Ephemeral Puddles—Potential Sites for Feeding and Reproduction of Hyporheic Copepoda
Abstract
:1. Introduction
2. Materials and Methods
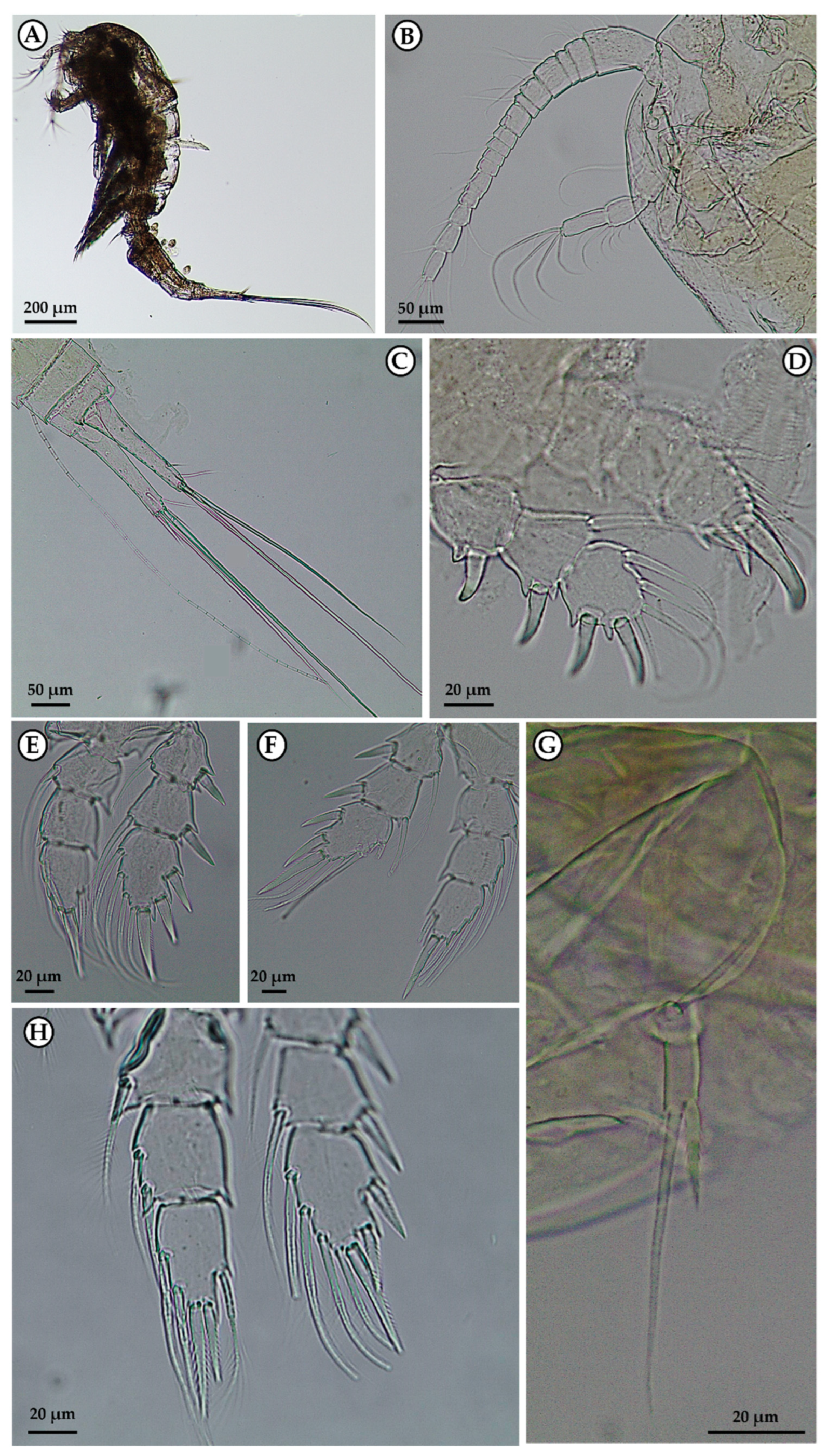
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zieliński, P.; Jekatierynczuk-Rudczyk, E. Dissolved organic matter transformation in the hyporheic zone of a small lowland river. Oceanol. Hydrobiol. Stud. 2010, 39, 97–103. [Google Scholar] [CrossRef]
- Mugnai, R.; Messana, G.; Di Lorenzo, T. The hyporheic zone and its functions: Revision and research status in Neotropical regions. Braz. J. Biol. 2015, 75, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Helešic, J.; Leichtfried, M.; Wagner, F.; Omesová, M. Investigations on gravel bars and the hyporheic zone in an alpine and two hercynian streams in Central Europe. Verhandlungen Int. Ver. Theor. Angew. Limnol. 2006, 29, 1511–1515. [Google Scholar] [CrossRef]
- Zhai, M.; Hřívová, D.; Peterka, T. The harpacticoid assemblages (Copepoda: Harpacticoida) in the Western Carpathian spring fens in relation to environmental variables and habitat age. Limnologica 2015, 53, 84–94. [Google Scholar] [CrossRef]
- Výravský, D.; Klímová Hřívová, D.; Bojková, J.; Horsák, M.; Zhai, M. Effects of thermal stability on microcrustacean assemblages in spring fens. Inland Waters 2023, 13, 86–100. [Google Scholar] [CrossRef]
- Galassi, D.M.P. Groundwater copepods: Diversity patterns over ecological and evolutionary scales. Hydrobiologia 2001, 453, 227–253. [Google Scholar] [CrossRef]
- Karpowicz, M.; Smolska, S.; Świsłocka, M.; Moroz, J. First insight into groundwater copepods of the Polish lowland. Water 2021, 13, 2086. [Google Scholar] [CrossRef]
- Smolska, S.; Karpowicz, M.; Świsłocka, M.; Jekatierynczuk-Rudczyk, E.; Więcko, A.; Tarasewicz, K. The patchy distribution of groundwater copepods in the lowland river valley. Ecohydrol. Hydrobiol. 2023. [Google Scholar] [CrossRef]
- Karpowicz, M. New data to the knowledge on the Harpacticoida (Crustacea, Copepoda) fauna in Poland. Fragm. Faun. 2016, 59, 87–98. [Google Scholar] [CrossRef]
- Einsle, U. Cyclops heberti n.sp. and Cyclops singularis n.sp., two new species within the genus Cyclops (‘strenuus-subgroup’) (Crust. Copepoda) from ephemeral ponds in southern Germany. Hydrobiologia 1996, 319, 167–177. [Google Scholar] [CrossRef]
- Alekseev, V.; Fefelova, F.; Dumont, H.J. Some noteworthy free-living copepods from surface freshwater in Belgium. Belg. J. Zool. 2002, 132, 133–139. [Google Scholar]
- Record, N.R.; Ji, R.; Maps, F.; Varpe, Ø.; Runge, J.A.; Petrik, C.M.; Johns, D. Copepod diapause and the biogeography of the marine lipidscape. J. Biogeogr. 2018, 45, 2238–2251. [Google Scholar] [CrossRef]
- Belmonte, G.; Miglietta, A.M.; Rubino, F.; Boero, F. Morphological convergence of resting stages produced by planktonic organisms: A review. Hydrobiologia 1997, 335, 159–165. [Google Scholar] [CrossRef]
- Belmonte, G. The suspected contradictory role of parental care in the adaption of planktonic Calanoida to temporary freshwater. Water 2021, 13, 100. [Google Scholar] [CrossRef]
- Louette, G.; De Meester, L. High dispersal capacity of cladoceran zooplankton in newly founded communities. Ecology 2005, 86, 353–359. [Google Scholar] [CrossRef]
- Michels, E.; Cottenie, K.; Neys, L.; De Meester, L. Zooplankton on the move: First results on the quantification of dispersal of zooplankton in a set of interconnected ponds. Hydrobiologia 2001, 442, 117–126. [Google Scholar] [CrossRef]
- Brendonk, L.; Riddoch, B.J. Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda). Biol. J. Linn. Soc. 1999, 67, 87–95. [Google Scholar] [CrossRef]
- Cáceres, C.E.; Soluk, D.A. Blowing in the wind: A field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 2002, 131, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Figuerola, J.; Green, A.J. Dispersal of aquatic organisms by waterbirds: A review of past research and priorities for future studies. Freshw. Biol. 2002, 47, 483–494. [Google Scholar] [CrossRef]
- Charalambidou, I.; Ketelaars, H.A.M.; Santamaría, L. Endozoochory by ducks: Influence of developmental stage of Bythotrephes diapause eggs on dispersal probability. Divers. Distrib. 2003, 9, 367–374. [Google Scholar] [CrossRef]
- Olmo, C.; Armengol, X.; Ortells, R. Re-establishment of zooplankton communities in temporary ponds after autumn flooding: Does restoration age matter? Limnologica 2012, 42, 310–319. [Google Scholar] [CrossRef]
- Coccia, C.; Almeida, B.A.; Badosa, A.; Diniz, L.P.; Brendock, L.; Frisch, D.; Green, A.J. Hydroperiod length, not pond age, determines zooplankton taxonomic and functional diversity in temporary ponds. Ecol. Indic. 2024, 159, 111632. [Google Scholar] [CrossRef]
- Einsle, U.K. Copepoda: Cyclopoida Genera Cyclops, Megacyclops, Acanthocyclops. In Guides to the Identificaton of the Microinvertebrates of the Continental Waters of the World; Dumont, H.J.F., Ed.; SPB Academic Publishing: Amsterdam, The Netherlands, 1996; Volume 10, pp. 1–83. [Google Scholar]
- Błędzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida). Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Miracle, M.R.; Alekseev, V.R.; Monchenko, V.; Sentandreu, V.; Vicente, E. Molecular-genetic-based contribution to the taxonomy of the Acanthocyclops robustus group. J. Nat. Hist. 2013, 47, 863–888. [Google Scholar] [CrossRef]
- Anufriieva, E.; Hołyńska, M.; Shadrin, N. Current invasions of Asian Cyclopid species (Copepoda: Cyclopidae) in Crimea, with taxonomical and zoogeographical remarks on the hypersaline and freshwater fauna. Ann. Zool. 2014, 64, 109–130. [Google Scholar] [CrossRef]
- Williams, P.; Whitfield, M.; Biggs, J.; Bray, S.; Fox, G.; Nicolet, P.; Sear, D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2004, 115, 329–341. [Google Scholar] [CrossRef]
- Oertli, B.; Biggs, J.; Céréghino, R.; Grillas, P.; Joly, P.; Lachavanne, J.B. Conservation and monitoring of pond biodiversity: Introduction. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 535–540. [Google Scholar] [CrossRef]
- Szałkiewicz, E.; Jusik, S.; Grygoruk, M. Status of and Perspectives on River Restoration in Europe: 310,000 Euros per Hectare of Restored River. Sustainability 2018, 10, 129. [Google Scholar] [CrossRef]
- Pociecha, A.; Karpowicz, M.; Namiotko, T.; Dumnicka, E.; Galas, J. Diversity of groundwater crustaceans in wells in various geologic formations of southern Poland. Water 2021, 13, 2193. [Google Scholar] [CrossRef]
- Martin, P.; De Broyer, C.; Fiers, F.; Michel, G.; Sablon, R.; Wouters, K. Biodiversity of Belgian groundwater fauna in relation to environmental conditions. Freshw. Biol. 2009, 54, 814–829. [Google Scholar] [CrossRef]
- Hobbs, H.H. Diversity Patterns in the United States. Encyclopedia of Caves; Academic Press: Chennai, India, 2012; pp. 251–264. [Google Scholar] [CrossRef]
- Strayer, D.L.; May, S.E.; Nielsen, P.; Wollheim, W.; Hausam, S. An endemic groundwater fauna in unglaciated eastern North America. Can. J. Zool. 1995, 73, 502–508. [Google Scholar] [CrossRef]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Biondi, M.; Di Cicco, M.; Galassi, D.M.P. Spatial distribution of stygobitic crustacean harpacticoids at the boundaries of groundwater habitat types in Europe. Sci. Rep. 2020, 10, 19043. [Google Scholar] [CrossRef]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Fiers, F.; Martin, P.; et al. Groundwater biodiversity in Europe. Freshw. Biol. 2009, 54, 709–726. [Google Scholar] [CrossRef]
- Gibert, J.; Culver, D.C. Assessing and conserving groundwater biodiversity: An introduction. Freshw. Biol. 2009, 54, 639–648. [Google Scholar] [CrossRef]
- Kalinowska, K.; Karpowicz, M. Ice-on and ice-off dynamics of ciliates and metazooplankton in the Łuczański Canal (Poland). Aquat. Ecol. 2020, 54, 1121–1134. [Google Scholar] [CrossRef]
- Svetlichny, L.; Obertegger, U. Influence of egg sacs on the swimming performance of freshwater cyclopoid copepods. J. Plankton Res. 2024, fbae007. [Google Scholar] [CrossRef]
- Gaviria, S. Checklist and distribution of the free-living copepods (Arthropoda: Crustacea) from Austria. Ann. Naturhistorischen Mus. Wien Ser. B Für Bot. Und Zool. 1998, 100, 539–594. [Google Scholar]
- Yevdokimov, N.A.; Yermokhin, M.V. Zooplankton crustaceans of ephemeral waterbodies on the territory of various natural zones in Saratov oblast. Inland Water Biol. 2009, 2, 59–66. [Google Scholar] [CrossRef]
- Pasternak, A.F.; Arashkevich, E.G. Resting stages in the life cycle of Eudiaptomus graciloides (Lill.) (Copepoda: Calanoida) in Lake Glubokoe. J. Plankton Res. 1999, 21, 309–325. [Google Scholar] [CrossRef]
- Wierzbicka, M. Distribution of Cyclopoida copepodites in the resting stage in bottom sediments of astatic reservoirs. Pol. Arch. Hydrobiol. 1972, 19, 369–376. [Google Scholar]
- Marcus, N.H.; Lutz, R.; Burnett, W.; Cable, P. Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: Evidence of an egg bank. Limnol. Oceanogr. 1994, 39, 154–158. [Google Scholar] [CrossRef]
- Hairston, N.G. Zooplankton egg banks as biotic reservoirs in changing environments. Limnol. Oceanogr. 1996, 41, 1087–1092. [Google Scholar] [CrossRef]
- Cáceres, C.E. Interespecific variation in the abundance, production and emergence of Daphnia diapausing eggs. Ecology 1998, 79, 1699–1710. [Google Scholar] [CrossRef]
- Brendonck, L.; De Meester, L. Egg banks in freshwater zooplankton: Evolutionary and ecological archives in the sediment. Hydrobiologia 2003, 491, 65–84. [Google Scholar] [CrossRef]
- Bruno, M.C.; Perry, S.A. Exchanges of copepod fauna between surface- and groundwater in the Rocky Glades of Everglades National Park (Florida, U.S.A.). Arch Hydrobiol. 2004, 159, 489–510. [Google Scholar] [CrossRef]
- Carver, S.; Spafford, H.; Storey, A.; Weinstein, P. Colonization of ephemeral water bodies in the Wheatbelt of Western Australia by assemblages of mosquitoes (Diptera: Culicidae): Role of environmental factors, habitat, and disturbance. Environ. Entomol. 2009, 38, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Marten, G.G.; Bordes, E.S.; Nguyen, M. Use of cyclopoid copepods for mosquito control. Hydrobiologia 1994, 292, 491–496. [Google Scholar] [CrossRef]
- Tuno, N.; Phong, T.V.; Takagi, M. Climate change may restrict the predation efficiency of Mesocyclops aspericornis (Copepoda: Cyclopidae) on Aedes aegypti (Diptera: Culicidae) Larvae. Insects 2020, 11, 307. [Google Scholar] [CrossRef]
- Pauly, I.; Jakoby, O.; Becker, N. Efficacy of native cyclopoid copepods in biological vector control with regard to their predatory behavior against the Asian tiger mosquito, Aedes albopictus. Parasites Vectors 2022, 15, 351. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpowicz, M.; Smolska, S. Ephemeral Puddles—Potential Sites for Feeding and Reproduction of Hyporheic Copepoda. Water 2024, 16, 1068. https://doi.org/10.3390/w16071068
Karpowicz M, Smolska S. Ephemeral Puddles—Potential Sites for Feeding and Reproduction of Hyporheic Copepoda. Water. 2024; 16(7):1068. https://doi.org/10.3390/w16071068
Chicago/Turabian StyleKarpowicz, Maciej, and Sabina Smolska. 2024. "Ephemeral Puddles—Potential Sites for Feeding and Reproduction of Hyporheic Copepoda" Water 16, no. 7: 1068. https://doi.org/10.3390/w16071068
APA StyleKarpowicz, M., & Smolska, S. (2024). Ephemeral Puddles—Potential Sites for Feeding and Reproduction of Hyporheic Copepoda. Water, 16(7), 1068. https://doi.org/10.3390/w16071068






