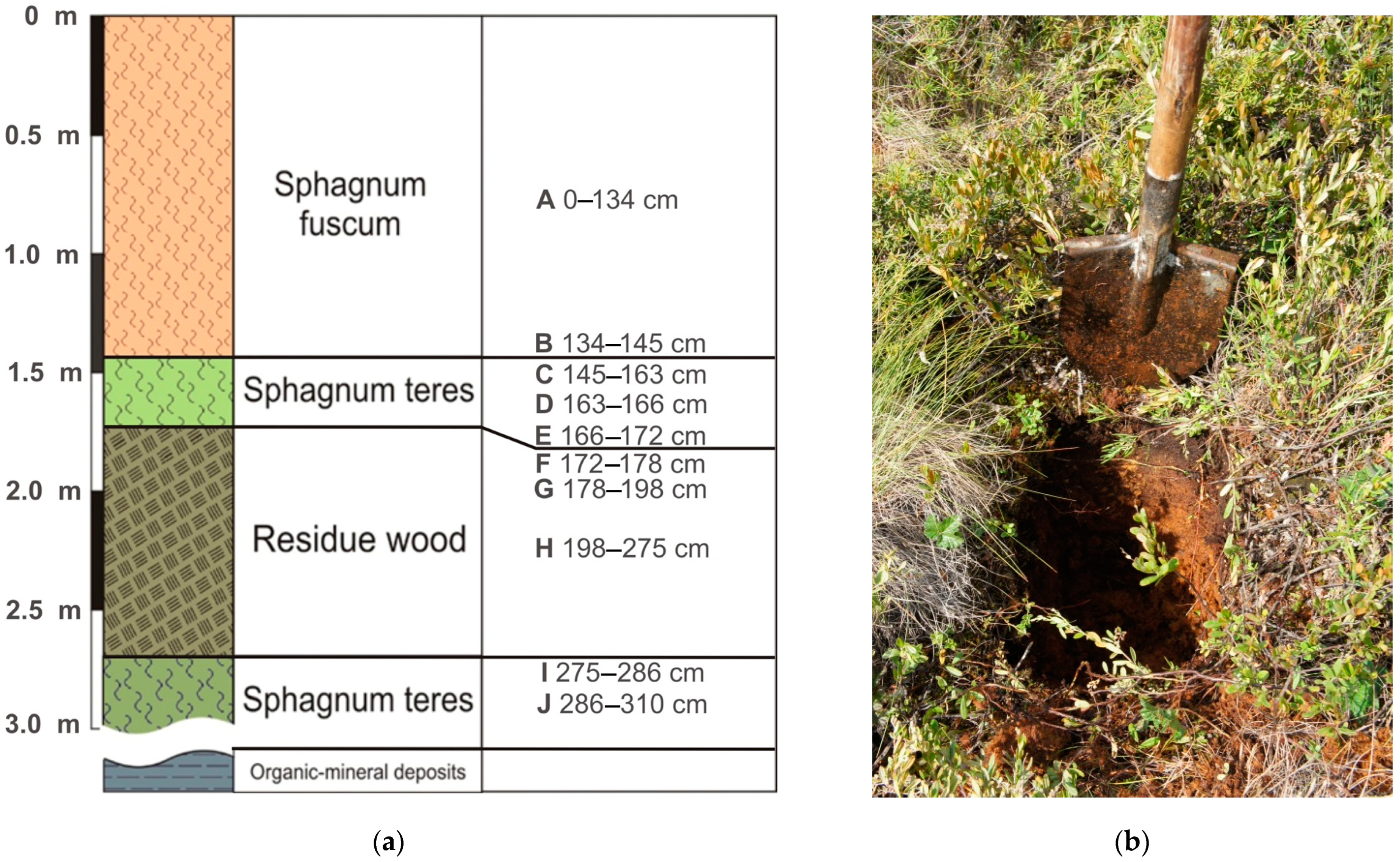
The object is a high pine–shrub–sphagnum bog represented by dark brown medium-decomposed plant remains (Figure 1). The ash content varies from 2 to 51%, increasing with depth. The upper part of the core (0–145 cm) consists of Sphagnum fuscum, the lower part (145–310 cm) comprises Sphagnum teres with an organic-rich woody layer (172–275 cm) and the organic–mineral sediments (under 310 cm) are represented by loam with peat inclusions.
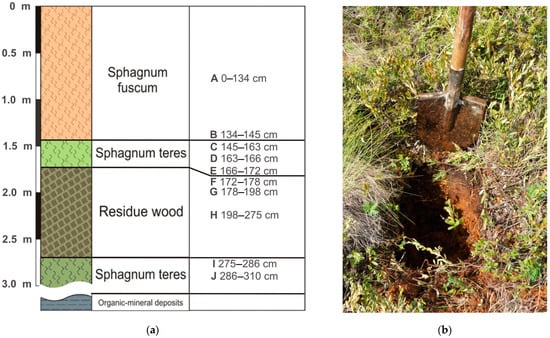
Figure 1.
Stratigraphy of the peat deposits and corresponding sampling horizons (A–J) (a). General view of the sample point (b).
2.1. Sampling and Laboratory Research
Two parallel peat cores were sampled in the central part of the bog with a peat sampler. In situ pH and redox potential (Eh, mV) were measured using a portable Hanna Instruments multiparameter analyzer immediately. The cores were cut into 10 intervals according to changes in the medium, color, humidity and density of the material. The first core samples were sealed in polyethylene film and delivered to the laboratory within 24 h, dried at in situ temperature, and finally pulverized and quartered for further analytical work. From the second parallel core, pore water was squeezed out at appropriate intervals at a pressure of 150 bar. When more than 50 mL was squeezed, the solution was analyzed for major and trace element composition; at a wringing volume of less than 50 mL, the solution was filtered through a 0.45 μm membrane filter and then acidified with pure-grade nitric acid to pH 2 for only trace element analysis.
The unstable parameters of bog waters were also measured in situ; then, 15 mL of the solution was filtered through a 0.45 µm membrane filter and acidified with pure-grade nitric acid to pH 2 for trace element analysis. For major elements and organic carbon content measurement, 1.5 L of water was taken in plastic bottles.
The cationic composition of water was analyzed using high-resolution mass spectrometry with inductively coupled plasma (ICP MS) (Element, Finnigan MAT, Bremen, Germany). The averaging error did not exceed 3%. The anionic composition was determined using the titrimetric method and capillary electrophoresis (Capel-105M, Lumex instruments, Saint-Petersburg, Russia). The measurement error did not exceed 5%. Total organic carbon (TOC) was measured using a Vario TOC Cube analyzer. The measurement error did not exceed 2%.
The solid samples were studied via X-ray fluorescent element analysis at the Siberian Synchrotron and Terahertz Radiation Center (INP SB RAS). The elemental and mineral compositions of the solid samples were analyzed using a Jeol JSM-6380LA (JEOL Ltd., Tokyo, Japan) scanning electron microscope (SEM) at the Analytical Center for Multi-elemental and Isotope Research of SB RAS. The samples were studied in the secondary electron mode, with an accelerating voltage of 30 kV. Energy dispersive X-ray spectroscopy (Oxford Instrument X-Max 80 EDS-system, Oxford Instruments plc, Abingdon, Oxon, UK) was used for the microanalysis of solid phases observed via SEM.
The concentration coefficients were calculated using the following formula:
where Ci—actual content of analyte in peat, mg/kg; Cbi—regional background content of analyte in peat, mg/kg.
Cc = Ci/Cbi
2.2. Speciation of Elements in Peat and Underlying Sediments
Selective extraction is an old-established way to characterize the way trace elements are attached/present in sediments. One of the earliest selective extraction methods is that presented by Tessier et al. [58], which provides as many as six different extracts: exchangeable metals, metals bound to carbonates, metals bound to Mn oxides, metals bound to Fe–Mn oxides, metals bound to organic matter and sulfides, and residual metals (largely in silicates).
Element speciation was investigated using the modified sequential extraction procedure; the following fractions were used to determine the speciation of elements in peat sediments: (1) water-soluble (H2O, V = 25 mL, T = 25 °C, t = 1 h); (2) exchangeable (1 M NH4OAc, pH 7, V = 25 mL, T = 25 °C, t = 1 h); (3) carbonate (elements associated with carbonates, 1 M NH4OAc, pH 5 buffered with HOAc, V = 25 mL, T = 25 °C, t = 5 h); (4) organic (elements associated with humic acids, 0.01 M NaOH, pH 11, V = 25 mL, T = 25 °C, t = 24 h); (5) reducible (elements associated with oxides of Fe and Mn, 2 M NH2OH·HCl in 25% HOAc, V = 25 mL, pH 2, T = 96 °C, t = 6 h); (6) oxidizable (elements associated with sulfides, 30% H2O2 with addition of 0.02 M HNO3 to pH 2, V = 20 mL, T = 85 °C, t = 24 h); and (7) residual (acid decomposition: HF–HNO3–HClO4–HCl). For the procedure, a 4 g sample of peat was taken.
After each stage of extraction, the residue was rinsed in 10 mL of distilled water and centrifuged. Concentrations of elements in solutions after sequential extraction experiments were determined using atomic absorption spectrophotometers using electrothermal (PerkinElmer 3030 Z, Boston, MA, USA) and flame (Pyi-Unicam SP-9, Philips, Cambridge, UK) atomization. The instrumental uncertainties were lower than 5%.
2.3. Computer Modeling
With an ICP-MS analysis, the presence of free ionic species and complexes can be calculated, along with the saturation with respect to solid phases. To calculate the chemical speciation of elements in the water–peat–organic matter system, we used HCh 4.6 speciation software [59,60]. It uses the Gibbs free energy minimization function to calculate mineral–fluid equilibrium. Chemical potentials of minerals are calculated using the equations of states included in HCh; activity coefficients of charged species are estimated using the Davies variant of the Debye–Hückel equation. The activity coefficients depend on the ionic strength, which itself depends on the molality of each solute in the solution [61].
Our model uses the well-established thermodynamic relationships among different chemical species to calculate the equilibrium distributions. The HCh database contains all the equilibrium constants for different reactions to predict ion speciation. The required analytical characteristics include the following: element concentrations in peat, pH, Eh, major and trace cations and anions in water. All these parameters were determined for the investigated intervals of peat deposits. The concentration of elements and other peat characteristics were used as input data for HCh software.
3. Results and Discussion
3.1. Geochemical Characteristics of Peat Depth Profile
3.1.1. Chemical Composition of Bog and Pore Waters
According to the prevailing ions, bog water belongs to the chloride-sulfate class of the calcium group; according to alkaline–acid conditions, to the acid class; according to the value of total dissolved solids (TDS), to ultra-fresh water (Table 1). The low pH values (3.4) of bog water are due to the decomposition of organic substances, leading to the release of CO2, fulvic and humic acids and other organic matter (OM) compounds into the water. The distribution of Eh-pH parameters and the main ions in the pore water of the upper part of the section up to 163 cm (horizons A–C) is presented in Table 1. Compared to the bog water, in the pore water, pH increases up to 5.5, and Eh decreases to the negative value of −134 mV (horizon C). A significant change in the TDS of pore water also occurs in horizon C due to an increase in the concentrations of anions NO3−, HCO3−, SO42− and Ca2+. It is obvious that the chemical composition of pore water changes strongly, and it becomes sulfate-nitrate-hydrocarbonate of the calcium group.

Table 1.
Main hydrochemical parameters of bog and pore water, ppm.
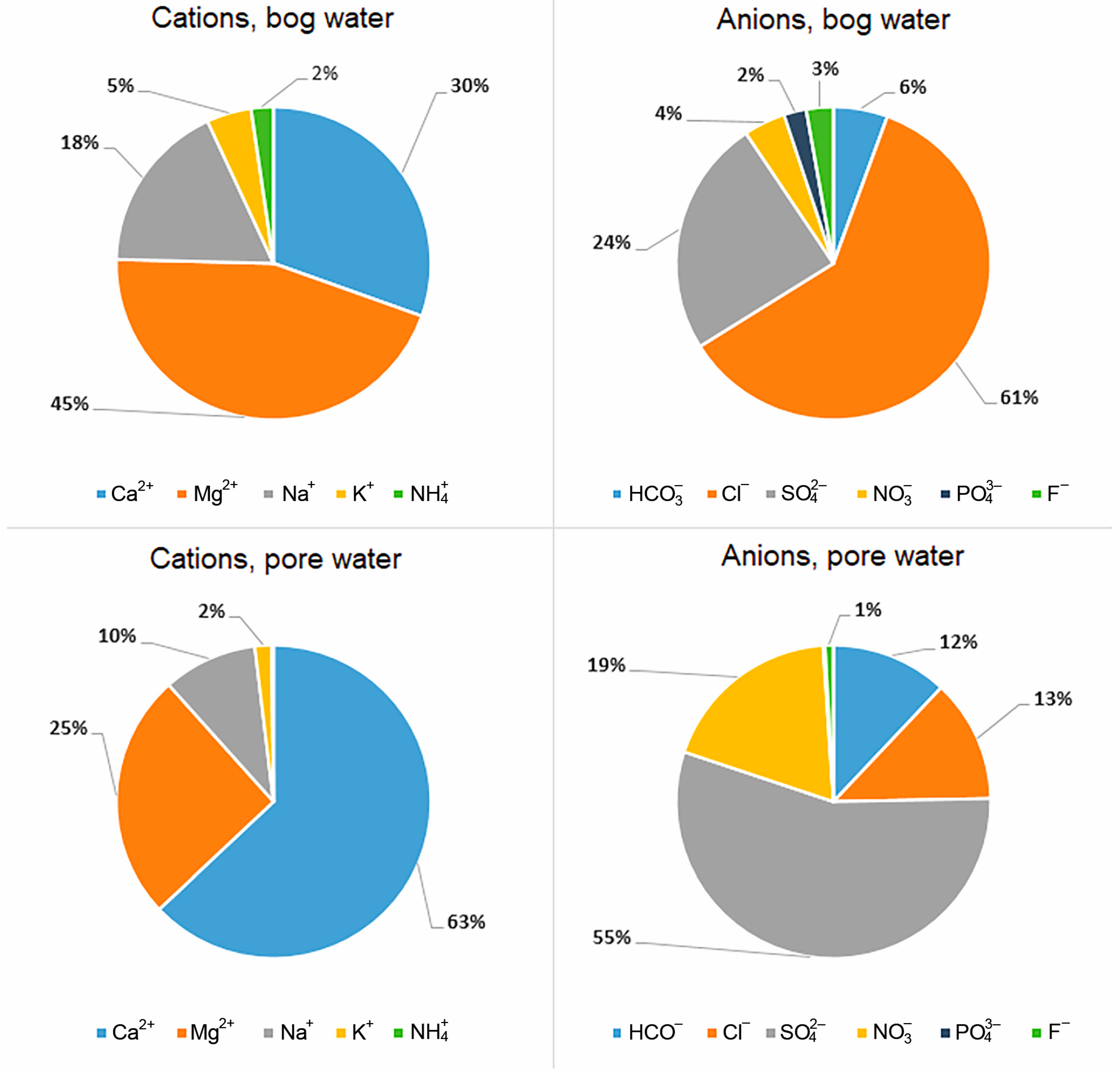
The increase in TDS with depth and the change in the relationship between the main cations and anions are a reflection of the diagenetic transformation of peat. The pie chart shows the ratios in % equivalent required to calculate ion-exchange and redox reactions, which allows one to more clearly see that Mg gives way to calcium with depth, and the chloride ion gives way to sulfate and nitrate (Figure 2).
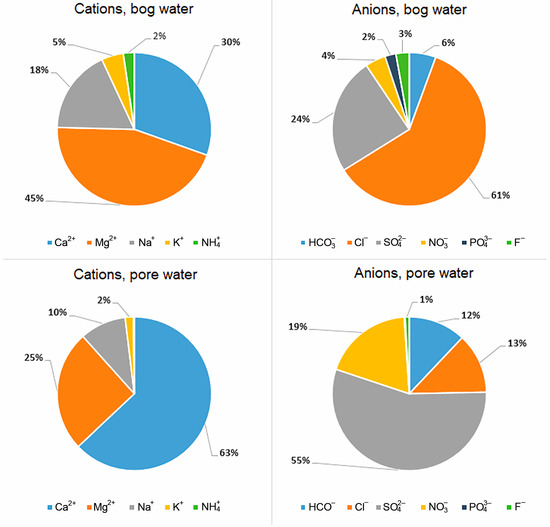
Figure 2.
Ionic composition of bog and pore (horizon C) water (% equiv.).
An increase in HCO3− concentrations in water down the peat section from 2.4 to 22.0 ppm was established. The content of the hydrocarbonate ion in the peat section is low and averages 16% of the total anions. For SO42−, a sharp increase in its concentration with depth from 8.5 to 80 ppm is also observed. The sulfate ion is one of the main anions in pore water and averages 51% of the total anions in the section. The concentration of Cl− practically does not change along the depth; however, in the range of 0–18 cm (horizon A), an increase in chlorine from 15.4 to 29.9 ppm is noted. Chlorine is on average 35% of the total anions. The content of Ca2+ in the pore water increases along the depth from 7.5 to 51 ppm. Calcium is the main cation; on average, it accounts for 55% of the total of all cations. In the distribution of Mg2+, Na+ and K+ cations, there is no clearly expressed trend of their increase with depth (Table 1), except an increase in K+ concentration in the depth of 0–18 cm (horizon A) to 7 ppm, which may be associated with active mineralization of peat-forming plants.
An increase with depth in the concentration of NO3−, one of the products of mineralization of OM during diagenesis, from 1.9 to 35 ppm was established. The decrease in ammonium nitrogen NH4+, on the contrary, is not so noticeable with depth. Microbiological activity is responsible for the oxidation of ammonia to nitrate (2):
NH4+ + 2O2 = NO3− + H2O + 2H+
For PO43−, concentrations decrease with depth from 0.52 to 0.17 ppm. Such a behavior may be due to the formation of iron phosphates in the reducing intervals of the peat bog. However, in comparison with mineral deposits, the content of phosphorus in the pore water is quite high, which reflects the processes of anaerobic degradation of organic matter.
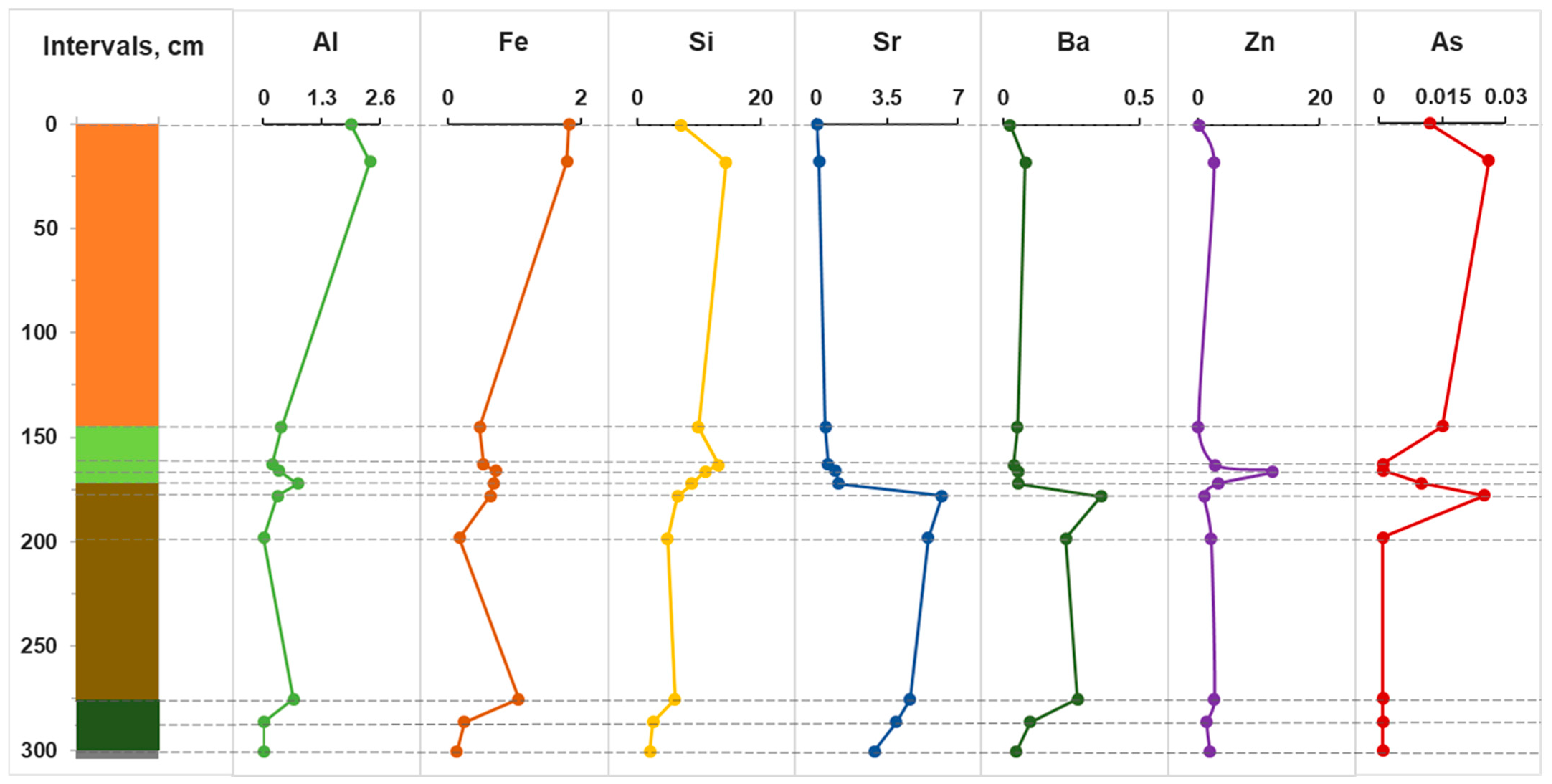
Figure 3 shows the distribution of some trace elements in pore water to the full depth of the section across the horizons. It should be noted that in pore water, the reducing environment in horizons C-J is co-stored, the pH reaches neutral values of 7.0, and the Eh minimum (−200 mV) occurs at a depth of 200 cm. Intense changes occur in the range of 160–200 cm, as can be seen from the example of all trace elements. Significant increases in concentrations are recorded for Al, Fe and Si. It is worth noting the distinct peaks for Sr, Ba, Zn and As. It is known that aluminum can play the role of an abiogenic indicator, i.e., terrigenous continental runoff [53]. Then, comparing the behavior of Al, As and Zn, we can conclude that they are a consequence of the anthropogenic load on the bog ecosystem in the 20th–21st centuries. In addition, As is often associated with sulfides, primarily pyrite; therefore, an increase in its content in the pore water of the upper horizons may also be a consequence of the dissolution of sulfides.
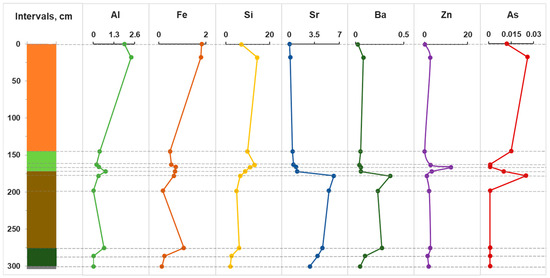
Figure 3.
Distribution of trace elements in bog and pore water, ppm.
It is worth mentioning the high concentrations of Al and Fe in bog water, the maximum allowable concentrations (MACs) of which for drinking water are 0.5 and 0.3 ppm, respectively. They form strong complexes with organic acids (TOC 128 mg/L), which may be the reason for their high concentrations. It should be noted that the extremely high concentrations of zinc in the pore water of the D horizon are up to 10 ppm (10 times higher than the MAC). High zinc contamination of oligotrophic bogs of the Baraba forest steppe was also noted earlier [13,44]. Thus, the destruction of a number of minerals and the diagenesis of peaty vegetation residues leads to an increase in the concentrations of Al, Fe and P in the slightly acidic (pH 4–4.5) pore water of the upper part (horizon A). The increase in the total P content in the upper intervals is a consequence of the active destruction of OM under aerobic conditions.
To identify sources and transformation of matter in bog water, Pearson’s correlation coefficients were calculated (Table 2). A strong positive correlation of Cu-Tl, Zn-V and Mn-As pairs confirms the anthropogenic load on the bog ecosystem. The most mobile element in bog waters is Zn, which is indicated by high values of Cc at the level of 26.8 (Table 3). The strong inverse correlation of the Al-B pair may be due to different sources of matter. Boron is a biophilic element, and its content in the ashes of dead plants is many times higher than that in the lithosphere and soils; aluminum is due to terrigenous drift, as mentioned above. In bog waters, the NH4+/NO3− ratio decreases with depth, which is a reflection of OM destruction processes. This is indicated by rather high values of Cc for NO3− at the level of 7.3 (Table 3).

Table 2.
Significant correlation coefficients of ions and trace elements in bog water.

Table 3.
Concentration coefficient (Cc) values of the main ions and trace elements in bog waters.
Thus, this study revealed significant diagenetic transformations in peat, evidenced by changes in total dissolved solids (TDS) and the shifting relationship between major cations and anions in water. This transformation is reflected in the transition from chloride-sulfate to sulfate-nitrate-hydrocarbonate of the calcium group with increasing depth.
3.1.2. Chemical Composition of Peat and Underlying Sediments
The terrestrial sources of inorganic solids in the peats may include both mineral matter physically incorporated from the underlying sediments and elements that were added to the peats by water–rock interactions [34]. The average chemical composition of the ash part of the peat deposits is shown in Table 4. We divided all the elements into five groups for the data description: (a) lithophilic metals of the Al group (Al, K, Na, Li, Ba); (b) siderophilic metals of the Fe group (Fe, Mn, Cr, Ni); (c) lithophilic elements of the Ca group (Ca, Mg); (d) chalcophilic elements (As, Cd, Cu, Zn, Pb); (e) lithophilic and siderophilic elements of mixed valence forming cations and anions (Ti, V, U, Mo).

Table 4.
Distribution of chemical elements in the peat profile.
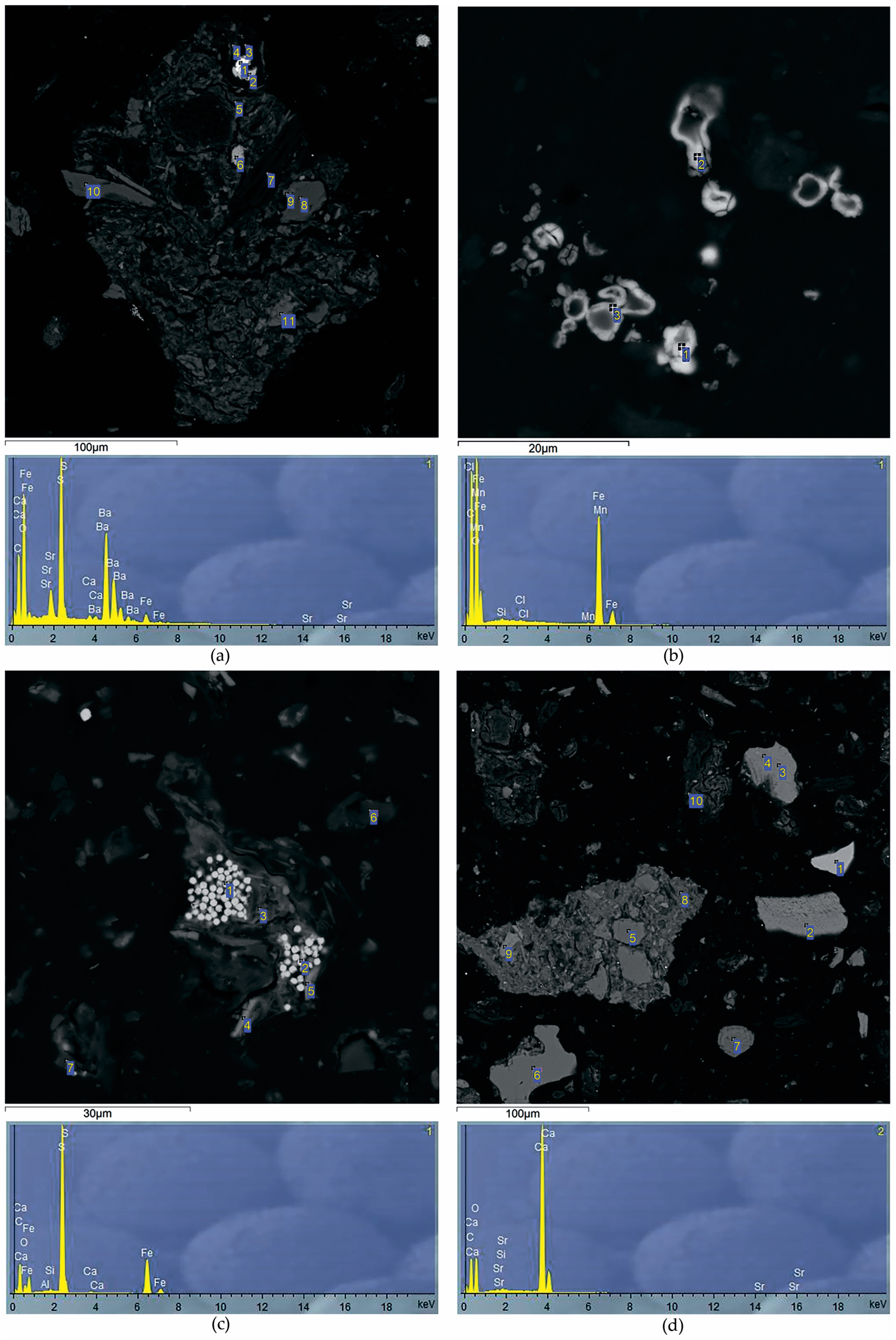
Since Al is one of the main components of peat ash, with an increase in ash content in peatland sections, Al concentrations increase synchronously. It was found that the minimum contents of Al (0.2 ppm), K (0.9%), Na (0.04 ppm) and Li (0.6 ppm) are characteristic of the upper peat horizons. According to the distribution similarity of Al, K, Na and Li in the Ubinskoye peatland, it can be concluded that these elements have a common source of input with atmospheric precipitation in the composition of silicate and aluminum silicate material. An increase in Ba content from 23 to 250 ppm in the lower peat horizons indicates the formation of authigenic Ba minerals in the diagenesis such as barite and barytocelestite detected in the peat interval 163–198 cm (horizons D-G) via the SEM method (Table 5, Figure 4a). The deposition mechanism of authigenic barite in the sediment is similar to the formation of Fe-Mn nodules. However, barite does not precipitate at the redox boundary, where Fe(II) and Mn(II) are oxidized and precipitated, but at the boundary of a sharp decrease in sulfate ions in bog waters. The precipitation zone of diagenetic barite is located just below the redox boundary, where active processes of bacterial sulfate reduction begin. The inclusion of Sr in the composition of barites clearly indicates their authigenic (diagenetic) nature—during the deposition process, such barites capture part of Sr from groundwater. In addition, the increase in the Ba/Al ratio from 0.0092 to 0.0125 in the grass transition type of peat indicates the leaching of barium from the underlying high-ash sites of peat and its deposition up the section.

Table 5.
Mineral composition of the peat ash.
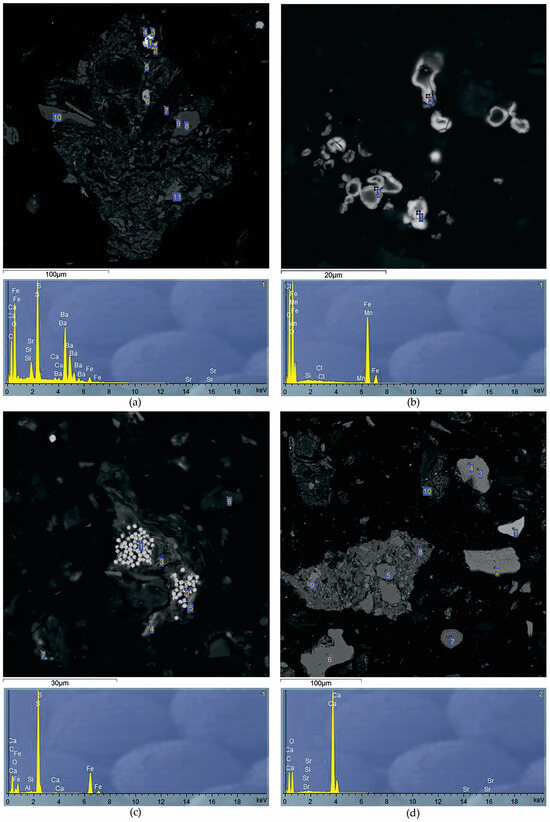
Figure 4.
SEM images of the peat deposits with the following minerals found at appropriate test intervals: (a) barytocelestite (166–172 cm); (b) siderite (60–80 cm); (c) pyrite (145–163 cm); (d) calcite (166–172 cm). The numbers show points of study of the mineral’s elemental composition.
All elements of the Fe group are closely correlated with each other, which reflects a similar direction of biogeochemical migration of elements both during the formation of the peat deposit of the Ubinskoye bog and during the diagenesis. Along with biochemical factors, the alkaline–acid and redox conditions of the medium play a huge role in their geochemistry. The upper peat intervals have oxidative medium conditions (+ 114 mV) and present an oxidative geochemical barrier on which elements of mixed valence are concentrated. Therefore, a significant increase in the content of Fe (1.4%), Cr (32 mg/kg), Ni (15 mg/kg), partially Mn (100 mg/kg) and Co (1.9 mg/kg) in the upper peat horizons can be associated with oxidation of their reduced forms coming with bog waters from the lower intervals of peatland with reducing conditions. In strong reducing conditions, Fe, Mn and Co actively change to a divalent state. Thus, the presence of mobile Fe and an unlimited reserve of carbonate ions leads to the formation of authigenic Fe carbonates, in particular siderite, during the diagenesis (Table 5, Figure 4a). In the section of the Ubinskoye peatland, siderite was found in the upper (0–145 cm) intervals of peat (Figure 4b). The conditions for the formation and location of siderite in modern peatlands are rather complicated since the entire mineral system, and especially the upper intervals of peat, is unstable. The formation of the solid siderite phase is possible with partial loss of peat in CO2 in deeper layers via the dissolution of bicarbonate iron ions due to a drop in the partial pressure of CO2. Siderite formation occurs mainly in summer, when the process of oxidation of OM in the upper layers is significantly intensified. An increase in the concentration of hydrogen ions, even if the Eh values remain unchanged, shifts the mineral system towards the formation of siderite. In winter, partial dissolution of siderite in the upper layers of peat is quite possible, when the oxidation of OM is minimal, and bog water is somewhat enriched by dissolved oxygen and may have higher pH values.
Part of the reduced Fe(II) enters the upper layers of peat via a concentration gradient, where it is oxidized to Fe(III) to form iron oxides and hydroxides of various compositions. In the section of peat of the Ubinskoye bog, the presence of iron oxides is established only in the upper horizons of peat (0–134 cm), which are included in the zone of seasonal fluctuations in the bog water level. The source of chemically reactive iron for the formation of iron oxides may be peat alternations containing siderite. The solution of ferrobicarbonate with siderite in equilibrium during the dry season of the year can hydrolyze with sufficient oxygen supply with the precipitation of the solid phase Fe (OH)2, which rapidly oxidizes to Fe2O3. In deeper, high-ash peat intervals, with an increase in the reducing settings, pyrite was established along the entire peatland section (Table 5, Figure 4c). Pyrite was formed in the early stages of diagenesis by the crystallization of amorphous Fe sulfides under the influence of H2S in the process of bacterial sulfate reduction.
A less noticeable increase in Mn contents than in Fe in the uppermost peat layers is due to the difference in the migration characteristics of these elements: the oxidation of dissolved iron (and as a consequence, its precipitation) occurs already with an Eh value ≥ 0, while the oxidation of Mn is observed at higher Eh values (+ 200… + 600 mV). The increase in manganese from 0.01 to 0.07 with depth indicates the predominance of its authigenic forms in the lower layers of the peatland, for example, as part of the digenetic carbonates, particularly calcite, which was found here according to SEM in the lower (163–172 cm) high-ash intervals (Figure 4d).
The group of Ca, Sr and partially Mg is characterized by their inclusion in the composition of authigenic carbonates formed during the peat formation. Increased concentrations of Ca (six times) and Mg (ten times) were detected in the lowest peatland intervals. In the upper part of the peat, the contents of this group of elements are minimal. We assume that the increase in Ca and Mg contents is a consequence of the formation of authigenic carbonates, in particular calcite and dolomite which are associated with both the change in water regime and the water content of the peatland at the early stages of its formation in the Holocene, and with the calcite redeposition during the diagenesis. Thus, the distribution of Ca and Mg is differentiated in a certain way along the peatland section—the upper horizons represented by the upper peat are depleted in Ca and Mg in contrast to the underlying layers of peat and organomineral deposits. In addition, a decrease in the contents of Ca in the upper intervals is also possible due to its removal by near-surface runoff, which leads to the partial calcium depletion of the upper peat of the Ubinskoye bog.
An increase in the concentrations of chalcophilic elements (As, Cd, Cu, Zn, Pb) in the upper peat intervals of the Ubinskoye bog was also found (Table 4). Thus, for the upper intervals of the peatland, the concentrations of these elements were as follows: Cu—47.1; Zn—101; As—17.2; Cd—0.68; Pb—45.6 ppm. The increase in the concentration of chalcophilic elements in the upper intervals of the Ubinskoye peatland is explained by the higher technogenic load on the bog ecosystem, specifically the proximity of the highway and a major urban area of the Ubinskoye rural community. One of the entering sources of this group of elements is atmospheric transport.
Uranium was not found in the peat material except for in the lowest intervals, where its concentrations were up to 2.3 ppm. In the diagenesis, U slightly migrates in strong reducing conditions and precipitates on a reducing geochemical barrier. It is likely that low ash values of the upper peat can also cause low U content.
The distribution of V in the diagenesis is defined by its multivalence—a change in the oxidation state in a wide range from +2 to +5. The data show a slight increase in concentrations of V in a peat deposit depth from 22 to 65 ppm from the range of 163 cm, which is also a consequence of an increase in reducing medium conditions. It is worth noting that in the uppermost interval of the peatland, high V levels were established (28 ppm). This can be preliminarily associated with a technogenic load on the bog ecosystem since the main sources of V are chemicals, pesticides and atmospheric precipitation. However, we believe that the main way V enters the upper bog is through aerosol precipitation.
Thus, the study of the chemical composition of peat and underlying sediments in the Ubinskoye bog enabled the identification of characteristic behavior patterns for distinct elemental groupings such as lithophilic metals, siderophilic metals, chalcophilic elements and mixed valence elements. The distribution of elements like Al, K, Na and Li suggests a common source from atmospheric precipitation, while an increase in Ba content indicates the diagenetic formation of authigenic Ba minerals.
Elements of the Fe group (Fe, Cr, Ni, Mn, Co) show a close correlation, reflecting biogeochemical migration during both peat formation and diagenesis. The study underscores the influence of alkaline–acid and redox conditions on their geochemistry, with the upper peat intervals exhibiting oxidative conditions and acting as a geochemical barrier.
The increase in concentrations of chalcophilic elements (As, Cd, Cu, Zn, Pb) in the upper peat layers is attributed to higher technogenic load, particularly due to the proximity to a highway and urban area. Atmospheric transport is identified as one of the sources for these elements.
3.2. Speciation of Elements in Water and Peat Deposits
3.2.1. Element Species in Peat and Underlying Sediments
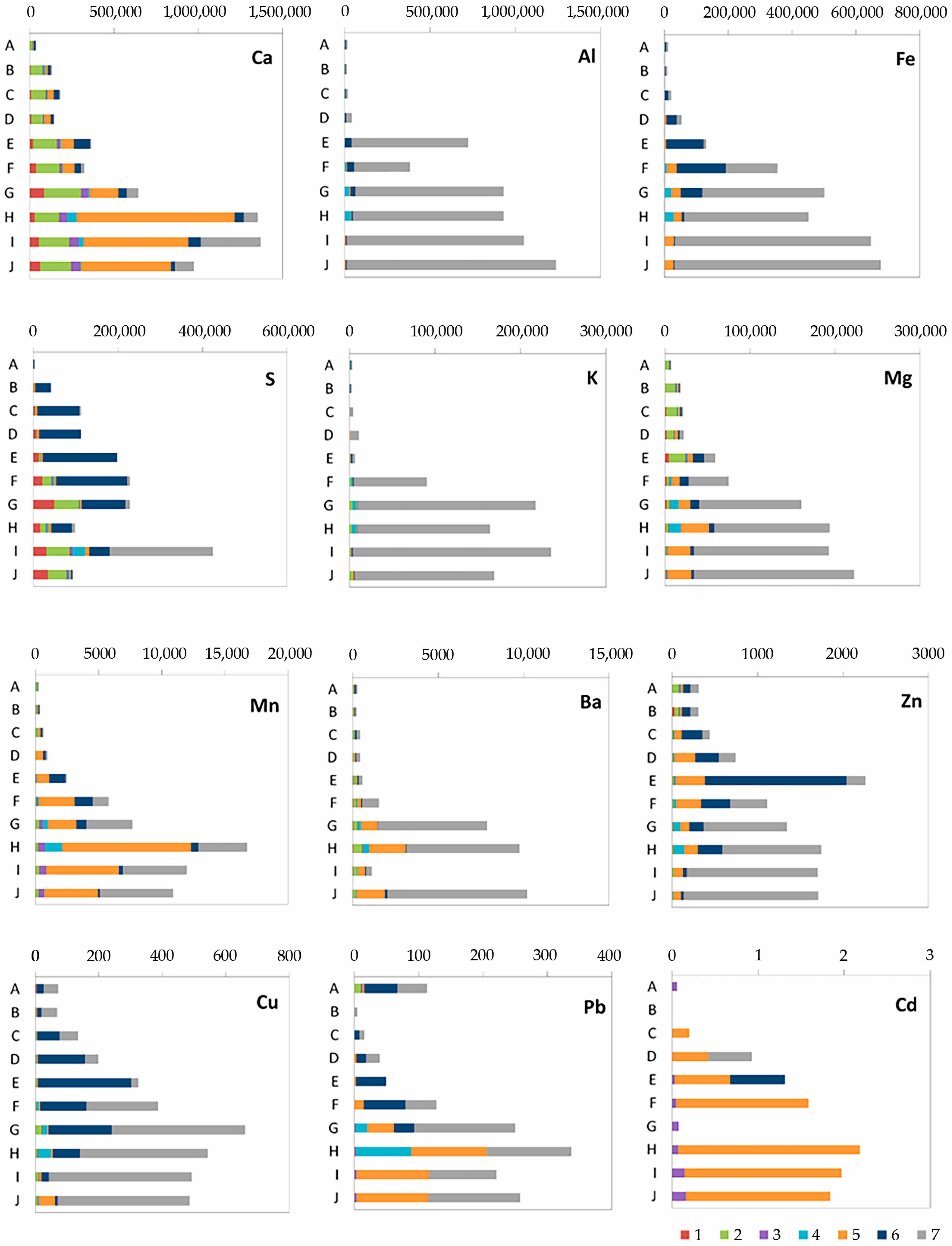
Experimental work on the assessment of the distribution of element speciation with depth has shown a wide variety of element species in peat deposits, while there is a stable increase in the content of almost all elements down the section (Figure 5).
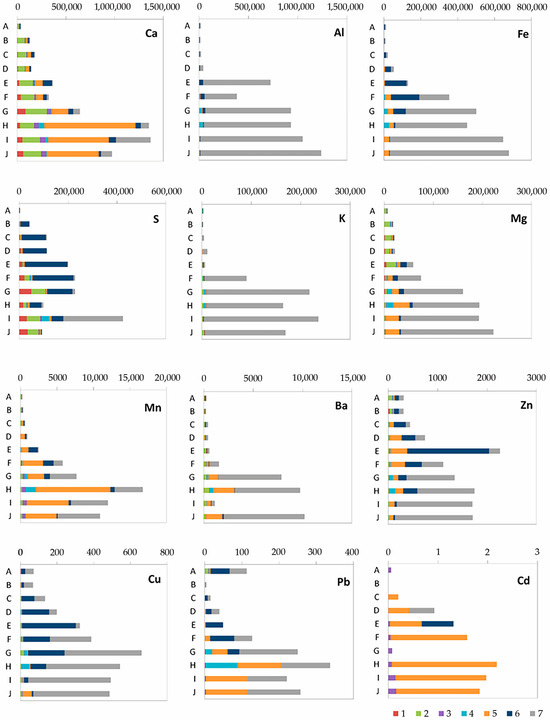
Figure 5.
The content, ppm and speciation of elements in peat sediments depending on the sequential fractions: (1) water-soluble; (2) exchangeable; (3) carbonate; (4) organic; (5) reducible; (6) oxidizable; and (7) residual.
The most characteristic thing that needs to be isolated is the belonging of Al and K to the residual fraction; i.e., they are cluster materials. They are close in form to the findings for Fe and Mg, with the differences that iron in horizons E and F is associated with sulfides (oxidizable fraction) and magnesium in the lower parts of the section is associated with oxides (~10%). A significant drop in Eh and the transition of iron from Fe(III) to Fe(II), which contributes to its mobility, with the simultaneous reduction of sulfur lead to the phenomenon of sulfides. Surprisingly, sulfur from horizon B (134–145) to G (178–198 cm) prevails in the sulfide-bound form. Previously, in [13] (p. 3), it was claimed that “no sulfur has been detected in the Ubinskoye bog peat till a depth of 187 cm; below this depth, S increases from 0.88 to 3.65% in peat and decreases to 1.0–1.4% in the organic-mineral and mineral layers”.
The distribution across fractions of heavy metals of Cu, Zn, Cd and Pb is also different. A similar behavior is characteristic of the first two belonging to the oxidizable fraction of sulfides and the residual one, soluble only in the mixture of strong acids (HF-HNO3−HClO4-HCl). Figure 5 shows that their maximum concentrations are in equilibrium with sulfides. At the same time, cadmium, which belongs to the zinc group, is associated with iron hydroxides, and lead is in both the residual and hydroxide-related fractions. The share of sulfides is also present in the special horizon E. In the interval of 166–172 cm, a peak in the content of Zn (more than 0.2%) was found, associated with anthropogenic emissions typical of most peat bogs in the territory.
Calcium and sulfur (in the lower horizons) are present in the water-soluble fraction, which probably indicates the formation of gypsum or anhydrite. Calcium is also present in the water-soluble and exchangeable fractions. A small part of Cd and Ca is associated with carbonates starting from the 178 cm interval, which confirms the presence of calcium carbonate in it.
Almost all analyzed elements are characterized by their presence in the organic fraction associated with the humic acids in the range from 172 to 275 cm, that is, where inclusions of poorly decomposed wood lignin rich in humic acids appear.
High levels of barium were found in the deposits, which are characteristic of the sub-terrestrial horizons of the forest-steppe zone of the research area. Deposits with high primary productivity are often enriched with barium-bearing solid phases. It is believed that the relationship between productivity and the amount of barium in the sediment is established by the formation of separate barite particles (BaSO4) in the water column associated with the decay of organic matter. Barium appears from a depth of 172 cm in the recoverable and residual fractions.
3.2.2. Computer Modeling of Element Redistribution in Water–Peat–Organic Matter System
Calculations of metal form distribution under various environmental factors involved determining the organic and inorganic forms of metals in water and understanding the role of organic acids in metal binding. The distribution and positioning of metals in complexes were predominantly influenced by the geochemical parameters of the environment such as the pH and salinity of water. The stability and accumulation series varied based on specific pH-Eh, TOC and Me/TOC ratios. The presence of organic acids, especially dissociating fulvic acids, played a significant role in the acidification of bog water.
An assessment of the distribution of metal forms under the influence of various environmental factors, taking into account high-molecular-weight organic acids, is presented in Table 6 and Table 7. We calculated the organic and inorganic forms of metals in water and determined the role of organic acids in the binding of metals. Obviously, the amount of metals bound into complexes and their certain positions in the series are determined not so much by the content of OM and the properties of the metals themselves but by the main geochemical parameters of the environment, namely the pH and salinity of water, and therefore, the series of stability and accumulation can only be conditional (for certain pH-Eh, TOC and Me/TOC ratios).

Table 6.
Equilibrium calculations for the Ubinskoye peat bog in the upper part of the section, indicating the formation of minerals (mol per 1 kg H2O) and the element species, mol/L.

Table 7.
Equilibrium calculations for the Ubinskoye peat bog in the lower part of the section, indicating the formation of minerals (mol per 1 kg H2O) and the element species, mol/L.
The data on the chemical composition of water make it possible to calculate equilibria in the presence of parameters measured in the field, such as T-Eh-pH-electrical conductivity (mSm/cm). Deeper in the section, the solutions gradually become neutral (3.4 < pH < 7.2); however, these values correspond to a sharp change in oxidizing conditions up to −379 mV (pH 7.1). The main cation of water is calcium, and the anion is sulfate. In bog water, up to a horizon of 18 cm, chloride anions are present in comparable amounts. The effect of the predominance of cations over anions was noted, which in bog water is often associated with the presence of organic acids, for example, dissociating fulvic acids:
>COOH = >COO− + H+
A consequence of reaction (3) is also the acidification of bog water to pH 3.4. According to chemical analyses, in bog water, the sum of the main cations (Ca + Mg + Na) is 15 ppm, and that of anions (Cl + SO4) is 6.45 ppm. Table 6 and Table 7 show the minerals that can precipitate from the sampled bog waters (close equilibrium) and the forms of occurrence of elements in solution. Those samples are shown by the depth of the core, which marks one or another significant change. All possible complexes were taken into account in the calculations, but only their significant concentrations are shown. Initially, the pH values were only slightly corrected in the course of model calculations and were close to the measured ones. Starting from a depth of 160 cm, where Eh is sharply negative and the solutions are rich in cations, the calculated pH is one unit higher than the measured values.
Down to a depth of 145 cm, where the values of the redox potential are weakly positive (the point of absolute neutrality of water at 25 °C—Eh 0.4 V, pH 7), the formation of a suspension of quartz > kaolinite > barite is observed. Sulfur is present in the form of sulfate. At first glance, it is surprising that Fe(III) hydroxides are not formed, but at such low, albeit positive, Eh, iron is present in solution in the form of Fe++ and FeSO40. The most interesting fact is the possibility of the formation of fulvo complexes both with the main ions of the solution (Ca, Mg) and with heavy metals (Cu, Zn, Fe, Al). Let us note that for copper and zinc, high-molecular-weight organic complexes can constitute the main form of occurrence.
At a depth of more than 145 cm, the pore water becomes reducing. Calculations show that at Eh < −135 V, various sulfides of a number of elements can be present in suspended matter and sediments—these are FeS2, ZnS, Cu2S, MoS2 and CoS2, as well as apatite, chlorite and barite. We checked the calculation of the saturation of the solutions in relation to gypsum; the SI (Saturation Index) values turned out to be about −2, demonstrating a significant undersaturation, but water is close to equilibrium with aluminum hydroxides, such as AlOOH (SI −0.66). Negative Eh affects the change in the forms of sulfur; such an environment is favorable for sulfate reductants, and H2S and HS− will be stable in solution.
The lower part of the section is dominated by an environment favorable for the accumulation of sulfides (barite is present among sulfates). As mentioned above, the calculated pH shifts towards more alkaline conditions. This contributes to the potential formation of carbonates—calcite and dolomite. In one case, even strontianite and rhodochrosite appear. This is the case of the most reducing solutions (Eh −0.38 V), when in the course of diagenesis, the supersaturation of water occurs with the release of gases such as methane and hydrogen sulfide.
Only at the very bottom of the section should we expect a return to the rocks of the sedimentary cover; here again, the redox potential increases, and ordinary clay minerals and goethite or other oxide iron hydroxides are stable. Pore waters are minimally undersaturated with respect to gypsum and celestite SrSO4; although quartz does not appear in the last two associations, the solutions are in equilibrium with it (SI −0.05).
A stable sulfide/sulfate equilibrium is observed in the solution with a predominant sulfate network. This issue was previously discussed in detail in [62]. Cationic species of metals prevail, as indicated by the low ionic strength of the solutions. The concentrations of zinc and iron are not indicated in the table, since in solutions, they are below significant limits during modeling due to their complete precipitation in pyrite and sphalerite. Copper is capable of forming pre-free stable hydrosulfide complexes, Cu(HS)2−, migrating with solutions.
4. Conclusions
In conclusion, the comprehensive analysis of bog water and peat chemistry provides valuable insights into the dynamic processes occurring within peat ecosystems. This study reveals a profound diagenetic transformation of peat, as evidenced by the variation in total dissolved solids (TDS) and the shifting relationship between major cations and anions in water. The concentration changes in ions such as HCO3−, SO42− and NO3− along the peat section signify the intricate dynamics of nutrient cycling during the mineralization of organic matter. The distribution of trace elements, particularly Al, Fe, Sr, Ba, Zn and As, highlights potential anthropogenic influences.
The study of the chemical composition of peat and underlying sediments in the Ubinskoye bog made it possible to identify characteristic behavioral features for distinct elemental groupings, such as lithophilic and siderophilic metals, chalcophilic elements and mixed valence elements.
This study highlights the influence of alkaline–acid and redox conditions on bog geochemistry, with the upper peat intervals exhibiting oxidative conditions and serving as a geochemical barrier.
Additionally, this research explores metal form distributions under various environmental conditions, particularly focusing on the role of organic acids in metal binding and the impact of geochemical parameters like pH and water salinity on metal complex formation.
In summary, this study enhances our understanding of the complex interactions in bog ecosystems, emphasizing the impact of both natural processes and human activities. The findings contribute to broader discussions on environmental management, water quality assessment and the delicate balance within peatland ecosystems.
Author Contributions
Conceptualization, O.S.; methodology, O.S., O.G., A.Y. and A.B.; validation, O.S., M.K. and D.M.; formal analysis, M.K., D.M. and A.B.; investigation, O.S., O.G. and A.Y.; writing—original draft preparation, O.S., A.Y. and O.G.; visualization, O.S. and A.Y.; supervision, O.S.; funding acquisition, O.S. All authors have read and agreed to the published version of the manuscript.
Funding
The fieldwork was funded by the academic leadership program of the University of Tyumen (strategic academic leadership program “Priority-2030”) and the West-Siberian Interregional Science and Education Center’s project No. 89-DON. Experimental work and analysis of peat and water samples were funded by the state assignment of IGM SB RAS (No. 122041400237-8). The physical–chemical modeling was performed within the state assignment in science for scientific projects carried out by teams of youth laboratories of educational institutions of higher education subordinated to the Ministry of Education and Science of Russia under the project “Development of a system for monitoring, assessing and predicting the complex state of the components of the ‘water-rock-gas-organic matter’ system during the exploitation of hydrocarbon fields” (FEWN-2023-0011, 2023–2024).
Data Availability Statement
The data presented in this article are not readily available because the data are part of an ongoing study. Requests to access the data should be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Veretennikova, E.E.; Kuryina, I.V.; Dyukarev, E.A.; Golovatskaya, E.A.; Smirnov, S.V. Geochemical Features of Peat Deposits at Oligotrophic Bogs in the Southern Taiga Subzone of West Siberia. Geochem. Int. 2021, 59, 618–631. [Google Scholar] [CrossRef]
- Kirpotin, S.; Berezin, A.; Bazanov, V.; Polishchuk, Y.; Vorobiov, S.; Mironycheva-Tokoreva, N.; Kosykh, N.; Volkova, I.; Dupre, B.; Pokrovsky, O.; et al. Western Siberia wetlands as indicator and regulator of climate change on the global scale. Int. J. Environ. Stud. 2009, 66, 409–421. [Google Scholar] [CrossRef]
- Groisman, P.Y.A.; Blyakharchuk, T.A.; Chernokulsky, A.V. Climate changes in Siberia. In Regional Environmental Changes in Siberia and Their Global Consequences; Groisman, P.Y.A., Gutman, G., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 57–109. [Google Scholar]
- Terentieva, I.E.; Glagolev, M.V.; Lapshina, E.D.; Sabrekov, A.F.; Maksyutov, S. Mapping of West Siberian taiga wetland complexes using Landsat imagery: Implications for methane emissions. Biogeosciences 2016, 13, 4615–4626. [Google Scholar] [CrossRef]
- Kharanzhevskaya, Y.A.; Voistinova, E.S.; Sinyutkina, A.A. Spatial and temporal variations in mire surface water chemistry as a function of geology, atmospheric circulation and zonal features in the South-Eastern part of Western Siberia. Sci. Total Environ. 2020, 733, 139343. [Google Scholar] [CrossRef] [PubMed]
- Bogush, A.A.; Leonova, G.A.; Krivonogov, S.K.; Bychinsky, V.A.; Bobrov, V.A.; Maltsev, A.E.; Tikhova, V.D.; Miroshnichenko, L.V.; Kondratyeva, L.M.; Kuzmina, A.E. Biogeochemistry and element speciation in sapropel from freshwater Lake Dukhovoe (East Baikal region, Russia). Appl. Geochem. 2022, 143, 105384. [Google Scholar] [CrossRef]
- Bogush, A.A.; Bobrov, V.A.; Klimin, M.A.; Bychinskii, V.A.; Leonova, G.A.; Krivonogov, S.K.; Kondrat’eva, L.M.; Preis, Y.I. Sedimentation and Accumulation of Elements in the Vydrino Peat Bog (southern Baikal region). Geol. Geoph. 2019, 60, 194–208. [Google Scholar] [CrossRef]
- Shotyk, W.; Noernberg, T. Sampling, handling, and preparation of peat cores from bogs: Review of recent progress and perspectives for trace element research. Can. J. Soil Sci. 2020, 100, 363–380. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Borrego, T.G.; Gallego, J.L.R.; Sánchez-Palencia, Y.; Urbanczyk, J.; Torres, T.; Domingo, L.; Estébanez, B. Biomarkers and inorganic proxies in the paleoenvironmental reconstruction of mires: The importance of landscape in Las Conchas (Asturias, Northern Spain). Org. Geochem. 2016, 95, 41–54. [Google Scholar] [CrossRef]
- Souch, C.J.; Filippelli, G.M.; Dollar, N.; Perkins, S.; Mastalerz, M. Accumulation rates of airborne heavy metals in wetlands. Phys. Geogr. 2002, 23, 21–43. [Google Scholar] [CrossRef]
- Leonova, G.A.; Melgunov, M.S.; Mezina, K.A.; Preis, Y.I.; Maltsev, A.E.; Shavekin, A.S.; Rubanov, M.V. Natural and manmade (137Cs) radioisotopes in Holocene sequence of the Sherstobitovsky raised bog in the Barabinsk forest-steppe (West Siberia). Appl. Geochem. 2022, 140, 105258. [Google Scholar] [CrossRef]
- Rudmin, M.; Ruban, A.; Savichev, O.; Mazurov, A.; Dauletova, A.; Savinova, O. Authigenic and detrital minerals in peat environ-ment of Vasyugan swamp, Western Siberia. Minerals 2018, 8, 500. [Google Scholar] [CrossRef]
- Leonova, G.A.; Maltsev, A.E.; Preis, Y.I.; Miroshnichenko, L.V. Biogeochemistry of Holocene peatlands in the Baraba forest-steppe (Southern West Siberia). Appl. Geochem. 2021, 124, 104811. [Google Scholar] [CrossRef]
- Savichev, O.; Soldatova, E.; Rudmin, M.; Mazurov, A. Geochemical barriers in oligotrophic peat bog (Western Siberia). Appl. Geochem. 2020, 113, 104519. [Google Scholar] [CrossRef]
- Naymushina, O.; Shvartsev, S.; Serebrennikova, O.; Ses, K.; Matveenko, I. Spatial distribution of chemical and organic compounds in the water of oligotrophic peatland of Tomsk region (Western Siberia). Procedia Chem. 2014, 10, 541–546. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Dupre, B.; Schott, J. Fe–Al-organic colloids control of trace elements in peat soil solutions: Results of ultrafiltration and dialysis. Aquat. Geochem. 2005, 11, 241–278. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.G.; Krickov, I.V.; Shirokova, L.S.; Kulizhskiy, S.P.; Golovatskaya, E.A.; Pokrovsky, O.S. Dissolved organic carbon and major and trace elements in peat porewater of sporadic, discontinuous, and continuous permafrost zones of Western Siberia. Biogeosciences 2017, 14, 3561–3584. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Kuzmina, D.M.; Shirokova, L.S.; Kulizhskiy, S.P.; Golovatskaya, E.A.; Pokrovsky, O.S. Colloidal organic carbon and trace elements in peat porewaters across a permafrost gradient in Western Siberia. Geoderma 2021, 390, 114971. [Google Scholar] [CrossRef]
- Naymushina, O.; Gaskova, O. Adsorption of Cu(II) from aqueous solution using raw peat: Preliminary results. E3S Web Conf. 2019, 98, 06010. [Google Scholar] [CrossRef]
- Boguslavsky, A.E.; Gaskova, O.L.; Naymushina, O.S.; Popova, N.M.; Safonov, A.V. Environmental monitoring of low-level radioactive waste disposal in electrochemical plant facilities in Zelenogorsk, Russia. Appl. Geochem. 2020, 119, 104598. [Google Scholar] [CrossRef]
- Shvartseva, O.; Skripkina, T.; Gaskova, O.; Podgorbunskikh, E. Modification of natural peat for removal of copper ions from aqueous solutions. Water 2022, 14, 2114. [Google Scholar] [CrossRef]
- Stepanova, V.A.; Pokrovsky, O.S.; Viers, J.; Mironycheva-Tokareva, N.P.; Kosykh, N.P.; Vishnyakova, E.K. Elemental composition of peat profiles in Western Siberia: Effect of the micro-landscape, latitude position and permafrost coverage. Appl. Geochem. 2014, 53, 53–70. [Google Scholar] [CrossRef]
- Efremova, T.T.; Efremov, S.P.; Kutsenogiy, K.P.; Onuchin, A.A.; Peresedov, V.F. Biogeochemistry of Fe, Mn, Cr, Ni, Co, Ti, V, Mo, Ta, W, U in lowland peat between the Ob and Tom rivers. Soil Sci. 2003, 5, 557–567. (In Russian) [Google Scholar]
- Arkhipov, V.S.; Bernatonis, V.K. Distribution of calcium and iron in the vertical profile of peat deposits in the taiga zone of Western Siberia. News Tomsk Polytech. Univ. 2013, 323, 173–178. (In Russian) [Google Scholar]
- Bobrov, V.A.; Preis, Y.I.; Budashkina, V.V. Estimation of mineral matter fluxes from the atmosphere based on the microelement composition of the peat deposit of the Bakchar-1 raised bog (southern taiga of Western Siberia). Probl. Biogeochem. Geochem. Ecol. 2013, 22, 20–29. (In Russian) [Google Scholar]
- Savichev, O.G.; Nalivaiko, N.G.; Rudmin, M.A.; Mazurov, A.K. Microbiological conditions of chemical elements distribution on peat deposit depth in ecosystems of the Vasyugan Swamp East part (Western Siberia). Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2019, 330, 184–194. [Google Scholar] [CrossRef]
- Savichev, O.G.; Rudmin, M.A.; Mazurov, A.K.; Nalivaiko, N.G.; Sergienko, V.I.; Semiletov, I.P. Mineralogical and geochemical features of a peat deposit in the eutrophic Obskoye fen under anthropogenic load (Western Siberia). Doklady Earth Sci. 2020, 492, 320–322. [Google Scholar] [CrossRef]
- Savichev, O.G.; Mazurov, A.K.; Semiletov, I.P.; Bazanov, V.A.; Guseva, N.V.; Khvashchevskaya, A.A.; Nalivayko, N.G. Hydrochemical conditions for the formation of oligotrophic swamp ecosystems. Izvestiya RAS 2016, 5, 60–69. (In Russian) [Google Scholar]
- Bobrov, V.A.; Budashkina, V.V.; Melgunov, M.S.; Leonova, G.A.; Maltsev, A.E. Natural and technogenic radioisotopes and microelements in the section of peat accumulation of the Dulikha peat bog (Cisbaikalia): Retrodictions of atmospheric inputs of mineral matter. Geol. Miner. Resour. Sib. 2019, 2, 93–102. [Google Scholar] [CrossRef]
- Berezin, A.E.; Bazanov, V.A.; Skugarev, A.A.; Rybina, T.A.; Parshina, N.V. Great Vasyugan Mire: Landscape structure and peat deposit structure features. Int. J. Environ. Stud. 2014, 71, 618–623. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Dinu, M.I.; Gashkina, N.A.; Jones, V.; Khoroshavin, V.Y.; Kremleva, T.A. Present status of water chemistry and acidification under nonpoint sources of pollution across European Russia and West Siberia. Environ. Res. Lett. 2018, 13, 105007. [Google Scholar] [CrossRef]
- Savichev, O.G.; Mazurov, A.K.; Pipko, I.I.; Sergienko, V.I.; Semiletova, I.P. Spatial patterns of the evolution of the chemical composition and discharge of river water in the Ob River basin. Dokl. Earth Sci. 2016, 466, 59–63. [Google Scholar] [CrossRef]
- Zyryanova, T.A.; Zinchenko, G.S.; Bezuglova, N.N. Assessing water balance components of the Great Vasyugan Mire. Geogr. Nat. Resour. 2007, 4, 68–74. (In Russian) [Google Scholar]
- Shvartsev, S.L.; Serebrennikova, O.V.; Zdvizhkov, M.A.; Savichev, O.G.; Naimushina, O.S. Geochemistry of wetland waters from the lower Tom Basin, southern Tomsk oblast. Geochem. Int. 2012, 50, 367–380. [Google Scholar] [CrossRef]
- Matveenko, I.A.; Savichev, O.G.; Bazanov, V.A.; Ivanova, Y.V. Spatial-temporal regularities in changing chemical composition of bog waters in taiga zone of Western Siberia. Procedia Chem. 2015, 15, 206–212. [Google Scholar] [CrossRef]
- Savichev, O.G. Geochemical parameters of bog waters in the taiga zone of the Western Siberia. Izv. RAS Seriya Geogr. 2015, 4, 47–57. [Google Scholar] [CrossRef][Green Version]
- Savichev, O.G.; Shmakov, A.V. Vertical zonation and intra-annual changes in the chemical composition of the waters of the Timiryazevsky swamp (Tomsk, Western Siberia). Bull. Tomsk Polytech. Univ. 2012, 320, 156–172. (In Russian) [Google Scholar]
- Ivanova, E.S.; Kharanzhevskaya, Y.A.; Mironov, A.A. Lateral distribution and migration of chemical elements in the waters of swamps of the Bakchar and Iksa River basins (Western Siberia). Bull. Moscow State Univ. 2017, 4, 55–64. (In Russian) [Google Scholar]
- Kharanzhevskaya, Y.A.; Sinyutkina, A.A. Investigating the role of bogs in the streamflow formation within the Middle Ob basin. Geogr. Nat. Resour. 2017, 38, 256–266. [Google Scholar] [CrossRef]
- Shvartsev, S.L. Geochemistry of natural waters in the area of the Great Vasyugan Swamp. In Integrated monitoring of the Great Vasyugan Swamp; TPU: Tomsk, Russia, 2002; pp. 139–149. (In Russian) [Google Scholar]
- Wertebach, T.-M.; Knorr, K.-H.; Lordieck, M.; Tretiakov, N.; Blodau, C.; Hölzel, N.; Kleinebecker, T. Relationships between vegetation succession, pore water chemistry and CH4 and CO2 production in a transitional mire of Western Siberia (Tyumen Oblast). Wetlands 2016, 36, 863–874. [Google Scholar] [CrossRef]
- Gorham, E.; Janssens, J.A. The distribution and accumulation of chemical elements in five peat cores from the mid-continent to the eastern coast of North America. Wetlands 2005, 25, 259–278. [Google Scholar] [CrossRef]
- Steinmann, P.; Shotyk, W. Geochemistry, mineralogy, and geochemical mass balance on major elements in two peat bog profiles (Jura Mountains, Switzerland). Chem. Geol. 1997, 138, 25–53. [Google Scholar] [CrossRef]
- Kolpakova, M.; Gaskova, O.; Borzenko, S.; Krivonogov, S.; Naymushina, O.; Rudaya, N. Distribution profile of chemical elements during the last 13 thousand years from the sediments of Maloye Yarovoe Lake (Western Siberia, Russia). Water 2020, 12, 3001. [Google Scholar] [CrossRef]
- Maltsev, A.E.; Leonova, G.A.; Miroshnichenko, L.V.; Kondratieva, L.M.; Bobrov, V.A.; Vosel, I.S.; Krivonogov, S.K. Geochemistry of porous water of organic-mineral deposits of Lake Kotokel (Eastern Pribaikalye) and their transformation in early diagenesis. Geol. Miner. Resour. Sib. 2019, 1, 71–85. [Google Scholar]
- Shevchenko, V.P.; Pokrovsky, O.S.; Vorobyev, S.N.; Krickov, I.V.; Manasypov, R.M.; Politova, N.V.; Kopysov, S.G.; Dara, O.M.; Auda, Y.; Shirokova, L.S.; et al. Impact of snow deposition on major and trace element concentrations and elementary fluxes in surface waters of the Western Siberian lowland across a 1700 km latitudinal gradient. Hydrol. Earth Syst. Sci. 2017, 21, 5725–5746. [Google Scholar] [CrossRef]
- Kalyuzhny, I.L. General features of the formation of the hydrochemical regime and the chemical composition of the waters of eutrophic wetland massifs. Water Manag. Russia 2016, 3, 30–46. (In Russian) [Google Scholar] [CrossRef]
- Gerdol, R.; Pontin, A.; Tomaselli, M.; Bombonato, L.; Brancaleoni, L.; Gualmini, M.; Petraglia, A.; Siffi, C.; Gargini, A. Hydrologic controls on water chemistry, vegetation and ecological patterns in two mires in the south-eastern Alps (Italy). Catena 2011, 86, 86–97. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Bu, Z.; Zeng, L. Spatial variations in the surface water chemistry of subtropical Peatlands (Central China) linked to anthropogenic pressures. Water 2017, 9, 505. [Google Scholar] [CrossRef]
- Avagyan, A.; Runkle, B.R.; Hartmann, J.; Kutzbach, L. Spatial variations in pore-water biogeochemistry greatly exceed temporal changes during Baseflow conditions in a Boreal River valley mire complex, Northwest Russia. Wetlands 2014, 34, 1171–1182. [Google Scholar] [CrossRef]
- Bourbonniere, R.A. Review of water chemistry research in natural and disturbed peatlands. Can. Water Resour. J. 2009, 34, 393–414. [Google Scholar] [CrossRef]
- Cowley, K.L.; Fryirs, K.A.; Hose, G.C. Identifying key sedimentary indicators of geomorphic structure and function of upland swamps in the Blue Mountains for use in condition assessment and monitoring. Catena 2016, 147, 564–577. [Google Scholar] [CrossRef]
- Helmer, E.H.; Urban, N.R.; Eisenreich, S.J. Aluminum geochemistry in peatland waters. Biogeochemistry 1990, 9, 247–276. [Google Scholar] [CrossRef]
- Olid, C.; Bindler, R.; Nilsson, M.B.; Eriksson, T.; Klaminder, J. Effects of warming and increased nitrogen and sulfur deposition on boreal mire geochemistry. Appl. Geochem. 2017, 78, 149–157. [Google Scholar] [CrossRef]
- Stanton, M.R.; Yager, D.B.; Fey, D.L.; Wright, W.G. Formation and geochemical significance of iron bog deposits. In Formation and Geochemical Significance of Iron Bog Deposits; Church, S.E., Guerard, P., Finger, S.E., Eds.; Geological Survey Professional Paper; Geological Survey: San Juan County, WA, USA; Golden, CO, USA, 2007; Volume 1651, pp. 689–720. [Google Scholar]
- Kempter, H.; Krachlera, M.; Shotyk, W.; Zaccone, C. Major and trace elements in Sphagnum moss from four southern German bogs, and comparison with available moss monitoring data. Ecol. Indic. 2017, 78, 19–25. [Google Scholar] [CrossRef]
- Eckstein, Y.; Savichev, O.G.; Pasechnik, E.Y. Two decades of trends in groundwater chemical composition in the Great Vasyugan mire, Western Siberia, Russia. Environ. Earth Sci. 2015, 73, 7329–7341. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- Shvarov, Y.V. HCh: New potentialities for the thermodynamic simulation of geochemical systems offered by windows. Geochem. Int. 2008, 46, 834–839. [Google Scholar] [CrossRef]
- Shvarov, Y.V. Algorithmization of the numeric equilibrium modeling of dynamic geochemical processes. Geochem. Int. 1999, 37, 571–576. [Google Scholar]
- Zhong, R.; Li, Y.; Etschmann, B.; Brugger, J.; Yu, C.; Cui, H. HighPGibbs, a practical tool for fluid-rock thermodynamic simulation in deep Earth and its application on calculating nitrogen speciation in subduction zone fluids. Geochem. Geophysi. Geosyst. 2020, 20, e2020GC008973. [Google Scholar] [CrossRef]
- Gaskova, O.L.; Strakhovenko, V.D.; Ermolaeva, N.I.; Zarubina, E.Y.; Ovdina, E.A. A simple method to model the reduced environment of lake bottom sapropel formation. Chin. J. Oceanol. Limnol. 2017, 35, 956–966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).