Algae in Recreational Waters: An Overview within a One Health Perspective
Abstract
:1. Introduction
2. Definition and Classification of Algae and Cyanobacteria
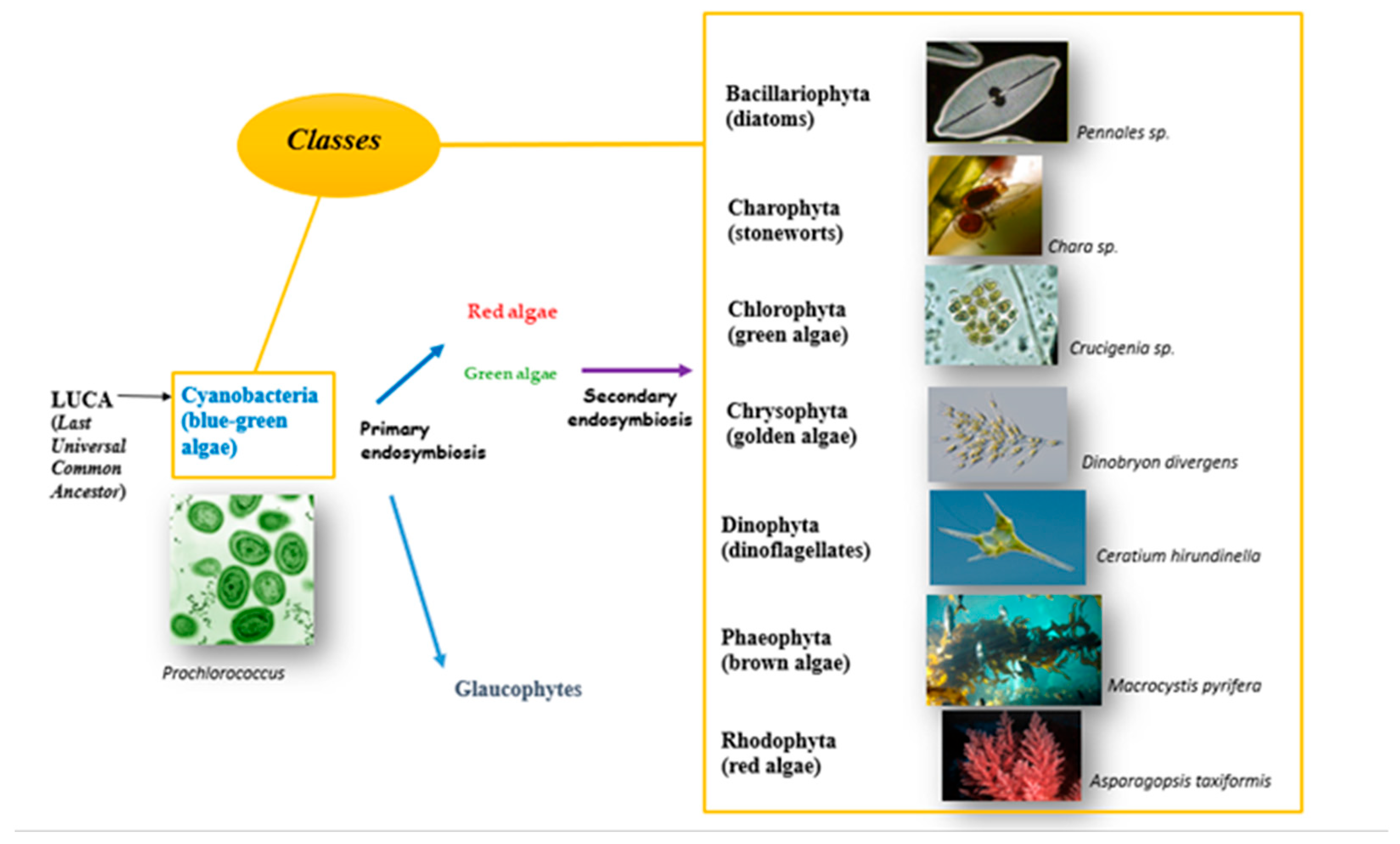
2.1. Evolution of Eukaryotic Photosynthetic Algae
2.2. Algal Taxonomy and Phylogeny
3. Cyanobacterial and Other Harmful Algae Blooms in Recreational Waters
3.1. Algae and Cyanobacteria in Spa and Thermal Spring Water Sources
3.2. Environmental Epidemiology and Ecotoxicity
3.3. Ecotoxicity of Algal Blooms and Related Toxins
3.4. Environmental Epidemiology of HAB, Algal, and Cyanobacterial Toxins
4. Clinical Epidemiology of HAB, Algal, and Cyanobacterial Toxins
5. Anti-Algae Treatments
5.1. Quaternary Ammonium Compounds (QACs)
5.2. Metallic Pool Algaecides
5.3. Algae Removal
6. Algae Detection Methods
7. Risk Assessment, Laws, and Water Safety Plans
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines on Recreational Water Quality: Volume 1 Coastal and Fresh Waters; WHO: Geneva, Switzerland, 2003; Available online: https://www.who.int/publications/i/item/9241545801 (accessed on 20 December 2023).
- Summers, E.J.; Ryder, J.L. A critical review of operational strategies for the management of harmful algal blooms (HABs) in inland reservoirs. J. Environ. Manag. 2023, 330, 117141. [Google Scholar] [CrossRef]
- Varga, C. To treat or not to treat? Misbeliefs in spa water disinfection. Int. J. Biometeorol. 2019, 63, 1135–1138. [Google Scholar] [CrossRef]
- Kiliç, S.; Kalkan, E.; Nadaroglu, H. Removal of algae from thermal mud pool: A case study in Koprukoy (Erzurum, Northeast (NE) Turkey) thermal spring area. Bull. Chem. Soc. Ethiop. 2022, 36, 545–553. [Google Scholar] [CrossRef]
- Xiao, F.; Xiao, P.; Wang, D. Influence of allochthonous organic matters on algae removal: Organic removal and floc characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123995. [Google Scholar] [CrossRef]
- Qi, J.; Lan, H.C.; Miao, S.Y.; Xu, Q.; Liu, R.; Liu, H.; Qu, J. KMnO4-Fe(II) pretreatment to enhance Microcystis aeruginosa removal by aluminum coagulation: Does it work after long distance transportation. Water Res. 2010, 44, 3617–3624. [Google Scholar]
- Gilmour, D.J. Diversity of algae and their biotechnological potential. Adv. Microb. Physiol. 2023, 82, 301–321. [Google Scholar]
- Cohen, P.A.; Kodner, R.B. The earliest history of eukaryotic life: Uncovering an evolutionary story through the integration of biological and geological data. Trends Ecol. Evol. 2022, 37, 246–256. [Google Scholar] [CrossRef]
- Butterfield, N.J. Bangiomorpha pubescens n. gen., n. sp.: Implication for the evolution of sex, multicellularity and the mesoproterozoic/neoproterozoic radiation of eukaryotes. J. Paleontol. 2011, 26, 386–404. [Google Scholar]
- Brocks, J.J. The transition from a cyanobacterial to alga world and the emergence of animals. Emerg. Top. Life Sci. 2018, 2, 181–190. [Google Scholar] [PubMed]
- Adl, S.M.; Simpson, A.G.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–493. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.J. What is aquatic botany?—And why algae are plants: The importance of non-taxonomic terms for groups of organisms. Aquat. Bot. 2016, 132, 1–4. [Google Scholar] [CrossRef]
- Ponce-Toledo, R.I.; Lopez-Garcia, P.; Moreira, D. Horizontal and endosymbiotic gene transfer in early plastid evolution. New Phytol. 2019, 224, 618–624. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The kingdom chromista: Origin and systematics. Prog. Phycol. Res. 1986, 4, 309–347. [Google Scholar]
- Javed, M.R.; Bilal, M.J.; Mehmood, M.A.; Nashat, N. Microalgae as a Feedstock for Biofuel Production: Current Status and Future Prospects. Energy Res. Dev. 2019, 3, 1–39. [Google Scholar]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Nation Museum of Natural Hystory. The Classification. Available online: https://naturalhistory.si.edu/research/botany/research/algae/algae-classification (accessed on 20 December 2023).
- Barsani, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Fritsch, F.E. The Structure and Reproduction of Algae; Cambridge University Press: London, UK, 1935; Volume I. [Google Scholar]
- Ghosh, A.; Kiran, B. Carbon Concentration in Algae: Reducing CO2 From Exhaust Gas. Trends Biotechnol. 2017, 35, 806–808. [Google Scholar] [CrossRef]
- Ambika, H.D. Positive and negative environmental impacts on algae. In Algae Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 343–353. [Google Scholar]
- World Health Organization. Guidelines on Recreational Water Quality: Volume 1 Coastal and Fresh Waters; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240031302 (accessed on 21 March 2024).
- Centers for Disease Control and Prevention. Avoid Harmful Algae and Cyanobacteria. 2023. Available online: https://www.cdc.gov/habs/be-aware-habs.html (accessed on 7 November 2023).
- Dallas, H. Water temperature and riverine ecosystems: An overview of knowledge and approaches for assessing biotic responses, with special reference to South Africa. Water SA 2008, 34, 393–404. [Google Scholar] [CrossRef]
- Sompong, U.; Hawkins, P.R.; Besley, C.; Peerapornpisal, Y. The distribution of cyanobacteria across physical and chemical gradients in hot springs in northern Thailand. FEMS Microbiol. Ecol. 2005, 52, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, M. The distribution of algae and insects in hot spring thermal gradients at Waimangu, New Zealand. N. Z. J. Mar. Freshw. Res. 1969, 3, 459–465. [Google Scholar] [CrossRef]
- Zhang, Y.; Whalen, J.K.; Cai, C.; Shan, K.; Zhou, H. Cyanobacteria-diatom/dinoflagellate blooms and their cyanotoxins in freshwaters: A nonnegligible chronic health and ecological hazard. Water Res. 2023, 233, 119807. [Google Scholar] [CrossRef]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.-F. A review of current knowledge on toxic benthic freshwater cyanobacteria ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar]
- Gere, D.; Róka, E.; Záray, G.; Vargha, M. Disinfection of Therapeutic Spa Waters: Applicability of Sodium Hypochlorite and Hydrogen Peroxide-Based Disinfectants. Water 2022, 14, 690. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013; ISBN 9789241506090. [Google Scholar]
- Balegová, J. The mineral spa of Rudnok as described by staff colonel Henrik Mayer. Comm. Hist. Art. Med. 2008, 54, 87–92. [Google Scholar]
- World Health Organization. Guidelines for Safe Recreational Water Environments; WHO Press: Geneva, Switzerland, 2006; Volume 2, Available online: http://apps.who.int/iris/bitstream/10665/43336/1/9241546808_eng.pdf (accessed on 6 November 2023).
- Health and Safety Executive. The Control of Legionella and Other Infectious Agents in Spa-Pool Systems. 2014. Available online: www.hse.gov.uk/pubns/books/hsg282.htm (accessed on 6 November 2023).
- EU Council. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184&from=ES (accessed on 7 November 2023).
- Ministero della Salute. Decreto 7 Febbraio 2012, n. 25 Disposizioni Tecniche Concernenti Apparecchiature Finalizzate al Trattamento dell’Acqua Destinata al Consumo Umano. (12G0044) (GU Serie Generale n. 69 del 22 March 2012). 2012. Available online: https://shorturl.at/gDM35 (accessed on 5 November 2023).
- Regione Toscana. Legge Regionale 27 Luglio 2004, n. 38 Norme per la Disciplina della Ricerca, della Coltivazione e dell’Utilizzazione delle Acque Minerali, di Sorgente e Termali. (GU 3a Serie Speciale Regioni n.45 del 13 November 2004). 2004. Available online: https://www.edizionieuropee.it/law/html/208/to4_04_012.html (accessed on 5 November 2023).
- Regione Campania. Legge Regionale n. 8 del 29 Luglio 2008. “Disciplina della Ricerca ed Utilizzazione delle Acque Minerali e Termali, delle Risorse Geotermiche e delle Acque di Sorgente”. 2008. Available online: https://www.regione.campania.it/normativa/userFile/documents/attachments/1953_8-2008Storico.pdf (accessed on 5 November 2023).
- Sevillano, D.; Romero-Lasta, C.I.; Alou, L.; Gonzáles, N.; González, N.; Collado, L.; Domínguez, A.A.; Arias, C.M.; Corvillo, I.; Armijo, F.; et al. Impact of the biotic and abiotic components of low mineralized natural mineral waters on the growth of pathogenic bacteria of human origin: A key to self-control of spa water quality. J. Hydrol. 2018, 566, 227–234. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Romano Spica, V.; Gianfranceschi, G.; Valeriani, F. Untouchability of natural spa waters: Perspectives for treatments within a personalized water safety plan. Environ. Int. 2019, 133, 105095. [Google Scholar] [CrossRef]
- Valeriani, F.; Gianfranceschi, G.; Romano Spica, V. The microbiota as a candidate biomarker for SPA pools and SPA thermal spring stability after seismic events. Environ. Int. 2020, 137, 105595. [Google Scholar] [CrossRef]
- Giampaoli, S.; Valeriani, F.; Gianfranceschi, G.; Vitali, M.; Delfini, M.; Festa, M.; Bottari, E.; Romano Spica, V. Hydrogen sulfide in thermal spring waters and its action on bacteria of human origin. Microchem. J. 2013, 108, 210–214. [Google Scholar] [CrossRef]
- Vachee, A.; Vincent, P.; Struijk, C.B.; Mossel, D.A.A.; Leclerc, H. Study of the fate of the autochtonous bacterial flora of still mineral waters by analysis of restriction fragment length polymorphism of genes coding for rRNA. Syst. Appl. Microbiol. 1997, 20, 492–503. [Google Scholar] [CrossRef]
- Lu, J.; Struewing, I.; Vereen, E.; Kirby, A.; Levy, K.; Moe, C.; Ashbolt, N. Molecular detection of Legionella spp. and their associations with Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in a drinking water distribution system. J. Appl. Microbiol. 2016, 120, 509–521. [Google Scholar] [CrossRef]
- Giorgio, A.; Carraturo, F.; Aliberti, F.; De Bonis, S.; Libralato, G.; Morra, M.; Guida, M. Characterization of microflora composition and antimicrobial activity of algal extracts from Italian thermal muds. J. Nat. Sci. Biol. Med. 2018, 9, 150–158. [Google Scholar]
- Kalkan, E. Algae potential of the Delicermik thermal spring area (Köprüköy-Erzurum, NE Turkey). Int. J. Lat. Technol. Eng. Manag. Appl. Sci. 2019, 8, 98–101. [Google Scholar]
- Pabuccu, K. A Research on Köprüköy-Deliçermik Algal Flora. Master’s Thesis, Graduate School of Natural and Applied Sciences, Atatürk University, Erzurum, Turkey, 1993; p. 87. [Google Scholar]
- Gerwick, L.; Gerwick, W.H.; Coates, R.C.; Engene, N.; Grindberg, R.V.; Jones, A.C.; Sorrels, C.M. Giant marine cyanobacteria produce exciting potential pharmaceuticals. Microbe 2008, 3, 277–284. [Google Scholar] [CrossRef]
- Sivonen, K.; Börner, T. Bioactive compounds produced by cyanobacteria. In The Cyanobacteria: Molecular Biology, Genomics and Evolution; Caister Academic Press: Norfolk, UK, 2008; p. 15997. [Google Scholar]
- Costa, M.; Garcia, M.; Costa Rodrigues, J.; Costa, M.S.; Ribeiro, M.J.; Fernandes, M.H.; Barros, P.; Barreiro, A.; Vasconcelos, V.; Martins, R. Exploring bioactive properties of marine cyanobacteria isolated from the Portuguese coast: High potential as a source of anticancer compounds. Mar. Drugs 2013, 12, 98–114. [Google Scholar] [CrossRef]
- Surakka, A.; Sihvonen, L.M.; Lehtimäki, J.M.; Wahlsten, M.; Vuorela, P.; Sivonen, K. Benthic cyanobacteria from the Baltic sea contain cytotoxic Anabaena, Nodularia, and Nostoc strains and a apoptosis inducing phormidium strain. Environ. Toxicol. 2005, 20, 285–292. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel extracellular diterpenoids with biological activity from the Cyanobacterium nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Metera, K.L.; Kumar, J.R.; Watson, L.V.; Girouard, G.S.; Guan, Y.; Carr, R.I.; Barrow, C.J.; Ewart, H.S. Immunostimulatory principles from Chlorella pyrenoidosa–Part 1: Isolation and biological assessment in vitro. Phytomedicine 2007, 14, 57–64. [Google Scholar] [CrossRef]
- Komatsu, T.; Kido, N.; Sugiyama, T.; Yokochi, T. Antiviral activity of acidic polysaccharides from Coccomyxa gloeobotrydiformi, a green alga, against an in vitro human influenza A virus infection. Immunopharmacol. Immunotoxicol. 2013, 35, 1–7. [Google Scholar] [CrossRef]
- Kust, A.; Urajová, P.; Hrouzek, P.; Čapková, K.; Štenclová, L.; Řeháková, K.; Kozlíková-Zapomělová, E.; Lepšová-Skácelová, O.; Lukešová, A.; Mareš, J. A new microcystin producing Nostoc strain discovered in broad toxicological screening of non-planktic Nostocaceae (cyanobacteria). Toxicon 2018, 150, 66–73. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Otero, P.; Alfonso, A.; Ramos, V.; Vasconcelos, V.; Aráoz, R.; Molgó, J.; Vieytes, M.R.; Botana, L.M. Detection of anatoxin-a and three analogs in Anabaena spp. cultures: New fluorescence polarization assay and toxin profile by LC-MS/MS. Toxins 2014, 6, 402–415. [Google Scholar] [CrossRef]
- Stoyneva, M. Survey on green algae of Bulgarian thermal springs. Biol. Bratisl. Sect. Bot. 2003, 58, 563–574. [Google Scholar]
- Lai, G.G.; Wetzel, C.E.; Ector, L.; Lugliè, A.; Padedda, B.M. Composition, structure, and distribution of diatom assemblages in Mediterranean thermal spring ecotones affected by natural and human disturbances. Aquat. Sci. 2023, 85, 55. [Google Scholar] [CrossRef]
- Ulcay, S.; Öztürk, M.; Kurt, O. Algae Flora of Germencik-Alangüllü (Aydın, Turkey) Thermal Water. Celal Bayar Univ. J. Sci. 2017, 13, 601–608. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Süßwasserflora von Mitteleuropa. In Cyanoprokaryota, 2nd Part: Oscillatoriales; Springer: Berlin/Heidelberg, Germany, 2005; Volume 19, p. 759. [Google Scholar]
- Veen, A.; Hof, C.H.J.; Kouwets, F.A.C.; Berkhout, T. Rijkswaterstaat Waterdienst, Informatiehuis Water [Taxa Watermanagement the Netherlands (TWN)]. Available online: http://ipt.nlbif.nl/ipt/resource?r=checklist-twn (accessed on 7 November 2023).
- Anagnostidis, K. Untersuchungen über Die Cyanophyceen Einiger Thermen in Griechenland; Institute Systematic Botany & Pflanzengeorge University: Thessaloniki, Greece, 1961; Volume 7, pp. 1–322. [Google Scholar]
- Shanab, M.M.A. Algal flora of Ain Helwan, I. Algae of the Worm Spring. Egypt. J. Phycol. 2006, 7, 209–231. [Google Scholar] [CrossRef]
- Gupta, R.K. Mini review: Thermal algae of India with special reference to Jharkhand. Biospectra 2017, 12, 15–20. [Google Scholar]
- Varga, C. Water hygiene and toxicology: Actual problems and new research trends in Hungary. Acta Biol. Debr. Oecol. Hung. 2012, 29, 9–120. [Google Scholar]
- Antunes, J.T.; Leao, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Kurmayer, R.; Azevedo, S.M.F.O.; Wood, S.A.; Chorus, I.; Welker, M. Understanding the occurrence of cyanobacteria and cyanotoxins. In Toxic Cyanobacteria in Water; CRC Press: Boca Raton, FL, USA, 2021; pp. 213–294. [Google Scholar]
- Centers for Disease Control and Prevention. Harmful Algal Bloom (HAB) Associated Illness. 27 May 2016. Available online: http://www.cdc.gov/habs/general.html (accessed on 7 November 2023).
- Environmental Protection Agency. Learn about Cyanobacteria and Cyanotoxins. 1 August 2022. Available online: https://www.epa.gov/cyanohabs/learn-aboutcyanobacteria-and-cyanotoxins (accessed on 13 November 2023).
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water—A Guide io Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Chatterjee, S.; More, M. Cyanobacterial Harmful Algal Bloom Toxin Microcystin and Increased Vibrio Occurrence as Climate-Change-Induced Biological Co-Stressors: Exposure and Disease Outcomes via Their Interaction with Gut-Liver-Brain Axis. Toxins 2023, 15, 2. [Google Scholar] [CrossRef]
- Olker, J.; Banerji, A.; Benesh, K.; Kinziger, B.; Scott, T.; Karschnik, T.; Frisch, J.; Feist, T.; Pilli, A.; Hoff, D. Evidence Map for Ecological Toxicity of Cyanotoxins Using ECOTOXicology Knowledgebase Systematic Protocols; Science Inventory: Nashville, TN, USA, 2023. [Google Scholar]
- Mehinto, A.C.; Smith, J.; Wenger, E.; Stanton, B.; Linville, R.; Brooks, B.W.; Sutula, M.A.; Howard, M.D.A. Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats–Evaluating the basis for developing thresholds protective of aquatic life in the United States. Sci. Total Environ. 2021, 795, 148864. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Poirier, D.G.; Almirall, X.O.; Bhavsar, S.P.; Sibley, P.K. Assessing the toxicity of cell-bound microcystins on freshwater pelagic and benthic invertebrates. Ecotoxicol. Environ. Saf. 2020, 188, 109945. [Google Scholar] [CrossRef]
- Smutná, M.; Babica, P.; Jarque, S.; Hilscherová, K.; Maršálek, B.; Haeba, M.; Bláha, L. Acute, chronic and reproductive toxicity of complex cyanobacterial blooms in Daphnia magna and the role of microcystins. Toxicon 2014, 79, 11–18. [Google Scholar] [CrossRef]
- Davis, T.W.; Koch, F.; Marcoval, M.A.; Wilhelm, S.W.; Gobler, C.J. Mesozooplankton and microzooplankton grazing during cyanobacterial blooms in the western basin of Lake Erie. Harmful Algae 2020, 15, 26–35. [Google Scholar] [CrossRef]
- Palíková, M.; Krejcí, R.; Hilscherová, K.; Babica, P.; Navrátil, S.; Kopp, R.; Bláha, L. Effect of different cyanobacterial biomasses and their fractions with variable microcystin content on embryonal development of carp (Cyprinus carpio L.). Aquat. Toxicol. 2007, 81, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Kasianchuk, N.; Siemens, E.; Henao, E.; Rzymski, P. A Review of Common Cyanotoxins and Their Effects on Fish. Toxics 2023, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Liu, W.X.; Wen, C.G.; Qian, G.M.; Hu, B.Q.; Jian, S.Q.; Yang, G.; Dong, J. Effect of microcystin on the expression of Nrf2 and its downstream antioxidant genes from Cristaria plicata. Aquat. Toxicol. 2020, 225, 105526. [Google Scholar] [CrossRef]
- Lundqvist, J.; Pekar, H.; Oskarsson, A. Microcystins activate nuclear factor erythroid 2-related factor 2 (Nrf2) in human liver cells in vitro—Implications for an oxidative stress induction by microcystins. Toxicon 2017, 126, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.R.; Seawright, A.A.; Moore, M.R.; Lam, P.K.S. Cylindrospermopsin, a cyanobacterial alkaloid: Evaluation of its toxicological activity. Ther. Drug Monit. 2000, 22, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Jiang, H.; Yuen, C.N.T.; Wang, W.; He, J.; Zhang, H.; Liu, G.; Wei, L.; Chen, L.; Wu, H. Microcystin-leucine arginine induces skin barrier damage and reduces resistance to pathogenic bacteria in Lithobates catesbeianus tadpoles. Ecotoxicol. Environ. Saf. 2022, 238, 113584. [Google Scholar] [CrossRef]
- Xia, H.; Song, T.; Wang, L.; Jiang, L.; Zhou, Q.; Wang, W.; Liu, L.; Yang, P.; Zhang, X. Effects of dietary toxic cyanobacteria and ammonia exposure on immune function of blunt snout bream (Megalabrama amblycephala). Fish Shellfish Immun. 2018, 78, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Liang, H.; Zhang, X. Effect of cyanobacteria on immune function of crucian carp (Carassius auratus) via chronic exposure in diet. Chemosphere 2013, 90, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Patiño, R.; Christensen, V.G.; Graham, J.L.; Rogosch, J.S.; Rosen, B.H. Toxic Algae in Inland Waters of the Conterminous United States—A Review and Synthesis. Water 2023, 15, 2808. [Google Scholar] [CrossRef]
- Rattner, B.A.; Wazniak, C.E.; Lankton, J.S.; McGowan, P.C.; Drovetski, S.V.; Egerton, T.A. Review of harmful algal bloom effects on birds with implications for avian wildlife in the Chesapeake Bay region. Harmful Algae 2022, 120, 102319. [Google Scholar] [CrossRef]
- European Parliament. Water Protection and Management. Available online: https://www.europarl.europa.eu/factsheets/en/sheet/74/water-protection-and-management (accessed on 13 November 2023).
- Li, J.; Parkefelt, L.; Persson, K.M.; Pekar, H. Improving cyanobacteria and cyanotoxin monitoring in surface waters for drinking water supply. J. Water Secur. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal Bloom and Its Economic Impact; JRC Technical Reports; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- IARC. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Hurtado, I.; Pouget, L.; Fernández, S.; Cascales, P. Monitoring and forecasting cyanobacteria risk for a drinking water plant in Spain. Water Supply 2022, 22, 6296–6307. [Google Scholar] [CrossRef]
- Lim, C.C.; Yoon, J.; Reynolds, K.; Gerald, L.B.; Ault, A.P.; Heo, S.; Bell, M.L. Harmful algal bloom aerosols and human health. EBioMedicine 2023, 93, 104604. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Summary Report—One Health Harmful Algal Bloom System (OHHABS), United States, 2021; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/habs/data/2021-ohhabs-data-summary.html (accessed on 11 November 2023).
- Harmful Algae Event Database. HAEDAT. IOC-ICES-PICES. 2023. Available online: http://haedat.iode.org/ (accessed on 13 November 2023).
- Tito, J.C.R.; Luna, L.M.G.; Noppe, W.N.; Hubert, I.A. First Report on Microcystin-LR Occurrence in Water Reservoirs of Eastern Cuba, and Environmental Trigger Factors. Toxins 2022, 14, 209. [Google Scholar] [CrossRef]
- Oliveira, E.D.C.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First Detection of Microcystin-LR in the Amazon River at the Drinking Water Treatment Plant of the Municipality of Macapa, Brazil. Toxins 2019, 11, 669. [Google Scholar] [CrossRef]
- Chatziefthimiou, A.D.; Metcalf, J.S.; Glover, W.B.; Banack, S.A.; Dargham, S.R.; Richer, R.A. Cyanobacteria and cyanotoxins are present in drinking water impoundments and groundwater wells in desert environments. Toxicon 2016, 114, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Douma, M.; Ouahid, Y.; del Campo, F.F.; Loudiki, M.; Mouhri, K.; Oudra, B. Identification and quantification of cyanobacterial toxins (microcystins) in two Moroccan drinking-water reservoirs (Mansour Eddahbi, Almassira). Environ. Monit. Assess. 2010, 160, 439–450. [Google Scholar] [CrossRef]
- Bruno, M.; Gucci, P.M.B.; Pierdominici, E.; Sestili, P.; Joppolo, A.; Volterra, L. Anatoxin-a and previously unknown toxin in Anabaena planctonica from blooms found in Lake Mulargia (Italy). Toxicon 1994, 32, 369–373. [Google Scholar] [CrossRef]
- Bruno, M.; Gucci, P.M.B.; Pierdominici, E.; Sestili, P.; Joppolo, A.; Volterra, L. Production of microcystinlike toxins in different freshwater species of Oscillatoria. Toxicon 1992, 32, 1307–1311. [Google Scholar] [CrossRef]
- Messineo, V.; Bogialli, S.; Melchiorre, S.; Sechi, N.; Luglié, A.; Casiddu, P.; Mariani, M.A.; Padedda, B.M.; Di Corcia, A.; Mazza, R.; et al. Cyanobacterial toxins in Italian freshwaters. Limnologica 2009, 39, 95–106. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. Cyanobacteria in water for human consumption. In State of Knowledge for Risk Assessment; Lucentini, L., Ottaviani, M., Eds.; Rapporti ISTISAN 11/35 Pt. 1; Istituto Superiore di Sanità: Rome, Italy, 2011; Volume 1, xxii; 165p. [Google Scholar]
- European Union. S-3 EUROHAB Project. Sentinel-3 Satellite Products for Detecting Eutrophication and Harmful Algal Bloom Events in the French-English Channel. Official Project Website. 2023. Available online: https://www.s3eurohab.eu/ (accessed on 14 November 2023).
- Boopathi, T.; Ki, J.S. Impact of environmental factors on the regulation of cyanotoxin production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Weirich, C.A.; Miller, T.R. Freshwater harmful algal blooms: Toxins and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 2–24. [Google Scholar] [CrossRef]
- Humpage, A.R.; Falconer, I.R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environ. Toxicol. 2003, 18, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Astrachan, N.B.; Archer, B.G.; Hilbelink, D.R. Evaluation of the subacute toxicity and teratogenicity of anatoxin-a. Toxicon 1980, 18, 684–688. [Google Scholar] [CrossRef]
- Bumke-Vogt, C.; Mailahn, W.; Chorus, I. Anatoxin-a and neurotoxic cyanobacteria in German lakes and reservoirs. Environ. Toxicol. 1999, 14, 117–125. [Google Scholar] [CrossRef]
- van Apeldoorn, M.E.; van Egmond, H.P.; Speijers, G.J.; Bakker, G.J. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Strichartz, G. Structural determinants of the affinity of saxitoxin for neuronal sodium channels. Electrophysiological studies on frog peripheral nerve. J. Gen. Physiol. 1984, 84, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Holtcamp, W. The emerging science of BMAA: Do cyanobacteria contribute to neurodegenerative disease? Environ. Health Perspect. 2012, 120, A110–A116. [Google Scholar] [CrossRef]
- Jiang, L.; Kiselova, N.; Rosén, J.; Ilag, L.L. Quantification of neurotoxin BMAA (beta-N-methylamino-Lalanine) in seafood from Swedish markets. Sci. Rep. 2014, 4, 6931. [Google Scholar] [CrossRef]
- Torokne, A.; Palovics, A.; Bankine, M. Allergenic (sensitization, skin and eye irritation) effects of freshwater cyanobacteria—Experimental evidence. Environ. Toxicol. 2001, 16, 512–516. [Google Scholar] [CrossRef]
- Bláhová, L.; Adamovský, O.; Kubala, L.; Švihálková Šindlerová, L.; Zounková, R.; Bláha, L. The isolation and characterization of lipopolysaccharides from Microcystis aeruginosa, a prominent toxic water bloom forming cyanobacteria. Toxicon 2013, 76, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.T.; Webb, P.M.; Shaw, G.R. The toxins of Lyngbya majuscule and their human and ecological health effects. Environ. Int. 2001, 27, 381–392. [Google Scholar] [CrossRef]
- Arthur, K.; Limpus, C.; Balazs, G.; Capper, A.; Udy, J.; Shaw, G.; Keuper-Bennett, U.; Bennett, P. The exposure of green turtles (Chelonia mydas) to tumour promoting compounds produced by the cyanobacterium Lyngbya majuscula and their potential role in the aetiology of fibropapillomatosis. Harmful Algae 2008, 7, 114–125. [Google Scholar] [CrossRef]
- Churro, C.; Dias, E.; Valrio, E. Risk Assessment of Cyanobacteria and Cyanotoxins, the Particularities and Challenges of Planktothrix spp. Monitoring. In Novel Approaches and Their Applications in Risk Assessment; IntechOpen: London, UK, 2012. [Google Scholar]
- Simon, N.; Cras, A.L.; Foulon, E.; Lemée, R. Diversity and evolution of marine phytoplankton. Comptes Rendus Biol. 2009, 332, 159–170. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. Experimental immunologyFirst report of cylindrospermopsin effect on human peripheral blood lymphocytes proliferation in vitro. Cent. Eur. J. Immunol. 2012, 37, 314–317. [Google Scholar] [CrossRef]
- Kouakou, C.R.C.; Poder, T.G. Economic impact of harmful algal blooms on human health: A systematic review. J. Water Health 2019, 17, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health Glob. Access Sci. Source 2008, 7, S4. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human exposure to cyanotoxins and their effects on health. Arh. Hig. Rada Toksikol. 2013, 64, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ananya, K.A.; Iffat, A. Cyanobacteria “the blue green algae” and its novel applications: A brief review. Int. J. Innov. Appl. Stud. 2014, 7, 251–261. [Google Scholar]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef]
- Codd, G.A.; Steffensen, D.A. Toxic blooms of cyanobacteria in Lake Alexandrina, South Australia–Learning from history. Mar. Freshw. Res. 1994, 45, 731–736. [Google Scholar] [CrossRef]
- Dillenberg, H.O.; Dehnel, M.K. Toxic waterbloom in Saskatchewan, 1959. Can. Med. Assoc. J. 1960, 83, 1151–1154. [Google Scholar]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 2003, 18, 78–93. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Runnegar, M.T. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, B.; Barlow, T. Amnesic shellfish poison. Food Chem. Toxicol. 2004, 42, 545–557. [Google Scholar] [CrossRef]
- Teixeira, M.D.G.; Costa, M.D.C. Gastroenteritis epidemic in the area of the Itaparica Dam, Bahia, Brazil. Bull. Pan Am. Health Organ. 1993, 27, 244–253. [Google Scholar]
- Azevedo, S.M.; Carmichael, W.W. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 441–446, 181–182. [Google Scholar] [CrossRef]
- Backer, L.C.; Fleming, L.E. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae 2003, 2, 19–28. [Google Scholar] [CrossRef]
- Stewart, I.; Webb, P.M. Epidemiology of recreational exposure to freshwater cyanobacteria—An international prospective cohort study. BMC Public Health 2006, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.A.; Fleming, L.E. Florida Red Tide Toxins (Brevetoxins) and Longitudinal Respiratory Effects in Asthmatics. Harmful Algae 2011, 10, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Fleming, L.E. Aerosolized red tide toxins (brevetoxins) and asthma: Continued health effects after 1 h beach exposure. Harmful Algae 2011, 10, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Ma, J.; Cao, J.; McCarron, P. Toxins in mussels (Mytilus galloprovincialis) associated with diarrhetic shellfish poisoning episodes in China. Toxicon 2012, 60, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, L.; Sedan, D. An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, B.; Gervais, M.C. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci. Total Environ. 2014, 1, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Trevino-Garrison, I.; DeMent, J. Human illnesses and animal deaths associated with freshwater harmful algal blooms-Kansas. Toxins 2015, 7, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Pavaux, A. Environmental, human health and socioeconomic impacts of Ostreopsis spp. Blooms in the NW Mediterranean. Harmful Algae 2022, 119, 102320. [Google Scholar] [CrossRef] [PubMed]
- Veldee, M.V. An Epidemiological Study of Suspected Water-borne Gastroenteritis. Am. J. Public Health Nations Health 1931, 21, 1227–1235. [Google Scholar] [CrossRef]
- Backer, L.C.; Kirkpatrick, B. Occupational exposure to aerosolized brevetoxins during Florida red tide events: Effects on a healthy worker population. Environ. Health Perspect. 2005, 113, 644–649. [Google Scholar] [CrossRef]
- Lee, J.; Woo, S.Y.; Kim, Y.W.; Kim, S.J.; Pyo, J.; Cho, K.H. Dynamic calibration of phytoplankton blooms using the modified SWAT model. J. Clean. Prod. 2022, 343, 131005. [Google Scholar] [CrossRef]
- Figgatt, M.; Muscatiello, N. Harmful Algal Bloom-Associated Illness Surveillance: Lessons From Reported Hospital Visits in New York, 2008–2014. Am. J. Public Health 2016, 106, 440–442. [Google Scholar] [CrossRef]
- Wu, J.; Hilborn, E.D. Acute health effects associated with satellite-determined cyanobacterial blooms in a drinking water source in Massachusetts. Environ. Health 2021, 20, 83. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Roberts, V.A. Centers for Disease Control and Prevention (CDC). Algal bloom-associated disease outbreaks among users of freshwater lakes–United States, 2009–2010. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 11–15. [Google Scholar]
- Roberts, V.A.; Vigar, M.; Backer, L.; Veytsel, G.E.; Hilborn, E.D.; Hamelin, E.I.; Vanden Esschert, K.L.; Lively, J.Y.; Cope, J.R.; Hlavsa, M.C.; et al. Surveillance for Harmful Algal Bloom Events and Associated Human and Animal Illnesses—One Health Harmful Algal Bloom System, United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1889–1894. [Google Scholar] [CrossRef]
- Backer, L.C.; Carmichael, W. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef]
- Backer, L.C.; McNeel, S.V. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, R.P.; Li, Y. Quantifying Karenia brevis bloom severity and respiratory irritation impact along the shoreline of Southwest Florida. PLoS ONE 2022, 17, e0260755. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.E.; Friedman, M.A. Neurological illnesses associated with Florida red tide (Karenia brevis) blooms. Harmful Algae 2019, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemée, R.; Kantin, R.; Lasalle, J.L.; Drouet, G.; Rambaud, L.; Malfait, P.; et al. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean Sea: Experience of the French Mediterranean coast surveillance network from 2006 to 2009. Clin. Toxicol. 2010, 48, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Lavery, A.; Backer, L.; Daniel, J. Evaluation of Electronic Health Records to Monitor Illness from Harmful Algal Bloom Exposure in the United States. J. Environ. Health 2021, 839, 8–14. [Google Scholar] [PubMed]
- Abdullah, L.; Ferguson, S.; Niedospial, D.; Patterson, D.; Oberlin, S.; Nkiliza, A.; Bartenfelder, G.; Hahn-Townsend, C.; Parks, M.; Crawford, F.; et al. Exposure-response relationship between K. brevis blooms and reporting of upper respiratory and neurotoxin-associated symptoms. Harmful Algae 2022, 117, 102286. [Google Scholar] [CrossRef] [PubMed]
- Mchau, G.J.; Makule, E.; Machunda, R.; Gong, Y.Y.; Kimanya, M. Harmful algal bloom and associated health risks among users of Lake Victoria freshwater: Ukerewe Island, Tanzania. J. Water Health 2019, 17, 826–836. [Google Scholar] [CrossRef]
- Vila, M.; Abós-Herràndiz, R.; Isern-Fontanet, J.; Alvarez, J.; Berdalet, E. Establishing the link between Ostreopsis cf. Ovata blooms and human health impacts using ecology and epidemiology. Sci. Mar. 2016, 80, 107–115. [Google Scholar] [CrossRef]
- Lavery, A.M.; Backer, L.C.; Roberts, V.A.; DeVies, J.; Daniel, J. Evaluation of Syndromic Surveillance Data for Studying Harmful Algal Bloom-Associated Illnesses–United States, 2017–2019. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1191–1194. [Google Scholar] [CrossRef]
- Illoul, H.; Hernández, F.R.; Vila, M.; Adjas, N.; Younes, A.A.; Bournissa, M.; Koroghli, A.; Marouf, N.; Rabia, S.; Ameur, F.L.K. The genus Ostreopsis along the Algerian coastal waters (SW Mediterranean Sea) associated with a human respiratory intoxication episode. Cryptogam. Algol. 2012, 2, 209–216. [Google Scholar] [CrossRef]
- Figgatt, M.; Hyde, J.; Dziewulski, D.; Wiegert, E.; Kishbaugh, S.; Zelin, G.; Wilson, L. Harmful Algal Bloom-Associated Illnesses in Humans and Dogs Identified Through a Pilot Surveillance System–New York, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Dalpra, D.; Tamer, R.; Zaias, J.; Cheng, Y.S.; Pierce, R.; et al. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environ. Health Perspect. 2005, 113, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Bean, J.A.; Kirkpatrick, B.; Cheng, Y.S.; Pierce, R.; Naar, J.; Nierenberg, K.; Backer, L.C.; Wanner, A.; Reich, A.; et al. Exposure and effect assessment of aerosolized red tide toxins (brevetoxins) and asthma. Environ. Health Perspect. 2009, 117, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, P.; Jin, D.; Beet, A.; Kirkpatrick, B.; Reich, A.; Ullmann, S.; Fleming, L.E.; Kirkpatrick, G. The human health effects of Florida red tide (FRT) blooms: An expanded analysis. Environ. Int. 2014, 68, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, P.; Jin, D.; Polansky, L.Y.; Kirkpatrick, B.; Kirkpatrick, G.; Fleming, L.E.; Reich, A.; Watkins, S.M.; Ullmann, S.G.; Backer, L.C. The costs of respiratory illnesses arising from Florida gulf coast Karenia brevis blooms. Environ. Health Perspect. 2009, 117, 1239–1243. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fleming, L.E.; Backer, L.C.; Bean, J.A.; Tamer, R.; Kirkpatrick, G.; Kane, T.; Wanner, A.; Dalpra, D.; Reich, A.; et al. Environmental exposures to Florida red tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae 2006, 5, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Chomérat, N.; Antajan, E.; Auby, I.; Bilien, G.; Carpentier, L.; Casamajor, M.-N.d.; Ganthy, F.; Hervé, F.; Labadie, M.; Méteigner, C.; et al. First Characterization of Ostreopsis cf. ovata (Dinophyceae) and Detection of Ovatoxins during a Multispecific and Toxic Ostreopsis Bloom on French Atlantic Coast. Mar. Drugs 2022, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Bean, J.A.; Fleming, L.E.; Kirkpatrick, G.; Grief, L.; Nierenberg, K.; Reich, A.; Watkins, S.; Naar, J. Gastrointestinal Emergency Room Admissions and Florida Red Tide Blooms. Harmful Algae 2010, 9, 82–86. [Google Scholar] [CrossRef]
- Vila, M.; Masó, M.; Sampedro, N.; Illoul, H.; Arin, L.; Garcés, E.; Giacobbe, M.G.; Àlvarez, J.; Camp, J. The genus Ostreopsis in recreational waters of the Catalan Coast and Balearic Islands (NW Mediterranean Sea): Is this the origin of human respiratory difficulties? In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; UNESCO: Copenhagen, Denmark, 2008; pp. 334–336. [Google Scholar]
- Gallitelli, M.; Ungaro, N.; Addante, L.M.; Procacci, V.; Silveri, N.G.; Sabbà, C. Respiratory illness as a reaction to tropical algal blooms occurring in a temperate climate. JAMA 2005, 293, 2599–2600. [Google Scholar]
- Durando, P.; Ansaldi, F.; Oreste, P.; Moscatelli, P.; Marensi, L.; Grillo, C.; Gasparini, R.; Icardi, G.; Collaborative Group for the Ligurian Syndromic Algal Surveillance. Ostreopsis ovata and human health: Epidemiological and clinical features of respiratory syndrome outbreaks from a two-year syndromic surveillance, 2005–2006, in north-west Italy. Eurosurveillance 2007, 12, E070607. [Google Scholar]
- Milian, A.; Nierenberg, K.; Fleming, L.E.; Bean, J.A.; Wanner, A.; Reich, A.; Backer, L.C.; Jayroe, D.; Kirkpatrick, B. Reported respiratory symptom intensity in asthmatics during exposure to aerosolized Florida red tide toxins. J. Asthma Off. J. Assoc. Care Asthma 2007, 44, 583–587. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Bean, J.A.; Wanner, A.; Reich, A.; Zaias, J.; Cheng, Y.S.; Pierce, R.; Naar, J.; et al. Aerosolized red-tide toxins (brevetoxins) and asthma. Chest 2007, 131, 187–194. [Google Scholar] [CrossRef]
- Todd, E.C.D. Preliminary estimates of costs of foodborne disease in the United States. J. Food Prot. 1989, 52, 595–601. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Carter, R.A.A.; Joll, C.A. Occurrence and formation of disinfection by-products in the swimming pool environment: A critical review. J. Environ. Sci. 2017, 58, 19–50. [Google Scholar] [CrossRef]
- Heilgeist, S.; Sahin, O.; Sekine, R.; Stewart, R.A. Mapping the Complex Journey of Swimming Pool Contaminants: A Multi-Method Systems Approach. Water 2022, 14, 2062. [Google Scholar] [CrossRef]
- PWTAG–Pool Water Treatment Advisory Group. Swimming Pool Water: Treatment and Quality Standards for Pools and Spas; BC Publications: Victoria, BC, Canada, 2009; Available online: https://www.pwtag.org/standards/ (accessed on 13 November 2023).
- Bélanger, A.S.; Brouard, J.S.; Charlebois, P.; Otis, C.; Lemieux, C.; Turmel, M. Distinctive architecture of the chloroplast genome in the chlorophycean green alga Stigeoclonium helveticum. Mol. Genet. Genom. MGG 2006, 276, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Martić, A.; Čižmek, L.; Ul’yanovskii, N.V.; Paradžik, T.; Perković, L.; Matijević, G.; Vujović, T.; Baković, M.; Babić, S.; Kosyakov, D.S.; et al. Intra-Species Variations of Bioactive Compounds of Two Dictyota Species from the Adriatic Sea: Antioxidant, Antimicrobial, Dermatological, Dietary, and Neuroprotective Potential. Antioxidants 2023, 12, 857. [Google Scholar] [CrossRef]
- Data Bridge Market Research. Global Algae Products Market–Industry Trends and Forecast to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-algae-products-market (accessed on 27 February 2024).
- Bures, F. Quaternary ammonium compounds: Simple in structure, complex in application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Zhang, H.; Wang, N.; Ma, B.; Liu, X.; Niu, L.; Yang, F.; Xu, Y.; Zhang, X. Effects of copper sulfate algaecide on the cell growth, physiological characteristics, the metabolic activity of Microcystis aeruginosa and raw water application. J. Hazard. Mater. 2023, 445, 130604. [Google Scholar] [CrossRef] [PubMed]
- Juergensen, L.; Busnarda, J.; Caux, P.Y.; Kent, R.A. Fate, behavior, and aquatic toxicity of the fungicide DDAC in the Canadian environment. Environ. Toxicol. 2000, 15, 174–200. [Google Scholar] [CrossRef]
- Frank, T. Reregistration Eligibility Decision for Alkyl Dimethyl Benzyl Ammonium Chloride (ADBAC); Report; US Environmental Protection Agency Office of Prevention, Pesticides, and Toxic Substances: Washington, DC, USA, 2006; pp. 10–44. [Google Scholar]
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Alampi, R.; Vazzana, I.; Felice, M.R. Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat. Toxicol. 2006, 180, 258–265. [Google Scholar] [CrossRef]
- Michalek, K.; Ventura, A.; Sanders, T. Mytilus hybridisation and impact on aquaculture: A minireview. Mar. Genom. 2016, 27, 3–7. [Google Scholar] [CrossRef]
- Moore, G.T.; Kellerman, K.F. U.S. Department of Agriculture, Bureau of Plant Industry—Bulletin No. 76; Leopold Classic Library: Amsterdam, The Netherlands, 1994; Volume 64, p. 904. [Google Scholar]
- Bishop, W.M.; Johnson, B.M.; Rodgers, J.H., Jr. Comparative responses of target and nontarget species to exposures of a copper-based algaecide. J. Aquat. Plant Manag. 2014, 52, 65–70. [Google Scholar]
- Kang, L.; Mucci, M.; Fang, J.; Lürling, M. New is not always better: Toxicity of novel copper based algaecides to Daphnia magna. Ecotoxicol. Environ. Saf. 2022, 241, 113817. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, X.; Ren, B.; Zhang, Z.; Deng, X.; Yin, W.; Yang, S. Algae removal characteristics of the ultrasonic radiation enhanced drinking water treatment process. J. Water Process Eng. 2023, 55, 104154. [Google Scholar] [CrossRef]
- Sheng, D.; Bu, L.; Zhu, S.; Wu, R.; Shi, Z.; Zhou, S. Pre-oxidation coupled with charged covalent organic framework membranes for highly efficient removal of organic chloramines precursors in algae-containing water treatment. Chemosphere 2023, 333, 138982. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, P.; Zhao, S.; Kang, S.; Wang, P.; Zhou, M.; Lyu, J. Control and remediation methods for eutrophic lakes in the past 30 years. Water Sci. Technol. 2020, 81, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, V.; Commenges, D.; Jacqmin-Gadda, H.; Dartigues, J.F. Relation between aluminum concentrations in drinking water and Alzheimer’s disease: An 8-year follow-up study. Am. J. Epidemiol. 2000, 152, 59–66. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Tazart, Z.; Enaime, G.; Douma, M.; Ounas, A.; Loudiki, M. Nature-based coagulants for drinking water treatment: An ecotoxicological overview. Water Environ. Res. 2022, 94, e10782. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Libralato, G.; Douma, M.; Ounas, A.; Yaacoubi, A.; Lofrano, G.; Loudiki, M. A review of plant-based coagulants for turbidity and cyanobacteria blooms removal. Environ. Sci. Pollut. Res. 2022, 29, 42601–42615. [Google Scholar] [CrossRef]
- El Bouaidi, W.; Essalhi, S.; Douma, M.; Tazart, Z.; Ounas, A.; Enaime, G.; Yaacoubi, A.; Loudiki, M. Evaluation of the potentiality of Vicia faba and Opuntia ficus indica as eco-friendly coagulants to mitigate Microcystis aeruginosa blooms. Desalin. Water Treat. 2020, 196, 198–213. [Google Scholar] [CrossRef]
- Ma, J.; Jia, B.; Li, S.; Kong, Y.; Nie, Y.; Zhang, H.; Gao, T. Enhanced coagulation of covalent composite coagulant with potassium permanganate oxidation for algae laden water treatment: Algae and extracellular organic matter removal. Chem. Eng. J. Adv. 2023, 13, 100427. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, Y.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, L.; Ren, L. Photo-Fenton self-cleaning carbon fibers membrane supported with Zr-MOF@ Fe2O3 for effective phosphate removal from algae-rich water. Chemosphere 2023, 323, 138175. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, D.; Chang, Z.; Li, R.; Meng, S. Phosphorus removal from water by the metal-organic frameworks (MOFs)-based adsorbents: A review for structure, mechanism, and current progress. Environ. Res. 2024, 243, 117816. [Google Scholar] [CrossRef]
- Barkallah, M.; Elleuch, J.; Smith, K.F.; Chaari, S.; Ben Neila, I.; Fendri, I.; Michaud, P.; Abdelkafi, S. Development and application of a real-time PCR assay for the sensitive detection of diarrheic toxin producer Prorocentrum lima. J. Microbiol. Meth. 2020, 178, 106081. [Google Scholar] [CrossRef] [PubMed]
- Chin Chwan Chuong, J.J.; Rahman, M.; Ibrahim, N.; Heng, L.Y.; Tan, L.L.; Ahmad, A. Harmful Microalgae Detection: Biosensors versus Some Conventional Methods. Sensors 2022, 22, 3144. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, C.; Wang, Y.; Chen, G. A review of the current and emerging detection methods of marine harmful microalgae. Sci. Total Environ. 2022, 815, 152913. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y.; Kim, M.; Lee, W.H. A novel method for cell counting of microcystis colonies in water resources using a digital imaging flow cytometer and microscope. Environ. Eng. Res. 2019, 24, 397–403. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto António, D.; Loo, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; Joint Research Centre (JRC) Technical Reports; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Ghosh, P.; Mukherji, S. Fate, detection technologies and toxicity of heterocyclic PAHs in the aquatic and soil environments. Sci. Total Environ. 2023, 892, 164499. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zeng, L.H.; Ren, Z.H. Recent application of spectroscopy for the detection of microalgae life information: A review. Appl. Spectrosc. Rev. 2019, 55, 26–59. [Google Scholar] [CrossRef]
- Murdock, J.N.; Wetzel, D.L. FT-IR Microspectroscopy Enhances Biological and Ecological Analysis of Algae. Appl. Spectrosc. Rev. 2009, 44, 335–361. [Google Scholar] [CrossRef]
- Saleem, F.; Jiang, J.L.; Atrache, R.; Paschos, A.; Edge, T.A.; Schellhorn, H.E. Cyanobacterial Algal Bloom Monitoring: Molecular Methods and Technologies for Freshwater Ecosystems. Microorganisms 2023, 11, 851. [Google Scholar] [CrossRef]
- Marrone, B.L.; Banerjee, S.; Talapatra, A.; Gonzalez-Esquer, C.R.; Pilania, G. Toward a Predictive Understanding of Cyanobacterial Harmful Algal Blooms through AI Integration of Physical, Chemical, and Biological Data. ACS ES&T Water 2023, 4, 844–858. [Google Scholar]
- Jin, C.; Mesquita, M.M.F.; Deglint, J.L.; Emelko, M.B.; Wong, A. Quantification of Cyanobacterial Cells via a Novel Imaging-Driven Technique with an Integrated Fluorescence Signature. Sci. Rep. 2018, 8, 9055. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Determination of Cyanotoxins in Drinking and Ambient Freshwaters. Available online: https://www.epa.gov/cyanohabs/determination-cyanotoxins-drinking-and-ambient-freshwaters (accessed on 15 December 2023).
- Islam, M.S.; Aryasomayajula, A. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Pandur, Ž.; Dular, M.; Kostanjšek, R.; Stopar, D. Bacterial Cell Wall Material Properties Determine, E. coli Resistance to Sonolysis. Ultrason. Sonochem 2022, 83, 105919. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Audet, J. Current Techniques for Single-Cell Lysis. J. R. Soc. Interface 2008, 5, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Danaeifar, M. New Horizons in Developing Cell Lysis Methods: A Review. Biotechnol. Bioeng. 2022, 119, 3007–3021. [Google Scholar] [CrossRef]
- Dahlgren, K.K.; Gates, C.; Lee, T.; Cameron, J.C. Proximity-Based Proteomics Reveals the Thylakoid Lumen Proteome in the Cyanobacterium synechococcus sp. PCC 7002. Photosynth. Res. 2021, 147, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.K.; Evitt, N.H.; Swartz, J.R. Chemical Lysis of Cyanobacteria. J. Biol. Eng. 2015, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Vallaeys, T.; Hendrickx, L.; Natalie, L.; Wilmotte, A. An Efficient DNA Isolation Protocol for Filamentous Cyanobacteria of the Genus Arthrospira. J. Microbiol. Methods 2010, 80, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.S.; Chaffin, J.D.; DuFour, M.R.; Sherman, J.J.; Golnick, P.C.; Collier, C.D.; Nummer, S.A.; Margida, M.G. Quantifying and Reducing Uncertainty in Estimated Microcystin Concentrations from the ELISA Method. Environ. Sci. Technol. 2015, 49, 14221–14229. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.J.; Garthwaite, I.; Miles, C.O.; Ross, K.M.; Aggen, J.B.; Chamberlin, A.R.; Towers, N.R.; Dietrich, D.R. Congener-Independent Immunoassay for Microcystins and Nodularins. Environ. Sci. Technol. 2001, 35, 4849–4856. [Google Scholar] [CrossRef]
- Shoemaker, J.A.; Tettenhorst, D.R.; de la Cruz, A. Single Laboratory Validated Method for Determination of Microcystins and Nodularin in Ambient Freshwaters by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). Available online: https://www.epa.gov/sites/default/files/2017-11/documents/microcystin_method_for_ambient_water_nov_2017.pdf (accessed on 16 December 2023).
- Zaffiro, A.; Rosenblum, L.; Wendelken, S.C. Method 546: Determination of Total Microcystins and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/method-546-determination-total-microcystins-nodularins-drinking-water-ambient-water-adda-enzyme-linked-immunosorbent-assay.pdf (accessed on 16 December 2023).
- He, X.; Stanford, B.D.; Adams, C.; Rosenfeldt, E.J.; Wert, E.C. Varied Influence of Microcystin Structural Difference on ELISA Cross-Reactivity and Chlorination Efficiency of Congener Mixtures. Water Res. 2017, 126, 515–523. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Holland, P.; Sprosen, J. Method for Detecting Classes of Microcystins by Combination of Protein Phosphatase Inhibition Assay and ELISA: Comparison with LC-MS. Toxicon 2005, 45, 199–206. [Google Scholar] [CrossRef]
- Greer, B.; McNamee, S.E.; Boots, B.; Cimarelli, L.; Guillebault, D.; Helmi, K.; Marcheggiani, S.; Panaiotov, S.; Breitenbach, U.; Akçaalan, R.; et al. A Validated UPLC-MS/MS Method for the Surveillance of Ten Aquatic Biotoxins in European Brackish and Freshwater Systems. Harmful Algae 2016, 55, 31–40. [Google Scholar] [CrossRef]
- Coskun, O. Separation Techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156–160. [Google Scholar]
- Kumar, P.; Rautela, A.; Kesari, V.; Szlag, D.; Westrick, J.; Kumar, S. Recent Developments in the Methods of Quantitative Analysis of Microcystins. J. Biochem. Mol. Toxicol. 2020, 34, e22582. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Barón-Sola, Á.; Campo, F.F.d.; Sanz-Alférez, S. Dynamics of Cylindrospermopsin Production and Toxin Gene Expression in Aphanizomenon ovalisporum. Adv. Microbiol. 2016, 6, 381–390. [Google Scholar] [CrossRef]
- Jungblut, A.-D.; Neilan, B.A. Molecular Identification and Evolution of the Cyclic Peptide Hepatotoxins, Microcystin and Nodularin, Synthetase Genes in Three Orders of Cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef]
- Ngwa, F.; Madramootoo, C.; Jabaji, S. Monitoring Toxigenic Microcystis Strains in the Missisquoi Bay, Quebec, by PCR Targeting Multiple Toxic Gene Loci. Environ. Toxicol. 2014, 29, 440–451. [Google Scholar] [CrossRef]
- Furukawa, K.; Noda, N.; Tsuneda, S.; Saito, T.; Itayama, T.; Inamori, Y. Highly Sensitive Real-Time PCR Assay for Quantification of Toxic Cyanobacteria Based on Microcystin Synthetase A Gene. J. Biosci. Bioeng. 2006, 102, 90–96. [Google Scholar] [CrossRef]
- Gupta, R.S.; Mathews, D.W. Signature Proteins for the Major Clades of Cyanobacteria. BMC Evol. Biol. 2010, 10, 24. [Google Scholar] [CrossRef]
- Gupta, R.S. Impact of Genomics on Clarifying the Evolutionary Relationships amongst Mycobacteria: Identification of Molecular Signatures Specific for the Tuberculosis-Complex of Bacteria with Potential Applications for Novel Diagnostics and Therapeutics. High-Throughput 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, C.; Struewing, I.; Li, X.; Allen, J.; Lu, J. Cyanotoxin-Encoding Genes as Powerful Predictors of Cyanotoxin Production during Harmful Cyanobacterial Blooms in an Inland Freshwater Lake: Evaluating a Novel Early-Warning System. Sci. Total Environ. 2022, 830, 154568. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Struewing, I.; Wymer, L.; Tettenhorst, D.R.; Shoemaker, J.; Allen, J. Use of qPCR and RT-qPCR for Monitoring Variations of Microcystin Producers and as an Early Warning System to Predict Toxin Production in an Ohio Inland Lake. Water Res. 2020, 170, 115262. [Google Scholar] [CrossRef] [PubMed]
- Crevecoeur, S.; Edge, T.A.; Watson, L.C.; Watson, S.B.; Greer, C.W.; Ciborowski, J.J.H.; Diep, N.; Dove, A.; Drouillard, K.G.; Frenken, T.; et al. Spatio-Temporal Connectivity of the Aquatic Microbiome Associated with Cyanobacterial Blooms along a Great Lake Riverine-Lacustrine Continuum. Front. Microbiol. 2023, 14, 1073753. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C. Present and Future Opportunities in the Use of Atomic Force Microscopy to Address the Physico-Chemical Properties of Aquatic Ecosystems at the Nanoscale Level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar]
- Pillet, F.; Dague, E.; Ilić, J.P.; Ružić, I.; Rols, M.-P.; DeNardis, N.I. Changes in Nanomechanical Properties and Adhesion Dynamics of Algal Cells during Their Growth. Bioelectrochem. Amst. Neth. 2019, 127, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Marcuello, C.; Lostao, A.; Calvo-Begueria, L.; Velazquez-Campoy, A.; Bes, M.T.; Fillat, M.F.; Peleato, M.-L. Microcystin-LR Binds Iron, and Iron Promotes Self-Assembly. Environ. Sci. Technol. 2017, 51, 4841–4850. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Zhao, Y.-N.; Zhang, X.; Wei, X.; Chen, M.-L.; Chen, X.-W. Biochemical Analysis Based on Optical Detection Integrated Microfluidic Chip. TrAC Trends Anal. Chem. 2023, 158, 116865. [Google Scholar] [CrossRef]
- Knob, R.; Hanson, R.L.; Tateoka, O.B.; Wood, R.L.; Guerrero-Arguero, I.; Robison, R.A.; Pitt, W.G.; Woolley, A.T. Sequence-Specific Sepsis-Related DNA Capture and Fluorescent Labeling in Monoliths Prepared by Single-Step Photopolymerization in Microfluidic Devices. J. Chromatogr. A 2018, 1562, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Mao, S.; Li, W.; Lin, J.-M. Microfluidic Devices in the Fast-Growing Domain of Single-Cell Analysis. Chem. Eur. J. 2018, 24, 15398–15420. [Google Scholar] [CrossRef]
- Gaur, A.; Pant, G.; Jalal, A.S. Computer-Aided Cyanobacterial Harmful Algae Blooms (CyanoHABs) Studies Based on Fused Artificial Intelligence (AI) Models. Algal Res. 2022, 67, 102842. [Google Scholar] [CrossRef]
- Baek, S.-S.; Pyo, J.; Pachepsky, Y.; Park, Y.; Ligaray, M.; Ahn, C.-Y.; Kim, Y.-H.; Chun, J.A.; Cho, K.H. Identification and Enumeration of Cyanobacteria Species Using a Deep Neural Network. Ecol. Indic. 2020, 115, 106395. [Google Scholar] [CrossRef]
- Hennon, G.M.M.; Dyhrman, S.T. Progress and promise of omics for predicting the impacts of climate change on harmful algal blooms. Harmful Algae 2020, 91, 101587. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Yang, X.; Zhang, Y.; Zhang, L.; Ren, H.; Wu, B.; Ye, L. A Review of the Application of Machine Learning in Water Quality Evaluation. Eco-Environ. Health 2022, 1, 107–116. [Google Scholar] [CrossRef]
- Almuhtaram, H.; Kibuye, F.A.; Ajjampur, S.; Glover, C.M.; Hofmann, R.; Gaget, V.; Owen, C.; Wert, E.C.; Zamyadi, A. State of Knowledge on Early Warning Tools for Cyanobacteria Detection. Ecol. Indic. 2021, 133, 108442. [Google Scholar] [CrossRef]
- Burch, M.; Brookes, J.; Chorus, I. Assessing and controlling the risk of cyanobacterial blooms: Waterbody conditions. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- European Union. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Council Directive 92/43/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31992L0043 (accessed on 21 March 2024).
- European Union. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Directive 2008/56/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0056 (accessed on 21 March 2024).
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Directive 2000/60/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 (accessed on 21 March 2024).
- Pazos, Y.; Maneiro, J. Algal bloom detection, monitoring and prediction in the Galician Rías (NW Spain). In Algal Bloom Detection, Monitoring and Prediction; Catena, G., Funari, E., Eds.; 3rd Workshop on Public Health; Istituto Superiore di Sanitá: Roma, Italy, 1999; Volume 95. [Google Scholar]
- Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Directive 2006/7/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006L0007 (accessed on 21 March 2024).
- European Food Safety Authority. 2009 EU Report on Pesticide Residues. EFSA J. 2011, 9, 2430. [Google Scholar]
- UNI EN ISO 10253:2017; Water Quality. Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum tricornutum. ISO: Geneva, Switzerland, 2017.
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1907 (accessed on 21 March 2024).
- REACH—Registration, Evaluation, Authorisation and Restriction of Chemicals Regulation. Registered Substances Factsheets. Available online: https://echa.europa.eu/it/information-on-chemicals/registered-substances (accessed on 27 February 2024).
- Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 Concerning the Making Available on the Market and Use of Biocidal Products Text with EEA Relevance. Regulation (EU) No 528/2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32012R0528 (accessed on 21 March 2024).


| Toxin-Producing Genera | Toxin Category | Toxin Classification (Based on Effects on Humans and Animals) | WHO Guideline Value in Recreational Water | References |
|---|---|---|---|---|
| Anabaenopsis, Aphanizomenon, Dolichospermum (formerly, Ananbaena), Mycrocystis, Oscillatoria, Phormidium, Planktothrix | Microcystins | Hepatotoxins | 24 µg/L | [107,108] |
| Nodularia, Nostoc | Nodularins | Hepatotoxins | Not established | [107,108,109] |
| Aphanizomenon, Cylindrospermopsis, Dolichospermum (formerly, Ananbaena), Lyngbya, Oscillatoria, Raphidiopsis, Umezakia | Cylindrospermopsin | Cytotoxins | 6 µg/L | [107,110] |
| Aphanizomenon, Cylindrospermopsis, Dolichospermum (formerly, Ananbaena), Oscillatoria | Anatoxins | Neurotoxins | 60 µg/L | [111,112,113] |
| Aphanizomenon, Cylindrospermopsis, Dolichospermum (formerly, Ananbaena), Lyngbya, Planktothrix Raphidiopsis | Saxitoxins | Neurotoxins | 30 µg/L | [107,113,114] |
| Aphanizomenon, Dolichospermum (formerly, Ananbaena), Mycrocistis, Nodularia, Nostoc | β-Methylamino L-Alanine (BMAA) | Neurotoxins | Not established | [115,116] |
| Anacystis, Dolichospermum (formerly, Ananbaena), Microcystis, Oscillatoria, Schizothrix, Synechococcus | Lypopolysaccharides | Dermatoxins | Not established | [117,118] |
| Lyngbya | Lyngbyatoxins | Dermatoxins | Not established | [119,120] |
| Lyngbya, Oscillatoria, Schizothrix | Aplysiatoxins | Dermatoxins | Not established | [121] |
| Organism | Exposure Route | Population Size | Outcome Syndrome | Type of Recreational Water | References |
|---|---|---|---|---|---|
| Nodularia spumigena | Ingestion | 1 | Undescribed | Lake water | [130] |
| Anabaena circinalis | Ingestion | >2 | Gastrointestinal illness | Lake water | [131] |
| Raphidiopsis raciborskii | Ingestion | 150 | Kidney and liver disease | Freshwater | [132] |
| Raphidiopsis raciborskii | Ingestion | 138 | Hepatoenteritis | Freshwater | [133] |
| Pseudo-nitzschia spp. and Nitzschia spp. | Ingestion | 150 | Gastrointestinal and/or neurological illness | Seawater | [134] |
| Cyanobacteria | Ingestion | 2000 | Gastroenteritis and deaths | Freshwater | [135,136] |
| Karenia brevis | Inhalation | 129 | Hepatoenteritis | Seawater | [137] |
| Many Organisms | Ingestion | 1331 | Respiratory irritation | Lake water | [138] |
| Karenia brevis | Inhalation | 125 | Respiratory illness | Seawater | [139] |
| Karenia brevis | Inhalation | 52 | Respiratory illness | Seawater | [140] |
| Many organisms | Ingestion | >200 | Gastrointestinal symptoms | Seawater | [141] |
| Cyanobacteria | Ingestion | 1 | Intoxication | Fresh water | [142] |
| Cyanobacteria | Ingestion | 466 | Gastrointestinal symptoms | Lake water | [143] |
| Cyanobacteria | Direct contact, ingestion, inhalation | 13 | Gastrointestinal illness and Hepatoenteritis | Lake water | [144] |
| Ostreopsis spp. | Inhalation | 16 | Respiratory diseases | Seawater | [145] |
| Anabaena flos-aquae | Ingestion | >6 | Gastrointestinal illness | River water | [146] |
| Karenia brevis | Inhalation | 28 | Respiratory symptoms and headache | Seawater | [147] |
| Cyanobacteria | Ingestion and inhalation | - | Neurodegenerative diseases | Freshwater | [148] |
| Microcystin and Cylindrospermopsin | - | 228 | - | Freshwater | [149] |
| Cyanobacteria | Ingestion | - | Gastrointestinal, respiratory, and dermal illnesses | Freshwater | [150] |
| Cyanobacteria | Ingestion, inhalation, dermal contact | 41 | Gastrointestinal, neurological, respiratory, and dermal illnesses | Lake water | [151] |
| Many organisms | Inhalation | 14 | Gastrointestinal, dermatologic, respiratory, neurological, cardiopulmonary, genitourinary diseases | Freshwater | [152] |
| Microcystis aeruginosa | Ingestion and inhalation | 97 | Respiratory and dermatological diseases | Lake water | [153] |
| Microcystis spp. | Inhalation | 81 | Respiratory and dermatological diseases | Lake water | [154] |
| Karenia brevis | Inhalation | - | Respiratory tract irritation | Seawater | [155] |
| Karenia brevis | Inhalation | - | Headache | Seawater | [156] |
| Ostreopsis spp. | Inhalation, dermal contact | 47 | Gastrointestinal, dermatologic, respiratory, neurological, cardiopulmonary, genitourinary diseases | Seawater | [157] |
| - | Ingestion, inhalation, dermal contact | 380 + 178 | General flu-like symptoms | Lake water | [158] |
| Karenia brevis | Inhalation | 258 | Gastrointestinal, respiratory, and dermal illnesses | Seawater | [159] |
| Many Organisms | Ingestion | 432 | Gastrointestinal, respiratory, and dermal illnesses | Lake water and well water | [160] |
| Ostreopsis spp. | Inhalation | 16 | General malaise and respiratory illness | Seawater | [161] |
| Cyanobacteria | Inhalation, ingestion or dermal contact | - | Respiratory, gastrointestinal, neurologic, and dermatologic illness | Lake water | [162] |
| Ostreopsis spp. | Inhalation | 300 | Respiratory and dermatological diseases | Seawater | [163] |
| Cyanobacteria | Inhalation, ingestion or dermal contact | 32 | Fatigue, dermatological, and neurologic illness | Freshwater | [164] |
| Karenia brevis | Inhalation | 59 | Respiratory diseases | Seawater | [165] |
| Karenia brevis | Inhalation | 87 | Respiratory, gastrointestinal, and dermatologic illness | Seawater | [166] |
| Karenia brevis | Inhalation, ingestion | - | Gastrointestinal and respiratory symptoms | Seawater | [167] |
| Karenia brevis | Inhalation | - | Respiratory diseases | Seawater | [168] |
| Red tide | Inhalation | - | Respiratory diseases | Seawater | [169] |
| Ostreopsis spp. | Inhalation, direct contact | 674 | Fatigue, respiratory, and dermatological diseases | Seawater | [170] |
| Red tide | Inhalation | - | Gastrointestinal illness | Seawater | [171] |
| Ostreopsis spp. | Inhalation | 62 | Respiratory diseases and migraine | Seawater | [172] |
| Ostreopsis spp. | Inhalation, ingestion or dermal contact | 28 | Respiratory diseases | Seawater | [173] |
| Ostreopsis ovata | Inhalation, ingestion or dermal contact | 209 | Respiratory diseases | Seawater | [174] |
| Red tide | Inhalation | 97 | Respiratory diseases | Seawater | [175] |
| Brevitoxin | Inhalation | 97 | Respiratory diseases | Seawater | [176] |
| QAC | Formula | CAS-Number | Molecular Weight | Half-Life | Log Koc | Log Dow/Kow |
|---|---|---|---|---|---|---|
| ATMACs | CH3(CH3)7-17 N(CH3)3Cl | C8:10108-86-8 | C8: 207.8 | - | C8: 2.78 | C8: −1.05 |
| C10: 10108-86-9 | C10: 235.8 | C10: 3.30 | C10: −0.189 | |||
| C12: 112-00-5 | C12: 263.9 | C12: 3.82 | C12: 0.857 | |||
| C14: 4574-04-3 | C14: 291.9 | C14: 4.34 | C14: 1.77 | |||
| C16: 112-02-7 | C16: 320.0 | C16: 4.36 | C16: 2.43 | |||
| C18: 112-03-8 | C18: 348.0 | C18: 5.38 | C18: 3.25 | |||
| DDACs | [CH3(CH3)7-17]2 N(CH3)2Cl | C8: 5538-94-3 | C8: 306.0 | 180 days in river water (EPA, 2017) 1048 days in soil [189] | C8: 4.64 | C8: 1.57 |
| C10: 7173-51-5 | C10: 362.1 | C10: 5.68 | C10: 2.59 | |||
| C12: 3401-74-9 | C12: 418.2 | C12: 6.73 | C12: 4.31 | |||
| C14: 10108-91-5 | C14: 474.3 | C14: 7.71 | C14: 6.25 | |||
| C16: 1812-53-9 | C16: 530.4 | C16: 8.81 | C16: 9.98 | |||
| C18:107-64-2 | C18: 586.5 | C18: 9.86 | C18: 9.52 | |||
| BACs | CH3(CH3)5-17N(Cl) (CH3)2 CH2 C6H5 | C6: 22559-57-5 | C6: 255.8 | 379 days (in water at pH 9) [190] | C6: 3.87 | C6: −0.763 |
| C8: 959-55-7 | C8: 283.9 | C8: 4.39 | C8: 0.233 | |||
| C10: 965-32-2 | C10: 311.9 | C10: 4.91 | C10: 1.31 | |||
| C12: 139-07-1 | C12: 340.0 | C12: 5.43 | C12: 2.10 | |||
| C14: 139-08-2 | C14: 368.0 | C14: 5.96 | C14: 2.78 | |||
| C16: 122-18-9 | C16: 396.1 | C16: 6.48 | C16: 3.54 | |||
| C18: 122-19-0 | C18: 424.1 | C18: 7.00 | C18: 4.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valeriani, F.; Carraturo, F.; Lofrano, G.; Volpini, V.; Izzo, M.G.; Bruno, A.; Guida, M.; Romano Spica, V. Algae in Recreational Waters: An Overview within a One Health Perspective. Water 2024, 16, 946. https://doi.org/10.3390/w16070946
Valeriani F, Carraturo F, Lofrano G, Volpini V, Izzo MG, Bruno A, Guida M, Romano Spica V. Algae in Recreational Waters: An Overview within a One Health Perspective. Water. 2024; 16(7):946. https://doi.org/10.3390/w16070946
Chicago/Turabian StyleValeriani, Federica, Federica Carraturo, Giusy Lofrano, Veronica Volpini, Michela Giovanna Izzo, Agnese Bruno, Marco Guida, and Vincenzo Romano Spica. 2024. "Algae in Recreational Waters: An Overview within a One Health Perspective" Water 16, no. 7: 946. https://doi.org/10.3390/w16070946
APA StyleValeriani, F., Carraturo, F., Lofrano, G., Volpini, V., Izzo, M. G., Bruno, A., Guida, M., & Romano Spica, V. (2024). Algae in Recreational Waters: An Overview within a One Health Perspective. Water, 16(7), 946. https://doi.org/10.3390/w16070946












