Spatial Variations in Microplastics in the Largest Shallow Lake of Central Europe and Its Protecting Wetland Area
Abstract
:1. Introduction
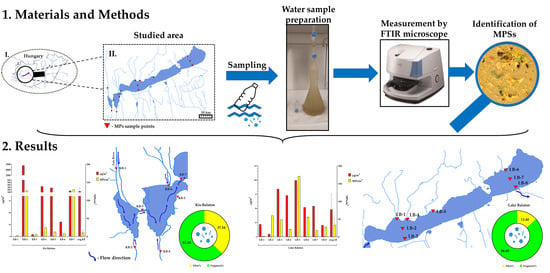
2. Material and Methods
2.1. Study Area and Sampling
2.2. Microplastic Extraction and Analysis
2.3. Contamination Prevention and Quality Control
3. Results
3.1. LOD and LOQ Values
3.2. MP Abundance in KB and Lake Balaton
3.3. Polymer Types of the Identified MPs
3.4. MP Distribution according to Their Shapes and Sizes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in Freshwater Systems: A Review of the Emerging Threats, Identification of Knowledge Gaps and Prioritisation of Research Needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Plastics—Environmental Aspects—State of Knowledge and Methodologies. Available online: https://cdn.standards.iteh.ai/samples/72300/65b73cb6030f442aa1bd7eaa97386292/ISO-TR-21960-2020.pdf (accessed on 27 March 2024).
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and Challenges in the Detection of Microplastics and Nanoplastics: A Mini-Review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative Assessment of Microplastics in Water and Sediment of a Large European River. Sci. Total Environ. 2020, 738, 139866. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Talbot, R.; Chang, H. Microplastics in Freshwater: A Global Review of Factors Affecting Spatial and Temporal Variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef] [PubMed]
- Svigruha, R.; Prikler, B.; Farkas, A.; Ács, A.; Fodor, I.; Tapolczai, K.; Schmidt, J.; Bordós, G.; Háhn, J.; Harkai, P.; et al. Presence, Variation, and Potential Ecological Impact of Microplastics in the Largest Shallow Lake of Central Europe. Sci. Total Environ. 2023, 883, 163537. [Google Scholar] [CrossRef] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic Hitchhikers—Assessing Pathogen Risks from Marine Microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Maasz, G.; Mayer, M.; Zrinyi, Z.; Molnar, E.; Kuzma, M.; Fodor, I.; Pirger, Z.; Takács, P. Spatiotemporal Variations of Pharmacologically Active Compounds in Surface Waters of a Summer Holiday Destination. Sci. Total Environ. 2019, 677, 545–555. [Google Scholar] [CrossRef]
- Molnar, E.; Maasz, G.; Pirger, Z. Environmental Risk Assessment of Pharmaceuticals at a Seasonal Holiday Destination in the Largest Freshwater Shallow Lake in Central Europe. Environ. Sci. Pollut. Res. 2021, 28, 59233–59243. [Google Scholar] [CrossRef]
- Kovács, J.; Hatvani, I.G.; Korponai, J.; Kovács, I.S. Morlet Wavelet and Autocorrelation Analysis of Long-Term Data Series of the Kis-Balaton Water Protection System (KBWPS). Ecol. Eng. 2010, 36, 1469–1477. [Google Scholar] [CrossRef]
- Istvánovics, V.; Clement, A.; Somlyódy, L.; Specziár, A.; G.-Tóth, L.; Padisák, J. Updating Water Quality Targets for Shallow Lake Balaton (Hungary), Recovering from Eutrophication. Hydrobiologia 2007, 581, 305–318. [Google Scholar] [CrossRef]
- Farkas, M.; Szoboszlay, S.; Vörös, L.; Lovász, Z.; Méhes, N.; Mátyás, K.; Sebők, R.; Kaszab, E.; Háhn, J.; Tóth, G.; et al. Bacterial Community Dynamics along a River-Wetland-Lake System. Water 2022, 14, 3519. [Google Scholar] [CrossRef]
- Dömötörfy, Z.; Reeder, D.; Pomogyi, P. Changes in the Macro-Vegetation of the Kis-Balaton Wetlands over the Last Two Centuries: A GIS Perspective. Hydrobiologia 2003, 506, 671–679. [Google Scholar] [CrossRef]
- Marinović, Z.; Tokodi, N.; Backović, D.D.; Šćekić, I.; Kitanović, N.; Simić, S.B.; Đorđević, N.B.; Ferincz, Á.; Staszny, Á.; Dulić, T.; et al. Does the Kis-balaton Water Protection System (Kbwps) Effectively Safeguard Lake Balaton from Toxic Cyanobacterial Blooms? Microorganisms 2021, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Bordós, G.; Gergely, S.; Háhn, J.; Palotai, Z.; Szabó, É.; Besenyő, G.; Salgó, A.; Harkai, P.; Kriszt, B.; Szoboszlay, S. Validation of Pressurized Fractionated Filtration Microplastic Sampling in Controlled Test Environment. Water Res. 2021, 189, 116572. [Google Scholar] [CrossRef] [PubMed]
- Mári, Á.; Bordós, G.; Gergely, S.; Büki, M.; Háhn, J.; Palotai, Z.; Besenyő, G.; Szabó, É.; Salgó, A.; Kriszt, B.; et al. Validation of Microplastic Sample Preparation Method for Freshwater Samples. Water Res. 2021, 202, 117409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lorenz, C.; Vianello, A.; Syberg, K.; Nielsen, A.H.; Nielsen, T.G.; Vollertsen, J. Exploration of Occurrence and Sources of Microplastics (>10 Μm) in Danish Marine Waters. Sci. Total Environ. 2023, 865, 161255. [Google Scholar] [CrossRef]
- Primpke, S.; Cross, R.K.; Mintenig, S.M.; Simon, M.; Vianello, A.; Gerdts, G.; Vollertsen, J. Toward the Systematic Identification of Microplastics in the Environment: Evaluation of a New Independent Software Tool (SiMPle) for Spectroscopic Analysis. Appl. Spectrosc. 2020, 74, 1127–1138. [Google Scholar] [CrossRef]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of Microplastic Mass and Removal Rates at Wastewater Treatment Plants Applying Focal Plane Array (FPA)-Based Fourier Transform Infrared (FT-IR) Imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity Assessment of Microplastic (MPs); a Threat to the Ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating Human Exposure to Indoor Airborne Microplastics Using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef] [PubMed]
- Cole, M. A Novel Method for Preparing Microplastic Fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-Automated Analysis of Microplastics in Complex Wastewater Samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Sedlak, M.; Lin, D.; Box, C.; Holleman, C.; Rochman, C.M.; Sutton, R. Recommended Best Practices for Collecting, Analyzing, and Reporting Microplastics in Environmental Media: Lessons Learned from Comprehensive Monitoring of San Francisco Bay. J. Hazard. Mater. 2021, 409, 124770. [Google Scholar] [CrossRef] [PubMed]
- Tanentzap, A.J.; Cottingham, S.; Fonvielle, J.; Riley, I.; Walker, L.M.; Woodman, S.G.; Kontou, D.; Pichler, C.M.; Reisner, E.; Lebreton, L. Microplastics and Anthropogenic Fibre Concentrations in Lakes Reflect Surrounding Land Use. PLoS Biol. 2021, 19, e3001389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Sun, C.; Cao, W.; Wang, M.; Jiang, F.; Ju, P. Comparative Study of Three Sampling Methods for Microplastics Analysis in Seawater. Sci. Total Environ. 2021, 765, 144495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Prikler, B.; Bordós, G.; Lorenz, C.; Vollertsen, J. Does Microplastic Analysis Method Affect Our Understanding of Microplastics in the Environment? Sci. Total Environ. 2023, 902, 166513. [Google Scholar] [CrossRef] [PubMed]
- Uurasjärvi, E.; Hartikainen, S.; Setälä, O.; Lehtiniemi, M.; Koistinen, A. Microplastic Concentrations, Size Distribution, and Polymer Types in the Surface Waters of a Northern European Lake. Water Environ. Res. 2020, 92, 149–156. [Google Scholar] [CrossRef]
- Bordós, G.; Urbányi, B.; Micsinai, A.; Kriszt, B.; Palotai, Z.; Szabó, I.; Hantosi, Z.; Szoboszlay, S. Identification of Microplastics in Fish Ponds and Natural Freshwater Environments of the Carpathian Basin, Europe. Chemosphere 2019, 216, 110–116. [Google Scholar] [CrossRef]
- Dalvand, M.; Hamidian, A.H. Occurrence and Distribution of Microplastics in Wetlands. Sci. Total Environ. 2023, 862, 160740. [Google Scholar] [CrossRef] [PubMed]
- Kaliszewicz, A.; Winczek, M.; Karaban, K.; Kurzydłowski, D.; Górska, M.; Koselak, W.; Romanowski, J. The Contamination of Inland Waters by Microplastic Fibres under Different Anthropogenic Pressure: Preliminary Study in Central Europe (Poland). Waste Manag. Res. 2020, 38, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Thornton Hampton, L.M.; Brander, S.M.; Coffin, S.; Cole, M.; Hermabessiere, L.; Koelmans, A.A.; Rochman, C.M. Characterizing Microplastic Hazards: Which Concentration Metrics and Particle Characteristics Are Most Informative for Understanding Toxicity in Aquatic Organisms? Microplastics Nanoplastics 2022, 2, 20. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in Water Systems: A Review of Their Impacts on the Environment and Their Potential Hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M. Paint Fragments as Polluting Microplastics: A Brief Review. Mar. Pollut. Bull. 2021, 162, 111847. [Google Scholar] [CrossRef]
- Turner, A. Paint Particles in the Marine Environment: An Overlooked Component of Microplastics. Water Res. X 2021, 12, 100110. [Google Scholar] [CrossRef]
- Kameda, Y.; Yamada, N.; Fujita, E. Source- and Polymer-Specific Size Distributions of Fine Microplastics in Surface Water in an Urban River. Environ. Pollut. 2021, 284, 117516. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.Z.; Al Kendi, R.; Thiemann, T. The Effect of Wastewater Treatment Plants on Retainment of Plastic Microparticles to Enhance Water Quality—A Review. J. Environ. Prot. 2021, 12, 161–195. [Google Scholar] [CrossRef]
- Ronda, A.C.; Menéndez, M.C.; Tombesi, N.; Álvarez, M.; Tomba, J.P.; Silva, L.I.; Arias, A.H. Microplastic Levels on Sandy Beaches: Are the Effects of Tourism and Coastal Recreation Really Important? Chemosphere 2023, 316, 137842. [Google Scholar] [CrossRef]
- Franco, A.A.; Iglesias-Arroyo, D.; Egea-Corbacho, Á.; Martín-García, A.P.; Quiroga, J.M.; Coello, M.D. Influence of Tourism on Microplastic Contamination at Wastewater Treatment Plants in the Coastal Municipality of Chiclana de La Frontera. Sci. Total Environ. 2023, 900, 165573. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Liu, K.; Zhu, L.; Wei, N.; Zong, C.; Li, D. Pelagic Microplastics in Surface Water of the Eastern Indian Ocean during Monsoon Transition Period: Abundance, Distribution, and Characteristics. Sci. Total Environ. 2021, 755, 142629. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, X.; Chen, R.; Liu, P.; Liang, W.; Wang, J.; Teng, M.; Wang, X.; Gao, S. Wastewater Treatment Plants Act as Essential Sources of Microplastic Formation in Aquatic Environments: A Critical Review. Water Res. 2022, 221, 118825. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Feng, Q.; Wang, J. Mini-Review of Microplastics in the Atmosphere and Their Risks to Humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef] [PubMed]
- Järlskog, I.; Strömvall, A.M.; Magnusson, K.; Galfi, H.; Björklund, K.; Polukarova, M.; Garção, R.; Markiewicz, A.; Aronsson, M.; Gustafsson, M.; et al. Traffic-Related Microplastic Particles, Metals, and Organic Pollutants in an Urban Area under Reconstruction. Sci. Total Environ. 2021, 774, 145503. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prikler, B.; Svigruha, R.; Háhn, J.; Harkai, P.; Fodor, I.; Kaszab, E.; Kriszt, B.; Tóth, G.; Szabó, I.; Csenki, Z.; et al. Spatial Variations in Microplastics in the Largest Shallow Lake of Central Europe and Its Protecting Wetland Area. Water 2024, 16, 1014. https://doi.org/10.3390/w16071014
Prikler B, Svigruha R, Háhn J, Harkai P, Fodor I, Kaszab E, Kriszt B, Tóth G, Szabó I, Csenki Z, et al. Spatial Variations in Microplastics in the Largest Shallow Lake of Central Europe and Its Protecting Wetland Area. Water. 2024; 16(7):1014. https://doi.org/10.3390/w16071014
Chicago/Turabian StylePrikler, Bence, Réka Svigruha, Judit Háhn, Péter Harkai, István Fodor, Edit Kaszab, Balázs Kriszt, Gergő Tóth, István Szabó, Zsolt Csenki, and et al. 2024. "Spatial Variations in Microplastics in the Largest Shallow Lake of Central Europe and Its Protecting Wetland Area" Water 16, no. 7: 1014. https://doi.org/10.3390/w16071014
APA StylePrikler, B., Svigruha, R., Háhn, J., Harkai, P., Fodor, I., Kaszab, E., Kriszt, B., Tóth, G., Szabó, I., Csenki, Z., Bordós, G., Micsinai, A., Nyírő-Fekete, B., Palotai, Z., Lovász, Z., Pirger, Z., & Szoboszlay, S. (2024). Spatial Variations in Microplastics in the Largest Shallow Lake of Central Europe and Its Protecting Wetland Area. Water, 16(7), 1014. https://doi.org/10.3390/w16071014