Abstract
To solve the problems of deep nitrogen removal in wastewater treatment plants and the high value utilization of steel slag in the metallurgical industry, this work aims to prepare a sulfur/steel slag-based filter using the melting method. The melt granulation method and the utilization of metallurgical waste were the main innovations of this work. On this basis, the nitrogen removal performance of the filter media in simulated wastewater and actual wastewater were systematically investigated. Furthermore, the factors affecting the nitrogen removal performance of the filter media were studied, and pilot experiments were carried out. The microbial community in the reactor was also analyzed. The results showed that when the mass ratio of sulfur and steel slag was 9:1, the filter media could remove up to 90% of TN in simulated wastewater at room temperature, with a hydraulic retention time (HRT) of 5–20 h and an influent TN of 21 mg/L. In the simulated wastewater, the effluent NO3−-N was less than 2 mg/L, the SO42− was less than 200 mg/L, and the pH was between 6 and 8. The removal of TN from actual wastewater was also greater than 90% at room temperature under a hydraulic retention time (HRT) of 8–20 h and an influent TN of 8 mg/L. Influence factor experiments were conducted at room temperature, with a C/N of 2:1, a DO of 0.9–1 mg/L, and an HRT of 4 h. The results of the pilot experiment confirmed that the effluent TN was stable below 10 mg/L. The filter media was compounded for practical engineering applications. Microbial community analysis showed that the sulfur autotrophic denitrifying bacterial species Thiobacillus accounted for 3.69% and 5.55% of the simulated and actual wastewater systems, respectively. This work provides a novel strategy for the application of solid metallurgical waste in the field of nitrate-containing wastewater treatment.
1. Introduction
Nitrogen is essential for the growth of all living organisms, but in excess, it will exert many adverse effects. Improper treatment of industrial wastewater, excessive use of nitrogen fertilizers, and substandard treatment of nitrogen oxides will lead to serious nitrogen pollution problems. Excessive levels of nitrogen in natural water bodies could cause the eutrophication and acidification of water bodies [1]. Currently, Kong et al. offer specific data related to nitrogen water pollution. Excess nitrates in drinking water adversely affect human health and increase the risk of serious diseases, such as infantile methemoglobinemia, non-Hodgkin’s lymphoma, and heart disease [2]. Nitrate is also a key component of the Nitrogen Pollution Program (NPD). Therefore, the efficient disposal of nitrogen-polluted water has become the focus of attention in the industry.
There are many traditional physical and chemical methods for nitrogen removal, but these methods possess the disadvantages of high operational costs and the production of secondary pollution. Although the heterotrophic denitrification technology used in the microbial method shows a better nitrogen removal effect, most of the tailwater exhibits the characteristic of low C/N, requiring an additional carbon source, which will significantly increase sludge production, thus increasing the operation costs [3].
In recent years, sulfur-based autotrophic denitrification technology has gained wide attention in the field of biological nitrogen removal. This technology offers the advantages of not requiring an additional carbon source, low mud production, and high economic benefits [4,5]. Yang et al. used sulfur-based autotrophic denitrification to degrade wastewater with an influent NO3−-N concentration of 90 mg/L, and the removal rate of NO3−-N was 40–60% after 15 d of operation [6]. Jian et al. used a novel lime sulfur–butanediol system to treat low C/N wastewater with an NO3−-N concentration of 30 mg/L, which exhibited the NO3−-N removal rate of 96.3% under a C/N value of 1 and a hydraulic retention time (HRT) of 3 h [7]. Meng et al. used sulfur-tourmaline-AD (STAD) as an innovative trial nitrate removal technique in wastewater. Complete denitrification of 75 mg/L NO3−-N occurred under a pH of 8.0, an HRT of 15 h, and a temperature of 30 °C [8].
Usually, in the process of sulfur autotrophic denitrification, NO3−-N is reduced to N2 by denitrifying bacteria (including thiobacillus denitrificans) under anoxic or anaerobic conditions, using sulfur as the electron donor [6,9]. The reaction equation is as follows [10]:
1.1S + NO3− + 0.76H2O + 0.40CO2 + 0.08NH4+ → 0.5N2 + 1.1SO42− + 1.28H+ + 0.08C5H7O2N
From Equation (1), it can be seen that sulfur autotrophic denitrification is an acid-producing process, and the reaction generates sulfate. Therefore, it is critical to maintain the pH stability of the reaction system and to control the sulfate content to meet the standard [11,12]. The reaction process can be used as a filter for the reaction system. Currently, limestone is commonly used as a filler to maintain the pH stability of the system [13]. Li et al. investigated the denitrification effect of a bio-filter based on a sulfur/limestone system and found that at a hydraulic retention time of 18 h, the removal rates of TN and NO3−-N at 10 °C were 81.1% and 85.3%, respectively [14]. However, the presence of limestone causes an increase in the hardness of the effluent water, so it is critical to develop novel autotrophic denitrification packings that do not increase the hardness, while also ensuring the proper concentration of sulfate, in the effluent water.
As a large metallurgical nation, China is among the world’s leading countries in the extraction and utilization of metallurgical minerals. Steel slag, which is the slag discharged from the steel making process, is a type of solid industrial waste. Steel slag consists mainly of calcium, iron, silicon, magnesium, and small amounts of aluminum, manganese, and phosphorus oxides [15]. According to the National Bureau of Statistics of China, in 2021, China produced more than 100 million tons of steel slag. Compared with other industrially developed countries, China’s steel slag utilization rate is 29.5%, compared with 87% in Europe, 98.4% in Japan, and 84.4% in the United States [16]. Underutilized steel slag, piled up without timely treatment, will occupy a large amount of arable land resources and result in serious environmental pollution problems [17]. Steel slag contains a large number of trace elements, exerting a positive effect on the growth of microorganisms. Thus, the treatment of steel slag has potential application value in wastewater denitrification field. Furthermore, steel slag exhibits a certain alkalinity, which could maintain pH stability in the filter.
In the denitrification process, NO3−-N must acquire electrons to complete denitrification; sulfur and iron are good electron donors for sulfur autotrophic denitrification and iron autotrophic denitrification, respectively, and the H+ and OH− produced by both can neutralize each other to maintain the pH stability of the system. Silicate and other substances present in the slag also provide nutrients for microbial growth.
The reuse of steel slag to fabricate the filter material can not only achieve the goal of high-value utilization for steel slag, but also reduce the cost of filter material. Up until now, there have been few report regarding the application of steel slag for filter preparation in the wastewater treatment field.
Based on this fact, this work organically combines the development of a sulfur-based autotrophic denitrification biological denitrification filter with the high-value utilization of steel slag, adopting the melt-mixed underwater granulation technology to prepare a steel slag/sulfur-based denitrification filter. Furthermore, the nitrogen removal effect of the steel slag/sulfur-based denitrification filter was investigated using wastewater simulation and actual wastewater. The nitrogen removal process was also investigated in regards to the changes in various effluent indicators and the factors affecting this process. On this basis, the novel denitrification filter was used in a pilot plant to investigate its operation, and the distribution of microorganisms in the system was investigated using high-throughput sequencing technology. It was found that the melt-mixed underwater granulation method was feasible for the preparation of sulfur/steel slag-based filter media. The prepared filter media exhibited excellent performance in nitrogen removal tests, not only in simulated wastewater, but also in actual wastewater, showing strong potential for practical application. The above work provides a new strategy for the development of novel nitrogen removal filter media and the high-value utilization of metallurgical solid waste.
2. Materials and Methods
2.1. Raw Materials for Experiments
Sulfur (600 mesh, purity 99.9%) was purchased from Luchuan Chemical Co., Ltd, Qingdao, China. Steel slag powder (400 mesh) was purchased from Magang (Group) Holding Company Ltd., Ma’anshan, China. The potassium nitrate, ammonium chloride, potassium dihydrogen phosphate, and sodium bicarbonate were all of AR grade, and were purchased from Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China.
2.2. Preparation of Denitrification Filter Media
The nitrogen removal filter media was fabricated using the melt granulation method. A total of 900 g of sulfur powder and 100 g of steel slag powder were mixed homogeneously and added to a beaker at 140 °C in an oil bath, heated to a molten state, and further stirred for 3 min to homogeneity at this temperature. Then, the well-mixed molten mixture was poured into a container with an aperture of 4 mm at the lower end; the mixture then flowed through the aperture into the cooling water to form a granular product. In this process, the temperature of the vessel was maintained at 130 °C to ensure that the mixture in the molten state did not rapidly cool and solidify upon entering the vessel. After cooling into granules, the obtained denitrified material was dried at room temperature until it achieved a constant weight. The granules with diameters of 3–6 mm were sieved for further testing.
2.3. Simulated Wastewater Denitrification Experiment
The simulated wastewater was manually distributed with a TN of 21 mg/L, an NO3−-N of 20 mg/L, and a COD of 18 mg/L, and the pH was maintained at 7.5 to 8.5. The specific composition of the simulated wastewater is shown in Table 1

Table 1.
Components of the simulated effluent.
2.4. Experimental Setup
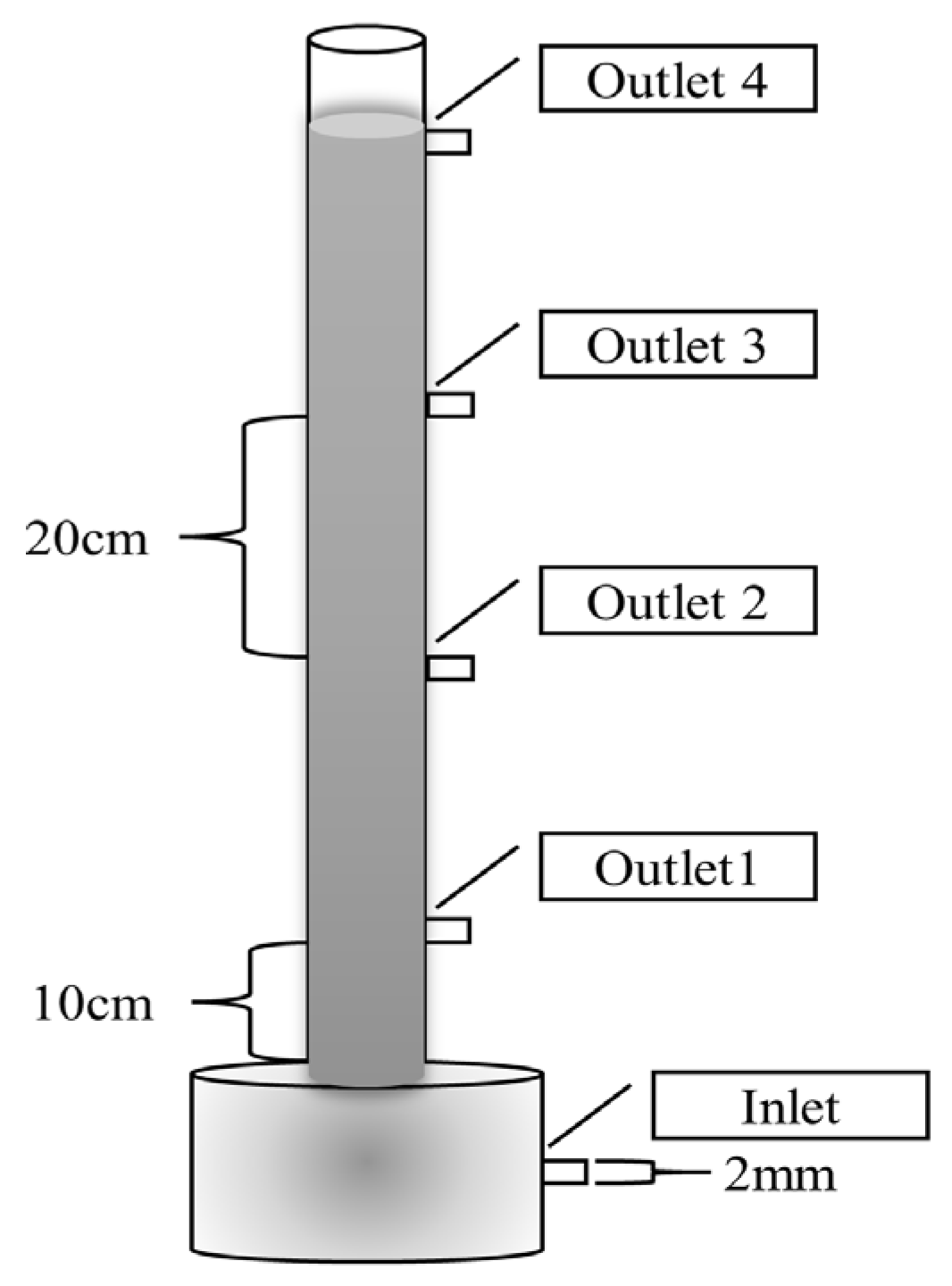
The experiments were conducted using an up-flow column reactor. As shown in Figure 1, the reactor material is plexiglass, with a main body wall thickness of 5 mm, a total height of 1 m, an outer diameter of 70 mm, an inner diameter of 60 mm, and an outlet aperture of 2 mm.
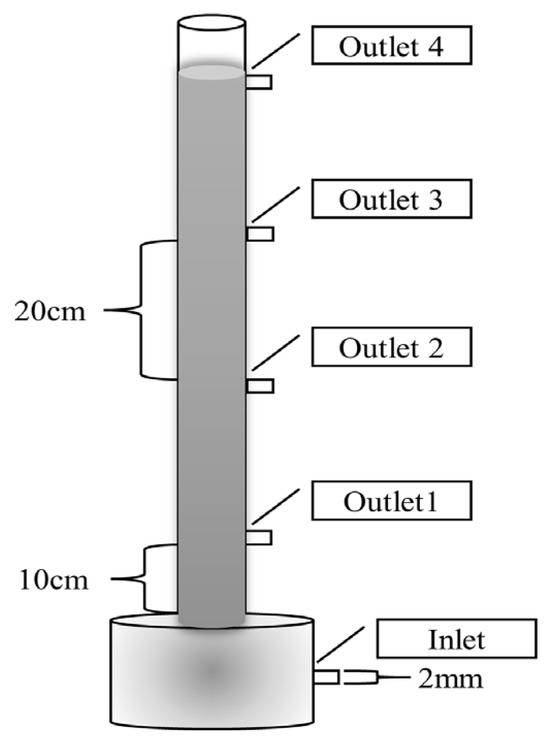
Figure 1.
Diagram of the column reactor.
2.5. Experimental Methodology
A total of 1475.04 g of the denitrification filter media was filled to the height of 60 cm in the reactor. The effective volume of the reactor was calculated to be 1680 mL. The initial retention time was set to be 20 h with the influent flow rate of 1.4 mL/min, and the volumetric loading was set to be 0.024 kg(TN)/(m3·d). Before water intake, 5 g of bacterial strain was added to 500 mL of anaerobic sludge and stirred evenly. Then, 300 mL of sludge was fed from the lower end of the reactor through peristaltic pump, and 200 mL of sludge was added to the upper end of the reactor. During the operation period, the hydraulic retention time (HRT) of the reactor was adjusted by changing the intake flow rate. The whole operation process was divided into five phases, and the specific parameters are shown in Table 2. In order to investigate the change rule regarding the functional material parameters for nitrogen removal throughout the process, four outlets were set up on the reactor from bottom to top, respectively, i.e., outlet 1, outlet 2, outlet 3, and outlet 4, and each outlet was 20 cm apart, of which outlet 1 was 10 cm away from the bottom of the reactor (the outlet is indicated in the reactor design drawing).

Table 2.
Parameter settings regarding hydraulic retention time for different stages or the process.
2.6. Testing and Characterization
TN was tested using alkaline potassium persulfate UV spectrophotometry (UV spectrophotometer model UV-6800A) according to the GB3838-2002 standard [18].
NO3−-N was tested using the spectrophotometric method according to the HJ/T346-2007 standard [19].
NO2−-N was tested using the spectrophotometric method according to the GB7493-87 standard [20].
SO42− was tested using ion chromatography according to the GB/T39305-2020 standard [21].
Fe3+ was tested using the CHFE-160 Rapid Detector (Mingbo Ltd, Qingdao, China).
The pH value of the effluent was measured using the PHSJ-3F type pH meter (Leici Ltd., Shanghai, China).
The microscopic morphology of the filter was obtained by a JSM-6490LV scanning electron microscope (JEOL Ltd., Tokyo, Japan). Before observation, the samples were coated with a conductive layer. The accelerating voltage was 10 kV.
Microbial community analysis was tested using a 16s RNA high-throughput sequencing analysis by Meggie Bio Ltd., Shanghai, China.
3. Results
3.1. Investigation of Nitrogen Removal Performance for Simulated Wastewater
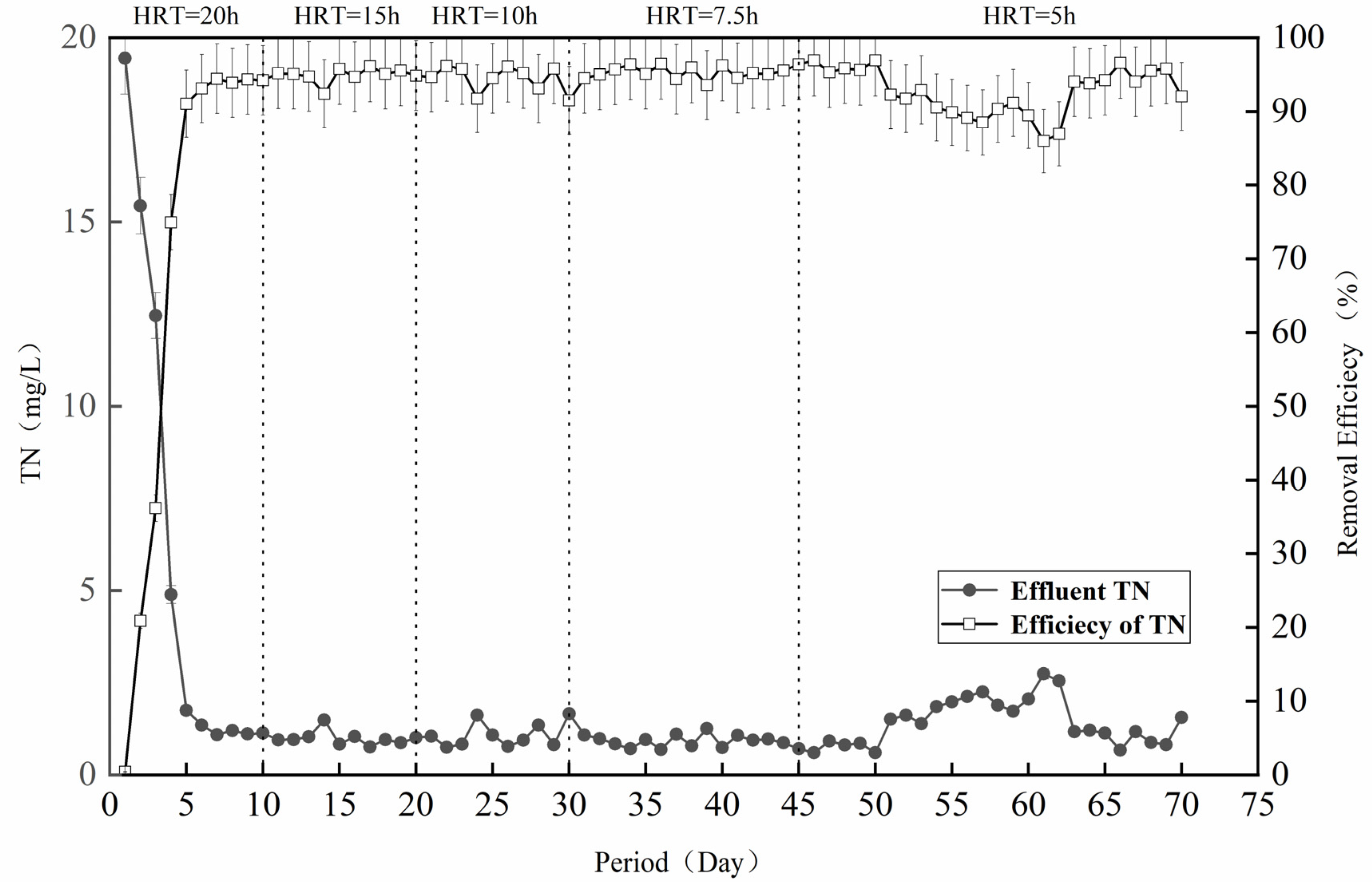
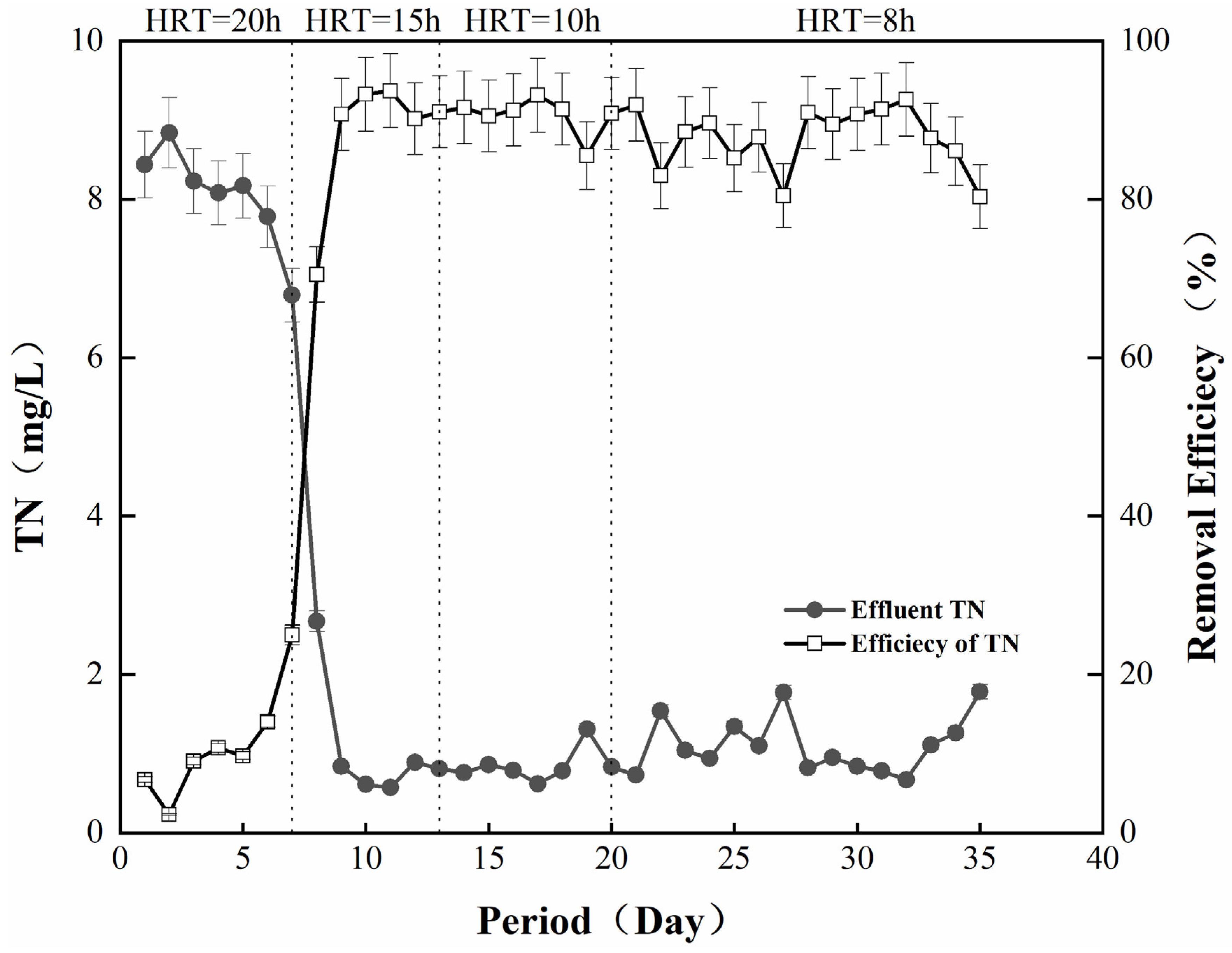
The treatment effect of the denitrification filter media on the simulated wastewater was further analyzed. As shown in Figure 2, the filter media operated well in the simulated wastewater environment, and the TN removal rate was stable at 90% after the filling reached operational stability on the 5th day. In the initial stage of 1–10 days (HRT = 20 h), the microorganisms gradually attached to the surface of the filter media to proliferate and grow, and the TN removal rate was not very high [22]. Accompanied by the continuous progress of the reaction, the TN removal rate also gradually increased and stabilized at about 90%, which was mainly due to good growth of the bacterial flora on the surface of the filter media, carrying out sulfur autotrophic denitrification with the help of the filter media [23]. In the second stage (HRT = 15 h), the TN concentration of the effluent of the denitrification filter media was stabilized at lower than 1 mg/L in the stable operation stage after the successful startup, with few fluctuations between 1 mg/L and 2 mg/L, indicating the excellent denitrification effect of this system. The removal effect of the third stage (HRT = 10 h) and the fourth stage (HRT = 7.5 h) was also relatively stable at 90%. However, in the late fourth stage, with the adjustment of the hydraulic retention time, the removal effect exhibited slight fluctuations. This phenomenon was ascribed to the clogging resulting from microorganisms and sludge, reducing the denitrification capacity of the reactor [24].
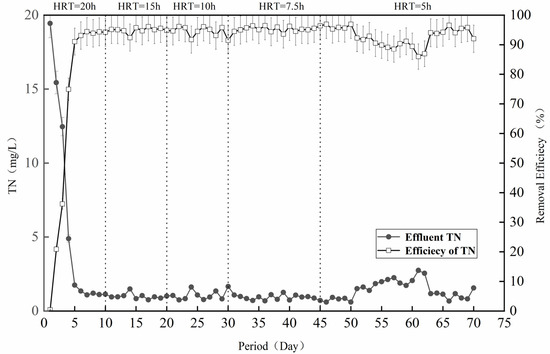
Figure 2.
Wastewater denitrification performance of the filter simulation.
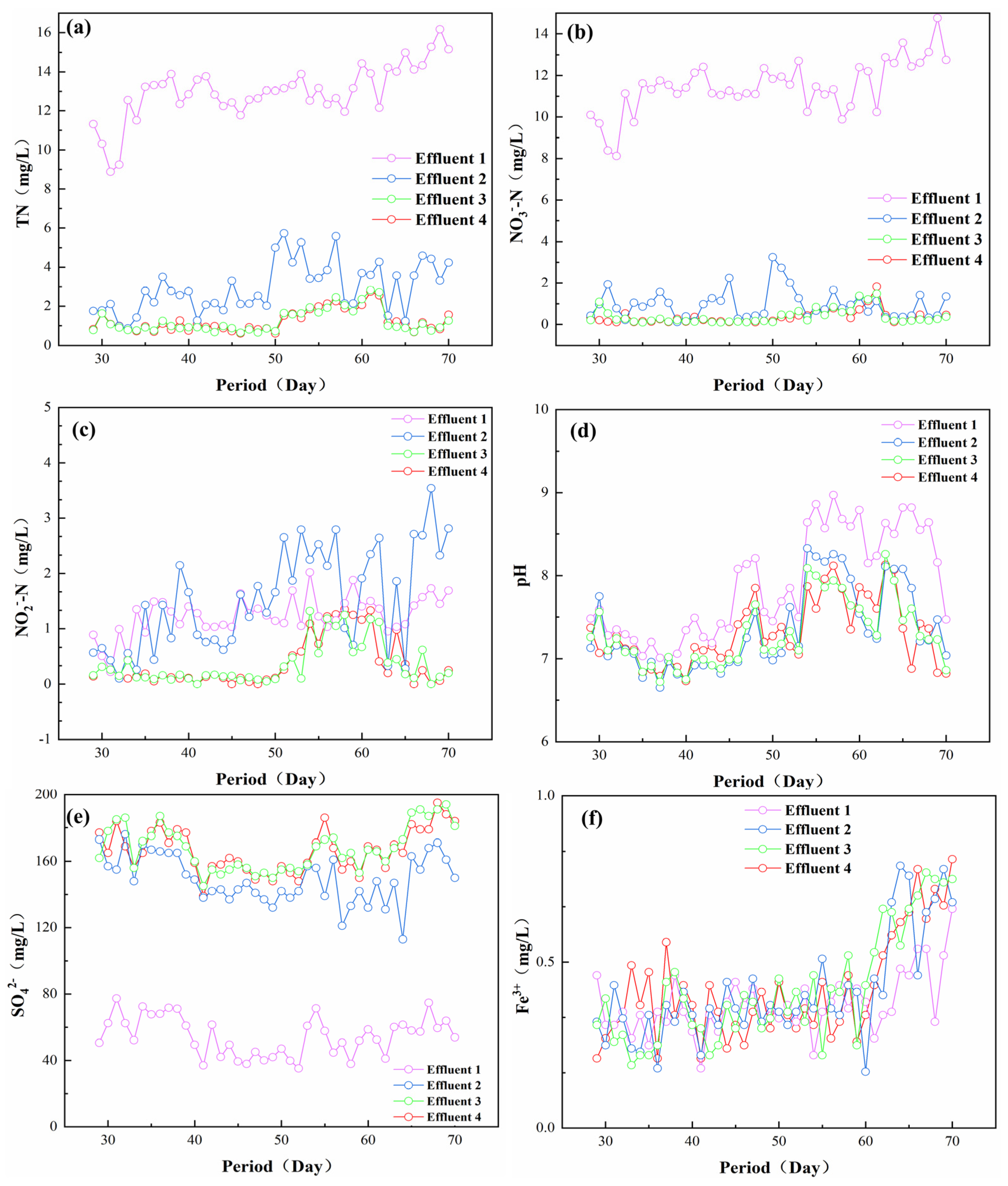
After the system was stably operated for a period of time (27 d), TN, NO3−-N, NO2−-N, pH, SO42−, Fe3+, and other parameters of the outlet at different heights during the denitrification process were detected. The main removal location of TN and NO3−-N and the accumulation location of NO2−-N were also analyzed according to the along-range changes, which provided support for the subsequent enhancement of the nitrogen removal effect and avoided the accumulation of excessive NO2−-N. The experimental results are shown in Figure 3. As shown in Figure 3a, the concentration of TN in the effluent gradually decreased with the height of the outlet, especially at outlet 3, where the difference between the concentration and outlet 4 was very small, indicating that the denitrification process in the reactor was mainly concentrated in the section from outlet 1 to outlet 2, which had the strongest denitrification capacity, removing most of the TN. In the uppermost section from outlet 3 to outlet 4, the TN concentration did not change significantly, indicating that the denitrification environment in this section is more stable. The denitrification reaction of the microorganisms was lower, contributing little to the removal of TN in this section [25]. As shown in Figure 3b, for the NO3−-N concentration along the course of the change, and the change rule of NO3−-N was similar to that of TN. With the increase in height, the NO3−-N concentration gradually decreased from outlet 1 to outlet 4, and most of the NO3−-N reduction reaction occurred at the bottom of the reactor. This was probably because the influent water was fed from the bottom to the top, the concentration of NO3−-N was higher in the middle and lower part of the reactor, and the microorganism concentration was more abundant in this area [26,27].
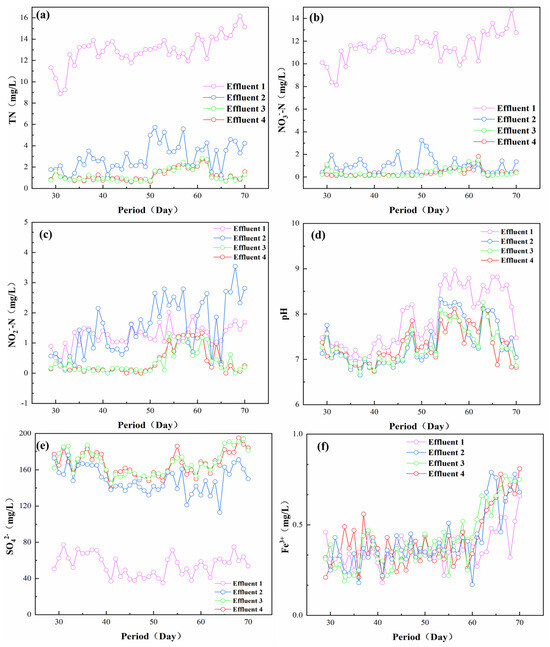
Figure 3.
Variation of different ion concentrations along the course of the process (a) TN; (b) NO3−-N; (c) NO2−-N; (d) pH; (e) SO42−; (f) Fe3+.
Figure 3c showed that before 50 d of operation, there was no NO2−-N accumulation problem in the effluent. After 50 d of operation, the NO2−-N concentration in effluent outlet 3 began to rise. This may result from the fact that with the shortening of the hydraulic retention time, the system’s treatment load increased, and the NO3−-N degradation also increased. Usually, the reaction rate of NO3−-N was faster than that of NO2−-N, which will result in the accumulation of some NO2−-N [28,29]. Figure 3d shows that the pH value of this system was always stabilized at 6.5–9 during the reactor operation, which was in the normal growth range of the microorganisms, indicating that steel slag could maintain the pH stabilization of this system [4,30]. As shown in Figure 3e, SO42−increased greatly between outlet 1 and outlet 3, which confirmed that this stage was the best stage for denitrification, which was also confirmed by the TN and NO3-N test results [31]. Theoretically, under the condition of the presence of a sufficient electron donor and autotrophic denitrifying bacteria, every 1 g of NO3−-N removal will produce 7.5 g of SO42− [32]. It was discovered that the SO42− of the effluent of this system was slightly lower than the theoretical value; this may be due to the fact that the reactor simultaneously carries out the sulfur autotrophic denitrification and also due to the existence of a certain number of iron particles in the steel slag, which will also synchronize with the iron autotrophic denitrification and thus generate Fe3+ [33,34]. Figure 3f shows the change of Fe3+ in the system; the steel slag contained some iron particles, which partly enhance of the reactor’s denitrification efficiency [29,35]. Usually, as the sulfur autotrophic denitrification is an acid-producing process, the pH of the system will gradually decrease as the reaction proceeds, while iron autotrophic denitrification is an alkaline-producing process, which can offset part of the acid produced by sulfur autotrophic denitrification to stabilize the pH of the whole system [36].
3.2. Impact Factor Investigation
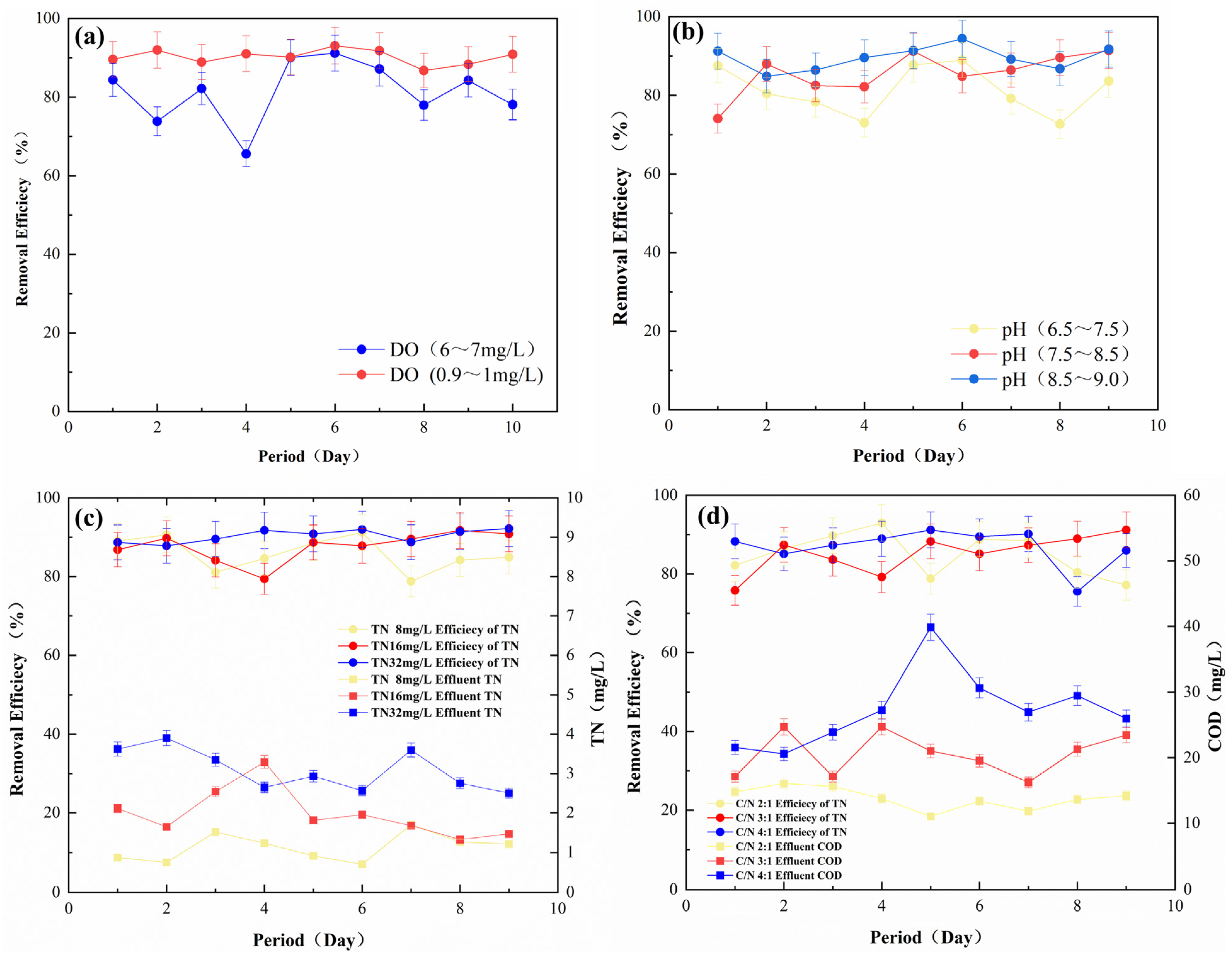
The effects of the dissolved oxygen (DO), pH, TN, and C/N of the influent water on nitrogen removal during reactor operation were further investigated. As shown in Figure 4a, the TN removal effect was better when DO was in the range of 0.9–1 mg/L after nitrogen blowdown of the influent water, indicating that dissolved oxygen had a negative effect on the removal efficiency, which was due to the fact that autotrophic denitrification was usually carried out under anoxic conditions [37]. As shown in Figure 4b, there was a slight fluctuation in the removal effect when the pH of the influent water was between 6.5 and 9, indicating that the effect of pH was small in this range. As shown in Figure 4c, when the influent TN increases to 32 mg/L, the outlet effluent TN will remain at 4 mg/L, and the overall removal rate was stabilized at 90%, indicating that the denitrification filter media possessed the excellent ability to resist load impact. As shown in Figure 4d, the denitrification effect did not change much when the C/N increased from 2:1 to 4:1, suggesting that the additional carbon source (such as the previous addition of a carbon source) exhibited little effect on the denitrification process of the filter. This phenomenon indirectly proved that the filter media could fulfill its role without a carbon source [38,39].
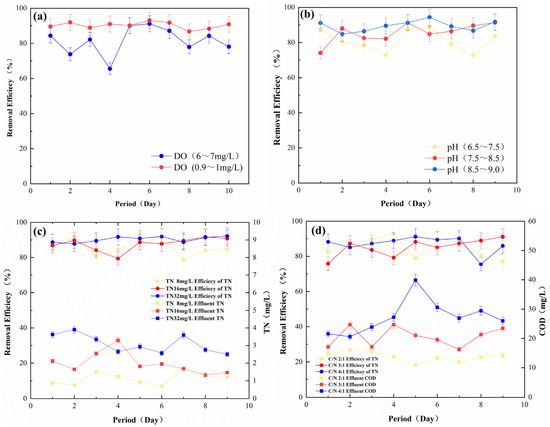
Figure 4.
Factors affecting the performance of nitrogen removal by (a) DO; (b) pH; (c) influent TN; (d) C/N (room temperature, HRT = 4 h, pH = 7, influent TN = 8 mg/L, C/N = 2:1, DO = 0.9–1 mg/L).
3.3. Analysis of the Effect of Tandem Nitrogen Removal
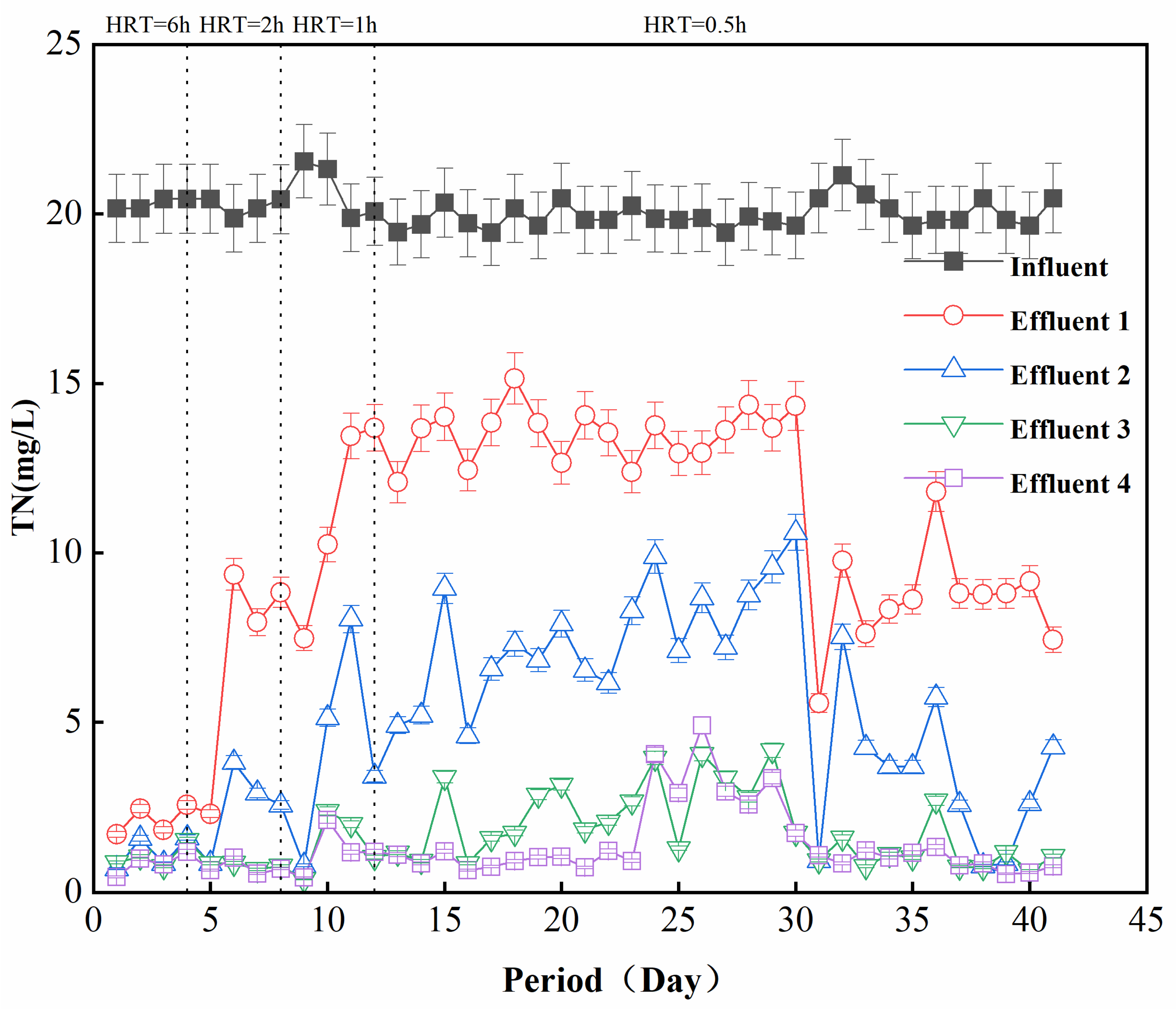
In order to further improve the performance of filter column denitrification, four groups of reactors were connected in series, with an HRT of 0.5 h for each column, and the TN of the effluent at each level was investigated. Figure 5 shows the curve of TN over time after four groups of reactors were connected in series. It can be seen that when the HRT was 6 h, the removal effect of one group of reactors was basically the same as that obtained with four groups in series. When the HRT was gradually reduced, each group of reactor effluents produced a difference in the TN concentration. The TN concentration in the effluent of reactor 1 was 12–15 mg/L, that of reactor 2 was 5–10 mg/L, and that of reactor 3 was 1–2 mg/L. However, the TN concentration in the effluent of reactors 4 and 3 was basically the same, indicating that the influent had basically reached the removal limit of the filter media after passing through three reactors. When the HRT of each reactor in the series system was 0.5 h, the total retention time was 1.5 h, which can achieve a TN value of 1–2 mg/L.
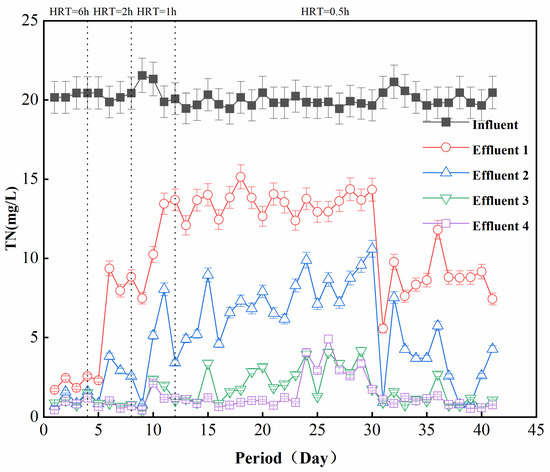
Figure 5.
Analysis of the effect of series connection (Effluent 1, 2, 3, and 4 were four sets of reactors connected in series).
3.4. Investigation of Nitrogen Removal Performance of Actual Wastewater
On this basis, the nitrogen removal effect of the nitrogen removal filter media on the actual wastewater was further investigated, and the results are shown in Figure 6. The influent used was the biochemical tail water of the Nanjing Chengbei Wastewater Treatment Plant, and the TN of the influent was 8–9 mg/L. The whole experiment was divided into 4 stages, the HRT value of the system was adjusted, and the changes of the effluent were observed in the steady state. In the stage with an HRT of 20 h, the overall removal rate was not high, which was due to the fact that at this time in the start-up phase of the system, the microorganisms were growing and attaching. In the stage with an HRT of 15 h, the removal effect was obviously improved: the TN of the effluent was stabilized at 1 mg/L, and the removal rate was also stabilized at 91.5%. This phenomenon indicated that this denitrification filter media possessed an excellent denitrification effect on the actual wastewater [40]. With the shortening of the HRT, the TN removal effect showed a slight fluctuation. When the HRT was 8 h, there was a period of time when the effluent TN reached 2 mg/L, and the removal rate decreased to 80.5%. This may be due to the fact that the change in HRT increased the system load, and the current denitrification capacity of the microorganisms was insufficient.
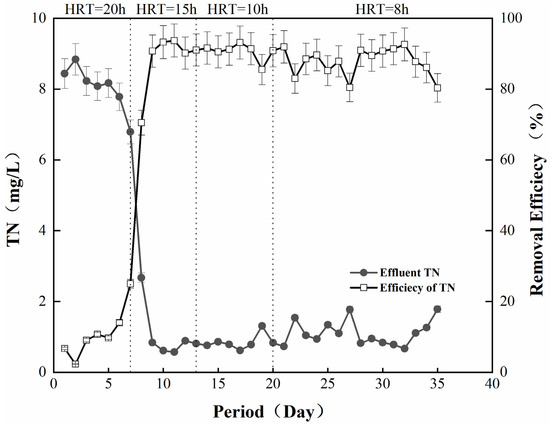
Figure 6.
Effect of filter media on nitrogen removal in actual wastewater.
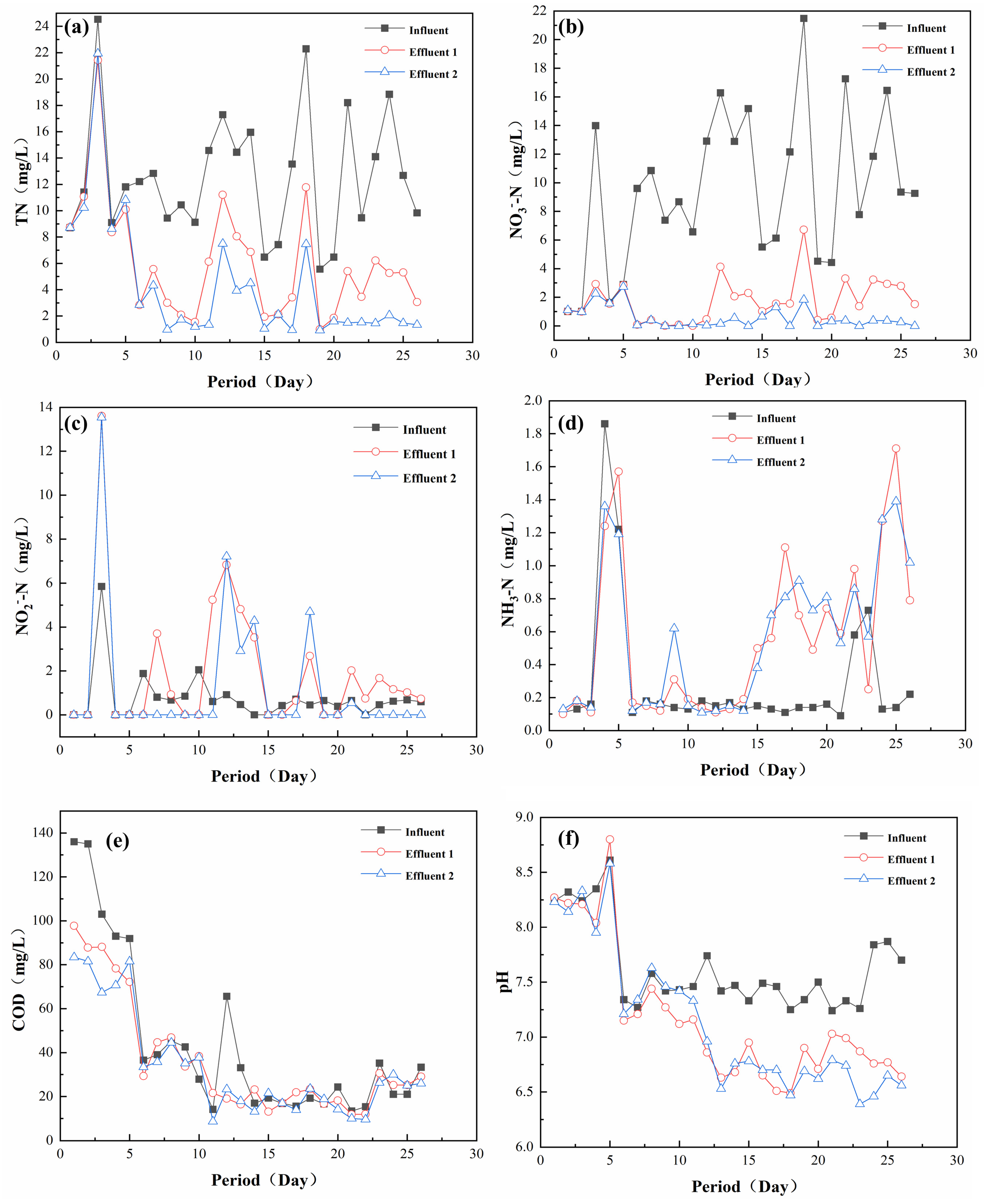
In order to further investigate the effect of a practical application of the filter media, a two-stage series system was used to carry out pilot experiments. Figure 7 showed the effect of the two-stage effluent in the pilot experiment. As shown in Figure 7a, the TN concentration in the influent water was 6–24 mg/L, while the TN in the first stage of the effluent water was 1–12 mg/L, compared with that of 1–10 mg/L in the second stage. It can be seen that there was not much difference in the TN of the effluent water in the two stages, indicating that most of the TN had been removed in the first stage, and the calculation of the removal effect of this system on influent water was about 16 mg/L. As shown in Figure 7b–d, NO3−-N was the main nitrogenous pollutant in the influent TN, and its influent concentration was 6–22 mg/L. The influent contained 0–6 mg/L of NO2−-N and 0.1–1.8 mg/L of NH3−-N. The system’s removal of NO2−-N and NH3−-N showed a similar pattern to that of TN. Meanwhile, it was found that there was only a small accumulation of NO2−-N in the experiment, which could be adjusted to no accumulation in a short time, indicating that the system possessed a good impact resistance to water quality fluctuations [41]. As shown in Figure 7e, the COD of the effluent water decreased slightly compared with that of the influent water, indicating that the system can contact the COD in the influent water to carry out partial heterotrophic denitrification without adding a carbon source [42]. As shown in Figure 7f, the pH value of the effluent water was lower than that of the influent water, which was due to the fact that the main reaction of the sulfur-based autotrophic denitrification was an acid-producing process. At the same time, it was found that the pH value of the system effluent was always maintained above 6.5, which was suitable for the growth of microorganisms. This phenomenon occurred because the steel slag was alkaline, enabling it to effectively maintain the stability of the system’s pH to ensure the stability of microbial denitrification [43].
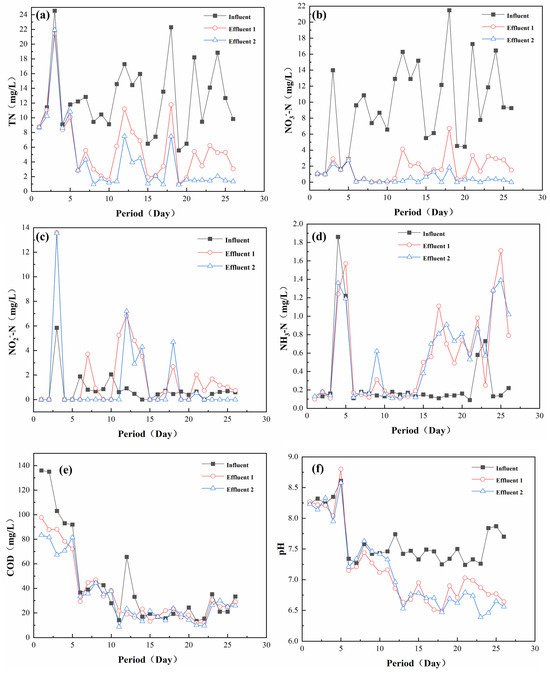
Figure 7.
Effectiveness of the secondary tandem system on real wastewater treatment: (a) TN; (b) NO3−-N; (c) NO2−-N; (d) NH3-N; (e) COD; (f) pH.
3.5. Microbiological Analysis
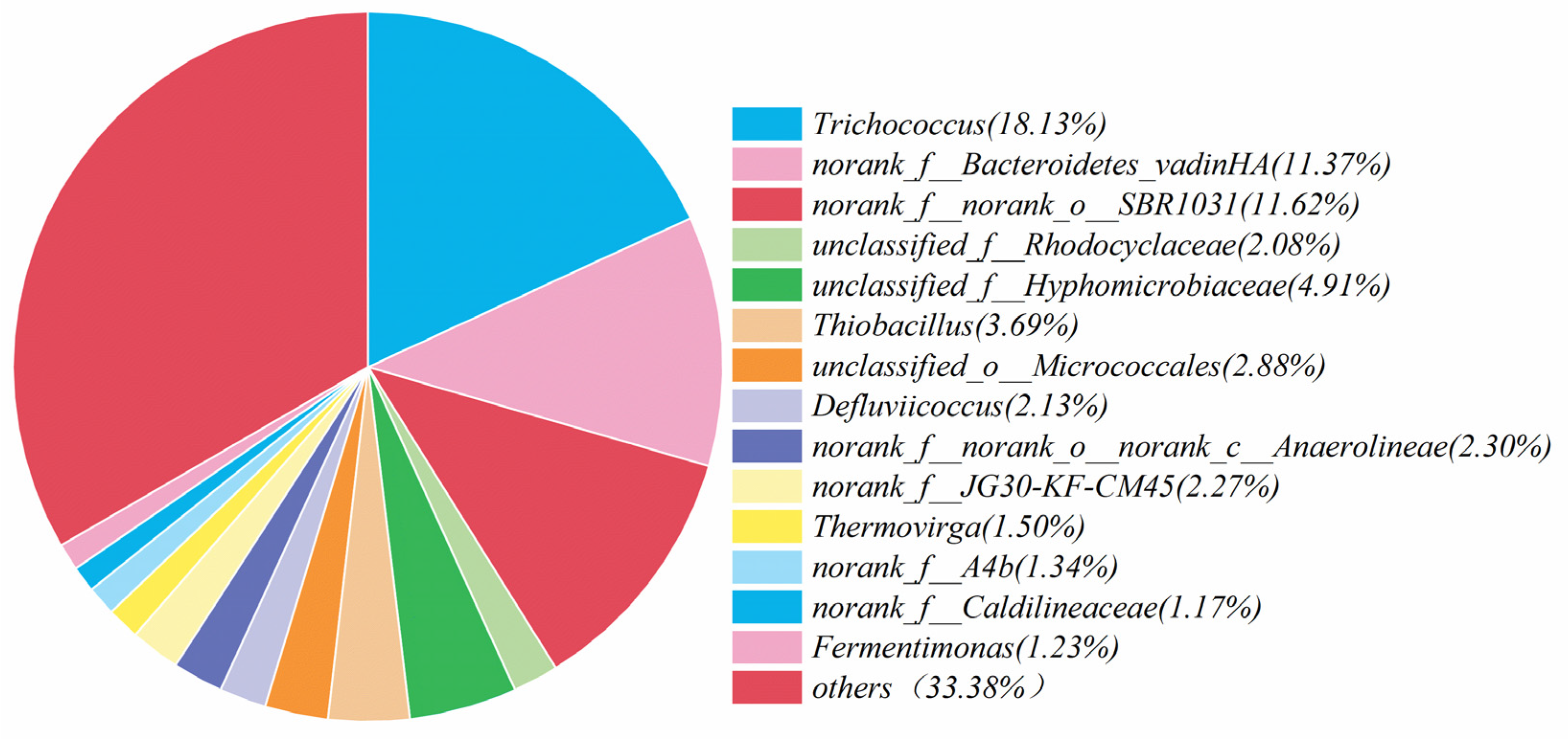
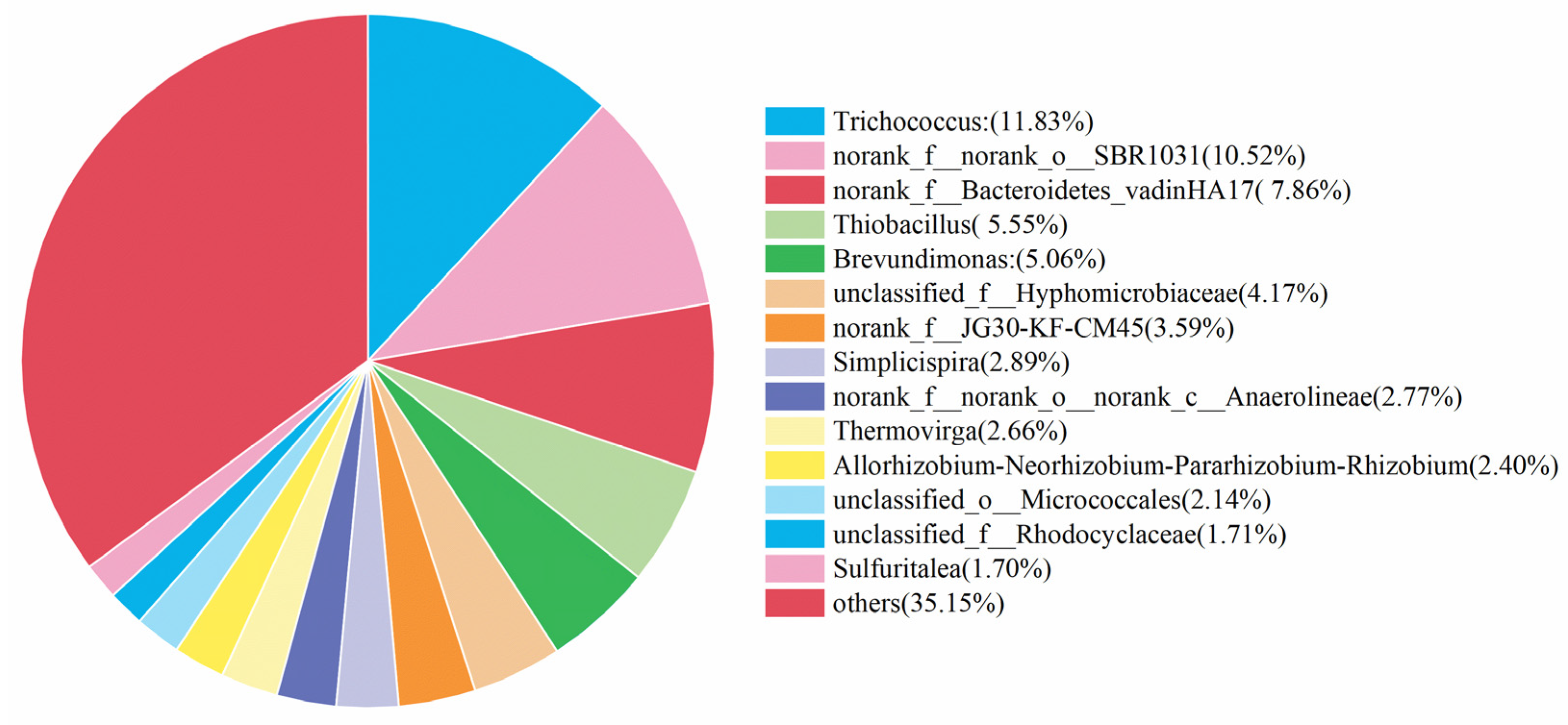
Microorganisms play vital role in the nitrogen removal process [44]. Based on this fact, the community analysis was carried out for the microorganisms in the simulated and actual wastewater, and the corresponding results are shown in Figure 8 and Figure 9. The most abundant microorganisms in both were Trichococcus, with an abundance of 18.13% and 11.83%, respectively. These two microorganisms, Trichococcus and norank_f__Bacteroidetes_vadinHA17, survived in the anaerobic environment, effectively increasing the activity of reductase in the denitrification process [45]. Norank_f__norank_o___SBR1031 mainly appeared in the heterotrophic denitrification system with carbon source utilization, and the contents were 11.62% and 10.52%, respectively, indicating that the system contained a small carbon source with a small portion of heterotrophic denitrification. Thiobacillus was considered as the main autotrophic denitrifying bacterium [46,47]. It was found that Thiobacillus was the dominant species in sulfur-based denitrification, and the contents of Thiobacillus in the simulated and actual wastewater were 3.69% and 5.55%, respectively.
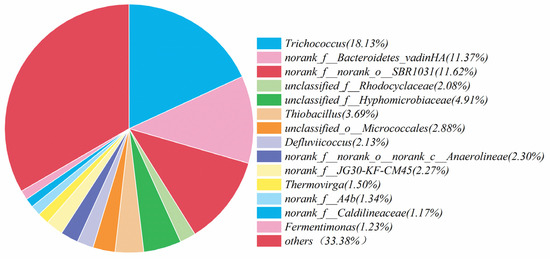
Figure 8.
Analysis of microbial communities in simulated wastewater denitrification experiments.
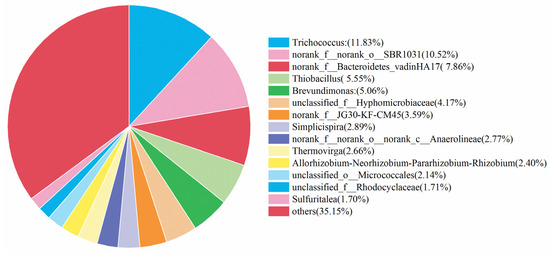
Figure 9.
Analysis of microbial communities in actual wastewater denitrification experiments.
3.6. Comparison of Microscopic Morphology of the Filters before and after Use
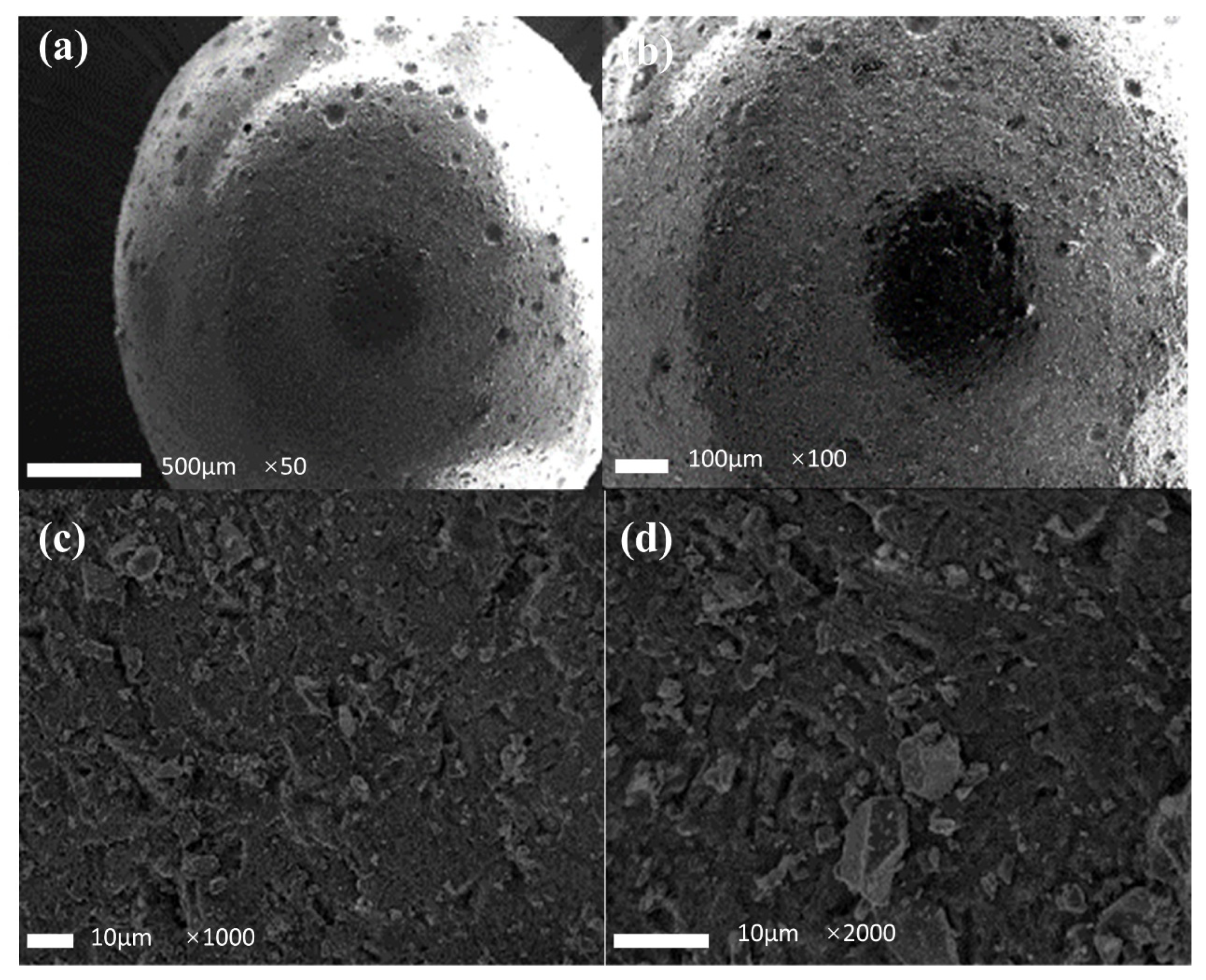
The microscopic morphology of the filter media before and after use were investigated using a scanning electron microscope (SEM), and these results are shown in Figure 10 and Figure 11, respectively. It can be seen that the filter media exhibited a spherical structure, with 5–10 μm holes on the surface. These holes were formed during the preparation process of the filter media due to the solidification and contraction of the molten sulfur/steel slag mixture into the cooling water. The holes were beneficial to the denitrification process. Firstly, they could increase the contact area between the filter media and water. Secondly, they provided a favorable location for the microorganisms to attach to the surface to enhance the nitrogen removal effect. Under higher magnification, as observed in Figure 10c,d, there were a lot of irregular particles on the surface of the filter media, with a particle size of 1–5 μm, ascribed to the existence of steel slag particles.
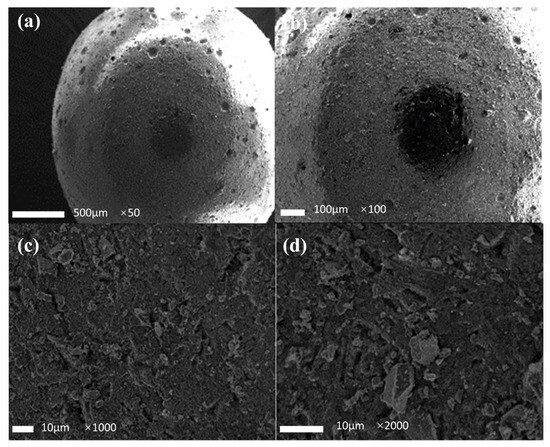
Figure 10.
SEM image of the filter media granule before use. (a) image ×50; (b) image ×100; (c) image ×1000; (d) image ×2000.
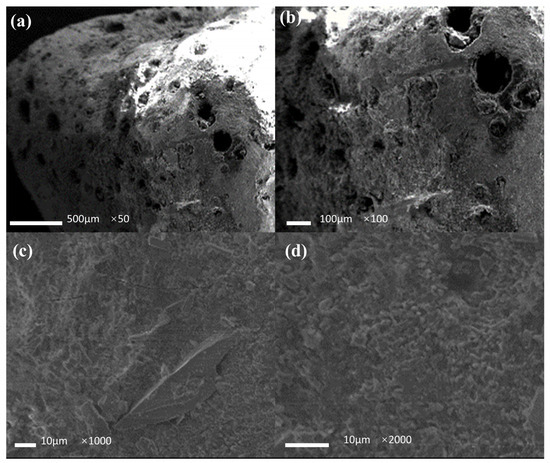
Figure 11.
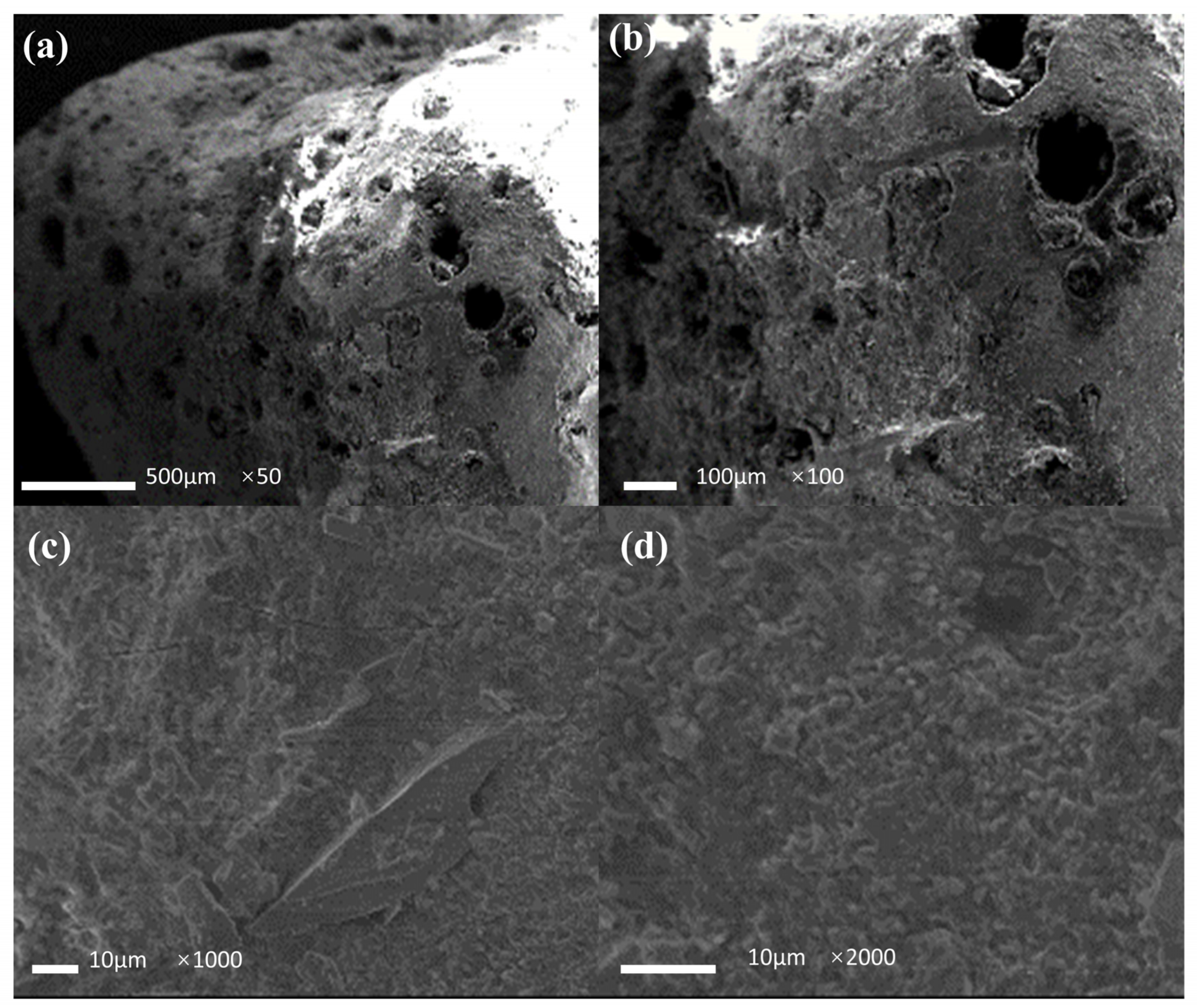
SEM image of the filter media particle after use. (a) image ×50; (b) image ×100; (c) image ×1000; (d) image ×2000.
Figure 11 shows the micrographs of the filter media after the denitrification experiments. As shown in the figure, the pore structure on the surface of the filter media deepened significantly after use, with skeletonized structures appearing in some cases, indicating that the microorganisms utilized the filter media as a donor and a nutrient for denitrification. As shown in Figure 11c,d, the gully structure on the surface of the filter media was more obvious under the action of the microorganisms. Moreover, a thin layer of biofilm structure was observed on the surface of the filter media granule, confirming the existence of microorganisms, which is vital to the denitrification process.
3.7. Work Mechanism of the Filter
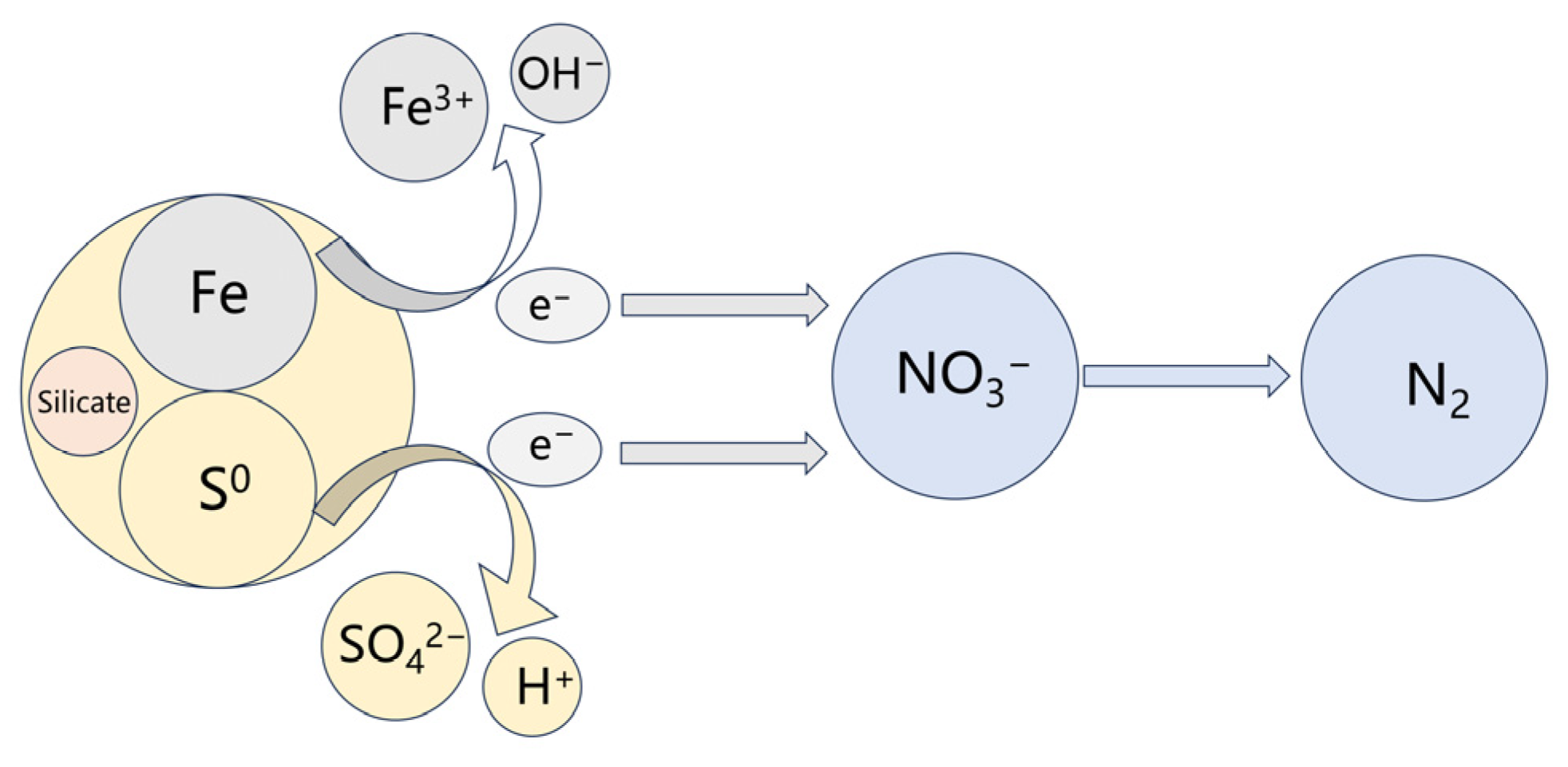
Based on the above investigation, the work mechanism of the steel slag-based filter was proposed. As shown in Figure 12, the sulfur and iron particles (in steel) in the filter act as electron donors to reduce NO3−-N into N2 during the operation process. During the reaction, sulfur autotrophic denitrification produced H+, and iron particles in the steel slag underwent iron autotrophic denitrification to produce OH−, which can neutralize part of the H+ to maintain the pH stability of the system and to benefit the denitrifying bacteria. Furthermore, the inorganic salt, including silicate, in steel slag could provide nutrients for the generation of denitrifying bacteria. Thus, the steel slag combines with sulfur, creating a synergistic effect in the filter.
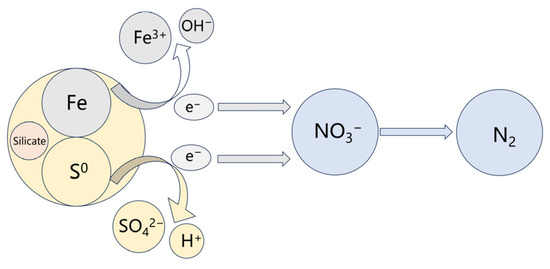
Figure 12.
Work mechanism of sulfur/steel slag-based filter.
4. Conclusions
Aiming to achieve the denitrification of tail water on a large scale and the high value utilization of metallurgical solid waste, steel slag/sulfur based denitrification filter media were prepared using the melt granulation method. Furthermore, the filter media were used for the investigation of denitrification using simulated and actual wastewater. The TN removal rate of the filter media was as high as 90% for simulated wastewater at room temperature, with an HRT of 5–20 h and influent TN of 21 mg/L. The TN removal rate of the actual wastewater at room temperature was also higher than 90%, with an HRT of 8–20 h. The results of the pilot experiment confirmed the successful application of filter media in practical engineering. Microbial community analysis showed that the sulfur autotrophic denitrifying bacterial species Thiobacillus levels accounted for 3.69% and 5.55% in the simulated and actual wastewater systems, respectively. The sulfur autotrophic denitrifying bacteria in the reactor were Thiobacillus, in addition to the presence of certain heterotrophic denitrifying bacterial colonies, norank_f__norank__o_SBR1031, which could significantly improve the nitrogen removal efficiency. Herein, this steel slag-based filter exhibited broad application prospects in the field of deep nitrogen removal from biochemical tailwater. The above investigation provides a novel strategy for the development of nitrogen removal filter media and the high value utilization of solid metallurgical waste.
Author Contributions
Investigation, conceptualization, methodology, validation, and writing—original draft, H.H.; writing—review and editing, resources, methodology, and supervision, M.Z.; investigation, data curation, and visualization, G.T.; conceptualization and supervision, J.L.; conceptualization, supervision, funding acquisition, and project administration, S.P.; project administration and methodology, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (21777069), the National Key Research and Development Program of China (2016YFE0112800), the National Key Research and Development Program of the Ningxia Hui Autonomous Region (2018DWHZ0243), and the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture, Nanqiao ‘Chunsun’ Talent Plan and ‘113′ Innovation Team Plan (Chuzhou, Anhui).
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lucchetti, R.; Onotri, L.; Clarizia, L.; Natale, F.D.; Somma, I.D.; Andreozzi, R.; Marotta, R. Removal of nitrate and simultaneous hydrogen generation through photocatalytic reforming of glycerol over “in situ” prepared zero-valent nano copper/P25. Appl. Catal. B Environ. 2017, 202, 539–549. [Google Scholar] [CrossRef]
- Espejo-Herrera, N.; Cantor, K.P.; Malats, N.; Silverman, D.T.; Tardón, A.; García-Closas, R.; Serra, C.; Kogevinas, M.; Villanueva, C.M. Nitrate in drinking water and bladder cancer risk in Spain. Environ. Res. 2015, 137, 299–307. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Kilic, A. Heterotrophic and elemental-sulfur-based autotrophic denitrification processes for simultaneous nitrate and Cr(VI) reduction. Water Res. 2014, 50, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zeng, Z.; Zhang, W.; Li, H.; Zhou, L.; Zhuang, W.Q. Functional bacteria and their genes encoding for key enzymes in hydrogen-driven autotrophic denitrification with sulfate loading. J. Clean. Prod. 2024, 440, 140901. [Google Scholar] [CrossRef]
- Yang, J.; Qin, Y.; Liu, X.; Yang, L.; Zheng, S.; Gong, S.; Liu, Z.; Wu, C.; Lin, X.; Lu, T.; et al. Effects of different electron donors on nitrogen removal performance and microbial community of denitrification system. J. Environ. Chem. Eng. 2022, 10, 107915. [Google Scholar] [CrossRef]
- Jian, C.; Hao, Y.; Liu, R.; Qi, X.; Chen, M.; Liu, N. Mixotrophic denitrification process driven by lime sulfur and butanediol: Denitrification performance and metagenomic analysis. Sci. Total Environ. 2023, 903, 166654. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Qiu, S.; Wang, J.; Zhu, Y.; Yuan, M.; Wang, L. Tourmaline mediated enhanced autotrophic denitrification: The mechanisms of electron transfer and Paracoccus enrichment. Sci. Total Environ. 2024, 915, 169847. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron donors for autotrophic denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Zheng, Z.; Chen, G.; Wang, H.; Yue, L.; Li, Q.; Liu, Y. Establishment and optimization of sulfur-based autotrophic-heterotrophic denitrification biofilters for advanced post-anaerobic treatment of effluent from kitchen wastewater and landfill leachate under low temperature. Bioresour. Technol. 2023, 393, 130155. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhou, W.; He, S.; Huang, J. Sulfur transformation in sulfur autotrophic denitrification using thiosulfate as electron donor. Environ. Pollut. 2021, 268, 115708. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Ahoranta, S.H.; Papirio, S.; Lens, P.N.L.; Esposito, G. Impacts of sulfur source and temperature on sulfur-driven denitrification by pure and mixed cultures of Thiobacillus. Process Biochem. 2016, 51, 1576–1584. [Google Scholar] [CrossRef]
- Liang, J.; Chen, N.; Tong, S.; Liu, Y.; Feng, C. Sulfur autotrophic denitrification (SAD) driven by homogeneous composite particles containing CaCO3-type kitchen waste for groundwater remediation. Chemosphere 2018, 212, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wan, D.; Li, B.; Zhang, P.; Wang, H. Pilot-scale application of sulfur-limestone autotrophic denitrification biofilter for municipal tailwater treatment: Performance and microbial community structure. Bioresour. Technol. 2020, 300, 122682. [Google Scholar] [CrossRef]
- Sarfo, P.; Wyss, G.; Ma, G.; Das, A.; Young, C. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass. Miner. Eng. 2017, 107, 8–19. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Wu, H.; Wu, M.; Wu, X.; Zhang, F.; Zhang, J.; Qiao, H.; Zhang, Y.; Zhang, Y. Study on the performance of collapsible loess subgrade improved by steel slag. J. Build. Eng. 2024, 84, 108642. [Google Scholar] [CrossRef]
- GB3838-2002; Environmental Quality Standards for Surface Water of the People’s Republic of China. PRC Environmental Protection Agency: Beijing, China, 2002.
- HJ/T346-2007; Determination of Nitrate Nitrogen in Water by Ultraviolet Spectrophotometric Method. PRC Environmental Protection Agency: Beijing, China, 2007.
- GB7493-87; Determination of Nitrite Nitrogen in Water by Spectrophotometric Method. PRC Environmental Protection Agency: Beijing, China, 1987.
- GB/T39305-2020; Determination of Fluorine, Chlorine, Nitrite, Nitrate and Sulphate in Reclaimed Water by Ion Chromatography. PRC Environmental Protection Agency: Beijing, China, 2020.
- Liu, J.; Zhang, P.; Li, H.; Tian, Y.; Wang, S.; Song, Y.; Zeng, G.; Sun, C.; Tian, Z. Denitrification of landfill leachate under different hydraulic retention time in a two-stage anoxic/oxic combined membrane bioreactor process: Performances and bacterial community. Bioresour. Technol. 2018, 250, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Wang, H.; Cheng, H.; Wang, A.; Zhang, Y.; Cui, C.; Sun, Y. Effects of salinity on sulfur-dominated autotrophic denitrification microorganisms: Microbial community succession, key microorganisms and response mechanisms. Chem. Eng. J. 2023, 478, 147308. [Google Scholar] [CrossRef]
- Wang, Y.; Bott, C.; Nerenberg, R. Sulfur-based denitrification: Effect of biofilm development on denitrification fluxes. Water Res. 2016, 100, 184–193. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Li, H.; Song, Y.; Chen, Z.; Lu, C.; Han, Y.; Hou, Y. Effect of dissolved oxygen on simultaneous removal of ammonia, nitrate and phosphorus via biological aerated filter with sulfur and pyrite as composite fillers. Bioresour. Technol. 2020, 296, 122340. [Google Scholar] [CrossRef]
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitrification, denitrification and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef]
- Shao, L.; Wang, D.; Chen, G.; Zhao, Z.; Fan, L. Advance in the sulfur-based electron donor autotrophic denitrification for nitrate nitrogen removal from wastewater. World J. Microbiol. Biotechnol. 2023, 40, 7. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Gu, C.; Huang, Y.; Yuan, Y. Selectivity control of nitrite and nitrate with the reaction of S(0) and achieved nitrite accumulation in the sulfur autotrophic denitrification process. Bioresour. Technol. 2018, 266, 211–219. [Google Scholar] [CrossRef]
- Blažková, Z. Influence of Fe3+ Ions on Nitrate Removal by Autotrophic Denitrification Using Thiobacillus denitrificans. Chem. Biochem. Eng. Q. 2017, 31, 167–172. [Google Scholar] [CrossRef]
- Fu, K.; Kang, J.; Zhao, J.; Bian, Y.; Li, X.; Yang, W.; Li, Z. Efficient nitrite accumulation in partial sulfide autotrophic denitrification (PSAD) system: Insights of S/N ratio, pH and temperature. Environ. Technol. 2023, 20, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Yang, X.; Ren, Z.; Huang, K.; Qian, F.; Li, J. Long-Term Operation of a Pilot-Scale Sulfur-Based Autotrophic Denitrification System for Deep Nitrogen Removal. Water 2023, 15, 428. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, H.; Zhou, S.; Sun, L.; Lu, H. Enhanced performance and electron transfer of sulfur-mediated biological process under polyethylene terephthalate microplastics exposure. Water Res. 2022, 223, 119038. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, S.; Zhussupbekova, A.; Shvets, I.V.; Lee, P.H.; Zhan, X. High-rate iron sulfide and sulfur-coupled autotrophic denitrification system: Nutrients removal performance and microbial characterization. Water Res. 2023, 231, 119619. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gu, X.; Zhang, M.; Yan, P.; Sun, S.; He, S. Simultaneously enhanced autotrophic-heterotrophic denitrification in iron-based ecological floating bed by plant biomass: Metagenomics insights into microbial communities, functional genes and nitrogen metabolic pathways. Water Res. 2023, 248, 120868. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, Y.; Chen, N.; He, Q.; Feng, C. High redox potential promotes oxidation of pyrite under neutral conditions: Implications for optimizing pyrite autotrophic denitrification. J. Hazard. Mater. 2021, 416, 125844. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, L.; Chen, F.; Song, Q.; Feng, C.; Liu, X.; Li, M. High efficient bio-denitrification of nitrate contaminated water with low ammonium and sulfate production by a sulfur/pyrite-based bioreactor. Bioresour. Technol. 2022, 346, 126669. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, Z.; Chen, L.; Ling, Q.; Zan, F.; Isawi, H.; Hao, T.; Ma, J.; Wang, Z.; Chen, G.; et al. Advances in elemental sulfur-driven bioprocesses for wastewater treatment: From metabolic study to application. Water Res. 2022, 213, 118143. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.-F.; Wu, D.; Huang, H.; Cui, Y.-X.; van Loosdrecht, M.C.M.; Chen, G.-H. Exploration and verification of the feasibility of sulfide-driven partial denitrification coupled with anammox for wastewater treatment. Water Res. 2021, 193, 116905. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, B.; Ma, L.; Jiao, H.; Tian, B.; Li, K.; Liang, J. Study on denitrification of hydroponic wastewater reverse osmosis concentrate using sulfur-autotrophic denitrification. J. Environ. Chem. Eng. 2023, 11, 111195. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Q.; Lu, H.; Ding, Z.; Meng, L.; Chen, G.-H. Sulfide-driven autotrophic denitrification significantly reduces N2O emissions. Water Res. 2016, 90, 176–184. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Liu, S.; Chen, N.; Ma, W.; Wang, R.; Wu, J.; Yu, Y. Nitrogen removal performance and bacterial flora analysis of a nitrification-sulphur autotrophic denitrification coupled with permeable reaction wall (NSAD+PRW) treating rare earth mine groundwater. J. Water Process Eng. 2023, 55, 104136. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, C.; Zhao, M.; Wang, Z.; Jiang, S.; Jin, Z.; Bei, K.; Zheng, X.; Wu, S.; Lin, P.; et al. A comprehensive review on mixotrophic denitrification processes for biological nitrogen removal. Chemosphere 2023, 313, 137474. [Google Scholar] [CrossRef]
- Weralupitiya, C.; Wanigatunge, R.; Joseph, S.; Athapattu, B.C.L.; Lee, T.-H.; Kumar Biswas, J.; Ginige, M.P.; Shiung Lam, S.; Senthil Kumar, P.; Vithanage, M. Anammox bacteria in treating ammonium rich wastewater: Recent perspective and appraisal. Bioresour. Technol. 2021, 334, 125240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-T.; Ye, L.; Ju, F.; Wang, Y.-L.; Zhang, T. Toward an Intensive Longitudinal Understanding of Activated Sludge Bacterial Assembly and Dynamics. Environ. Sci. Technol. 2018, 52, 8224–8232. [Google Scholar] [CrossRef] [PubMed]
- Booali, S.; Zoufan, P.; Bavani, M.R.Z. Effect of biofertilizer containing Thiobacillus bacteria along with different levels of chemical sulfur fertilizer on growth response and photochemical efficiency of small radish plants (Raphanus sativus L. var. shushtari) under greenhouse conditions. Sci. Hortic. 2024, 327, 112835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).