Influence of Technological Factors on the Formation and Transformation of Iron-Containing Phases in the Process of Ferritization of Exhausted Etching Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set
2.2. Study Materials
3. Results
3.1. The Study of pH Influence
3.2. Determination of the Effect of the Duration of Ferritization of the Reaction Mixture
3.3. Determination of the Effect of the Initial Concentration of Iron Ions in the Reaction Mixture
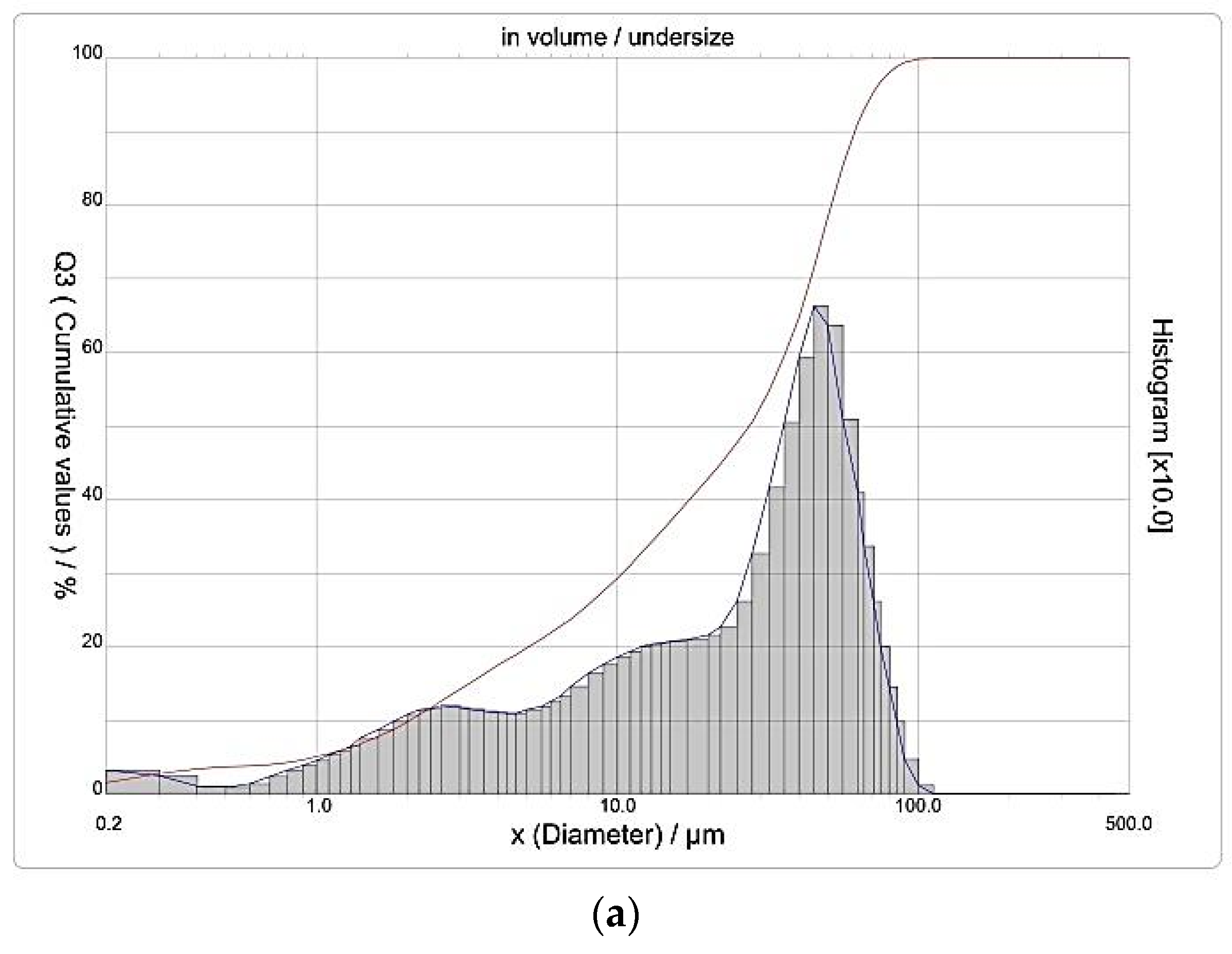
3.4. Determination of the Average Particle Size of Precipitate Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brück, F.; Fritzsche, A.; Totsche, K.U.; Weigand, H. Steel Pickling Rinse Water Sludge: Concealed Formation of Cr(VI) Driven by the Enhanced Oxidation of Nitrite. J. Environ. Chem. Eng. 2017, 5, 2163–2170. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Buchwald, T. Efficient Metals Removal from Waste Pickling Liquor Using Novel Task Specific Ionic Liquids—Classical Manner and Encapsulation in Polymer Shell. Sep. Purif. Technol. 2021, 262, 118239. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A Review on Methods of Regeneration of Spent Pickling Solutions from Steel Processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Wiśniewski, J.; Wiśniewska, G. Acids and Iron Salts Removal from Rinsing Water after Metal Etching. Desalination 1997, 109, 187–193. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Frank, J.J.; Poulakos, A.G.; Tornero-Velez, R.; Xue, J. Systematic Review and Meta-Analyses of Lead (Pb) Concentrations in Environmental Media (Soil, Dust, Water, Food, and Air) Reported in the United States from 1996 to 2016. Sci. Total Environ. 2019, 694, 133489. [Google Scholar] [CrossRef]
- Sharma, R.; Shelke, S.; Bagheri Kashani, M.; Morose, G.; Christuk, C.; Nagarajan, R. Safer and Effective Alternatives to Perfluoroalkyl-Based Surfactants in Etching Solutions for the Semiconductor Industry. J. Clean. Prod. 2023, 415, 137879. [Google Scholar] [CrossRef]
- Gueccia, R.; Winter, D.; Randazzo, S.; Cipollina, A.; Koschikowski, J.; Micale, G.D.M. An Integrated Approach for the HCl and Metals Recovery from Waste Pickling Solutions: Pilot Plant and Design Operations. Chem. Eng. Res. Des. 2021, 168, 383–396. [Google Scholar] [CrossRef]
- Fadillah, G.; Yudha, S.P.; Sagadevan, S.; Fatimah, I.; Muraza, O. Magnetic Iron Oxide/Clay Nanocomposites for Adsorption and Catalytic Oxidation in Water Treatment Applications. Open Chem. 2020, 18, 1148–1166. [Google Scholar] [CrossRef]
- Maliki, M.; Omorogbe, S.O.; Ifijen, I.H.; Aghedo, O.N.; Ighodaro, A. Incisive Review on Magnetic Iron Oxide Nanoparticles and Their Use in the Treatment of Bacterial Infections. In TMS 2023 152nd Annual Meeting & Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Society, Ed.; The Minerals, Metals & Materials Series; Springer Nature: Cham, Switzerland, 2023; pp. 487–498. ISBN 978-3-031-22523-9. [Google Scholar]
- Moreno, R.; Poyser, S.; Meilak, D.; Meo, A.; Jenkins, S.; Lazarov, V.K.; Vallejo-Fernandez, G.; Majetich, S.; Evans, R.F.L. The Role of Faceting and Elongation on the Magnetic Anisotropy of Magnetite Fe3O4 Nanocrystals. Sci. Rep. 2020, 10, 2722. [Google Scholar] [CrossRef]
- Trach, Y.; Tytkowska-Owerko, M.; Reczek, L.; Michel, M. Comparison the Adsorption Capacity of Ukrainian Tuff and Basalt with Zeolite–Manganese Removal from Water Solution. J. Ecol. Eng. 2021, 22, 161–168. [Google Scholar] [CrossRef]
- Trach, Y.; Melnychuk, V.; Michel, M.M.; Reczek, L.; Siwiec, T.; Trach, R. The Characterization of Ukrainian Volcanic Tuffs from the Khmelnytsky Region with the Theoretical Analysis of Their Application in Construction and Environmental Technologies. Materials 2021, 14, 7723. [Google Scholar] [CrossRef]
- Trach, Y.; Bujakowski, F.; Koda, E.; Mazur, Ł.; Nejbert, K.; Podlasek, A.; Vaverková, M.D. Characterization of Adsorbents from Ukrainian Kaolinite Clay for the Sorption of Nickel: Insight and Practical Application for Water Treatment in Conditions of Economic Constraints. Desalination Water Treat. 2022, 278, 1–12. [Google Scholar] [CrossRef]
- Tanyildizi, M.Ş. Modeling of Adsorption Isotherms and Kinetics of Reactive Dye from Aqueous Solution by Peanut Hull. Chem. Eng. J. 2011, 168, 1234–1240. [Google Scholar] [CrossRef]
- Šafaříková, M.; Šafařík, I. Magnetic Solid-Phase Extraction. J. Magn. Magn. Mater. 1999, 194, 108–112. [Google Scholar] [CrossRef]
- Vozniuk, M.; Shabliy, T.; Gomelya, M.; Sirenko, L.; Sidorov, D. Magnetosorption Purification of Water from Petroleum Products. J. Ecol. Eng. 2023, 24, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Aggarwal, I.; Kumar, H.; Prasad, L.; Kumar, A.; Sharma, A.; Vo, D.-V.N.; Van Thuan, D.; Mishra, V. Magnetite Nanoparticles as Sorbents for Dye Removal: A Review. Environ. Chem. Lett. 2021, 19, 2487–2525. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, X.; Kang, M.; Yang, G.; Yin, M.; Wang, J.; Gang, S. Factors Influencing the Reduction of U (VI) by Magnetite. Chemosphere 2020, 254, 126855. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhu, S.; Jin, C.; Sun, Z.; Wang, L.; Wen, Q.; Ma, F. Sorption Mechanisms of Antibiotic Sulfamethazine (SMT) on Magnetite-Coated Biochar: PH-Dependence and Redox Transformation. Chemosphere 2021, 268, 128805. [Google Scholar] [CrossRef]
- Drzymała, J. Mineral Processing: Foundations of Theory and Practice of Minerallurgy; University of Technology: Wroclaw, Poland, 2007; ISBN 83-7493-362-3. [Google Scholar]
- Natarajan, S.; Harini, K.; Gajula, G.P.; Sarmento, B.; Neves-Petersen, M.T.; Thiagarajan, V. Multifunctional Magnetic Iron Oxide Nanoparticles: Diverse Synthetic Approaches, Surface Modifications, Cytotoxicity towards Biomedical and Industrial Applications. BMC Mater. 2019, 1, 2. [Google Scholar] [CrossRef]
- Kouotou, P.M.; Tian, Z.-Y. Controlled Synthesis of α-Fe2O3@Fe3O4 Composite Catalysts for Exhaust Gas Purification. Proc. Combust. Inst. 2019, 37, 5445–5453. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Gingasu, D.; Culita, D.C.; Calderon Moreno, J.M.; Marinescu, G.; Bartha, C.; Oprea, O.; Preda, S.; Chifiriuc, M.C.; Popa, M. Synthesis of CoFe2O4 through Wet Ferritization Method Using an Aqueous Extract of Eucalyptus Leaves. Coatings 2023, 13, 1250. [Google Scholar] [CrossRef]
- Frolova, L.A. The Mechanism of Nickel Ferrite Formation by Glow Discharge Effect. Appl. Nanosci. 2019, 9, 845–852. [Google Scholar] [CrossRef]
- Kochetov, G.; Samchenko, D.; Lastivka, O.; Derecha, D. Determining the Rational Parameters for Processing Spent Etching Solutions by Ferritization Using Alternating Magnetic Fields. East. Eur. J. Enterp. Technol. 2022, 3, 21–28. [Google Scholar] [CrossRef]
- Samchenko, D.; Kochetov, G.; Derecha, D.O.; Skirta, Y.B. Sustainable Approach for Galvanic Waste Processing by Energy-Saving Ferritization with AC-Magnetic Field Activation. Cogent Eng. 2022, 9, 2143072. [Google Scholar] [CrossRef]
- Frolova, L.; Pivovarov, A.; Anisimova, L.; Yakubovskaya, Z.; Yakubovskii, A. The Extraction of Chromium (III) from Concentrated Solutions by Ferrite Method. Voprosy Khimii Khimicheskoi Tekhnologii 2017, 6, 110–115. [Google Scholar]
- Kochetov, G.; Samchenko, D.; Arhatenko, T. Determination of Influence of PH on Reaction Mixture of Ferritation Process with Electromagnetic Pulse Activation on the Processing of Galvanic Sludge. East. Eur. J. Enterp. Technol. 2021, 4, 24–30. [Google Scholar] [CrossRef]
- Heuss-Aßbichler, S.; John, M.; Klapper, D.; Bläß, U.W.; Kochetov, G. Recovery of Copper as Zero-Valent Phase and/or Copper Oxide Nanoparticles from Wastewater by Ferritization. J. Environ. Manag. 2016, 181, 1–7. [Google Scholar] [CrossRef]
- Ying, Y.; Xiong, X.; Wang, N.; Zheng, J.; Yu, J.; Li, W.; Qiao, L.; Cai, W.; Li, J.; Huang, H.; et al. Low Temperature Sintered MnZn Ferrites for Power Applications at the Frequency of 1 MHz. J. Eur. Ceram. Soc. 2021, 41, 5924–5930. [Google Scholar] [CrossRef]
- John, M.; Heuss-Aßbichler, S.; Tandon, K.; Ullrich, A. Recovery of Ag and Au from Synthetic and Industrial Wastewater by 2-Step Ferritization and Lt-Delafossite Process via Precipitation. J. Water Process Eng. 2019, 30, 100532. [Google Scholar] [CrossRef]
- Yemchura, B.; Kochetov, G.; Samchenko, D.; Prikhna, T. Ferritization-Based Treatment of Zinc-Containing Wastewater Flows: Influence of Aeration Rates. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 2nd ed.; Ksibi, M., Ghorbal, A., Chakraborty, S., Chaminé, H.I., Barbieri, M., Guerriero, G., Hentati, O., Negm, A., Lehmann, A., Römbke, J., et al., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2021; pp. 171–176. ISBN 978-3-030-51209-5. [Google Scholar]
- Shariati, S.; Faraji, M.; Yamini, Y.; Rajabi, A.A. Fe3O4 Magnetic Nanoparticles Modified with Sodium Dodecyl Sulfate for Removal of Safranin O Dye from Aqueous Solutions. Desalination 2011, 270, 160–165. [Google Scholar] [CrossRef]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic Review of the Preparation Techniques of Iron Oxide Magnetic Nanoparticles. Nanosci. Nanotechnol. 2013, 2, 148–158. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Shah, A.-H.A.; Bilal, S.; Rahman, G. Basic Blue Dye Adsorption from Water Using Polyaniline/Magnetite (Fe3O4) Composites: Kinetic and Thermodynamic Aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Yamini, Y.; Faraji, M.; Rajabi, A.A.; Nourmohammadian, F. Ultra Efficient Removal of Basic Blue 41 from Textile Industry’s Wastewaters by Sodium Dodecyl Sulphate Coated Magnetite Nanoparticles: Removal, Kinetic and Isotherm Study. Anal. Bioanal. Chem. Res. 2018, 5, 205–215. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A.; Jannesar, R. Comparative Study on Ultrasonic Assisted Adsorption of Dyes from Single System onto Fe3O4 Magnetite Nanoparticles Loaded on Activated Carbon: Experimental Design Methodology. Ultrason. Sonochem. 2017, 34, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Trach, Y.; Melnychuk, V.; Stadnyk, O.; Trach, R.; Bujakowski, F.; Kiersnowska, A.; Rutkowska, G.; Skakun, L.; Szer, J.; Koda, E. The Possibility of Implementation of West Ukrainian Paleogene Glauconite–Quartz Sands in the Building Industry: A Case Study. Sustainability 2023, 15, 1489. [Google Scholar] [CrossRef]
- McIllece, J.J. On Generalized Variance Functions for Sample Means and Medians; U.S. Bureau of Labor Statistics: Washington, DC, USA, 2018.
- Pereira, D.I.A.; Bruggraber, S.F.A.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate Iron(III) Oxo-Hydroxide Delivers Safe Iron That Is Well Absorbed and Utilised in Humans. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1877–1886. [Google Scholar] [CrossRef]
- Dong, W.; Ding, J.; Wang, W.; Zong, L.; Xu, J.; Wang, A. Magnetic Nano-Hybrids Adsorbents Formulated from Acidic Leachates of Clay Minerals. J. Clean. Prod. 2020, 256, 120383. [Google Scholar] [CrossRef]
- Lei, W.; Liu, Y.; Si, X.; Xu, J.; Du, W.; Yang, J.; Zhou, T.; Lin, J. Synthesis and Magnetic Properties of Octahedral Fe3O4 via a One-Pot Hydrothermal Route. Phys. Lett. A 2017, 381, 314–318. [Google Scholar] [CrossRef]
- Anigrahawati, P.; Sahar, M.R.; Sazali, E.S. Physical, Structural and Spectroscopic Analysis of Tellurite Glass Containing Natural Magnetite Fe3O4 Nanoparticles. Mater. Chem. Phys. 2022, 286, 126183. [Google Scholar] [CrossRef]
- Lv, W.; Liu, B.; Luo, Z.; Ren, X.; Zhang, P. XRD Studies on the Nanosized Copper Ferrite Powders Synthesized by Sonochemical Method. J. Alloys Compd. 2008, 465, 261–264. [Google Scholar] [CrossRef]
- Choudhary, A.; Khandelwal, N.; Ganie, Z.A.; Darbha, G.K. Influence of Magnetite and Its Weathering Originated Maghemite and Hematite Minerals on Sedimentation and Transport of Nanoplastics in the Aqueous and Subsurface Environments. Sci. Total Environ. 2024, 912, 169132. [Google Scholar] [CrossRef] [PubMed]
- Prikhna, T.; Monastyrov, M.; Soldatov, I.; Büchner, B.; Giebeler, L.; Sathyadharma Prasad, A.; Ostash, O.; Moshchil, V.; Podhurska, V.; Potapov, P.; et al. Corrosion-Resistant Polymer-Based Nanocomposite Materials with a High Level of Microwave Absorption and Soft Magnetic Materials Based on Iron Oxide Nanopowder Obtained by Electroerosion Dispersion. Ceram. Int. 2023, 49, 24322–24331. [Google Scholar] [CrossRef]
- Kallay, N.; Preočanin, T. Measurement of the Surface Potential of Individual Crystal Planes of Hematite. J. Colloid Interface Sci. 2008, 318, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Dimirkou, A.; Ioannou, A.; Doula, M. Preparation, Characterization and Sorption Properties for Phosphates of Hematite, Bentonite and Bentonite–Hematite Systems. Adv. Colloid Interface Sci. 2002, 97, 37–61. [Google Scholar] [CrossRef]
- Cromières, L.; Moulin, V.; Fourest, B.; Giffaut, E. Physico-Chemical Characterization of the Colloidal Hematite/Water Interface: Experimentation and Modelling. Colloids Surf. Physicochem. Eng. Asp. 2002, 202, 101–115. [Google Scholar] [CrossRef]






| Core Sample ID | pH of the Reaction Mixture | Concentration (Fe2+), g/dm3 | The Time of the Ferritization Process, min |
|---|---|---|---|
| A-1 | 8.5 | 16.6 | 15 |
| A-2 | 9.5 | 16.6 | 15 |
| A-3 | 10.5 | 16.6 | 15 |
| A-4 | 11.5 | 16.6 | 15 |
| B-1 | 11.5 | 16.6 | 5 |
| B-2 | 11.5 | 16.6 | 10 |
| B-3 | 11.5 | 16.6 | 15 |
| B-4 | 11.5 | 16.6 | 20 |
| B-5 | 11.5 | 16.6 | 25 |
| B-6 | 11.5 | 16.6 | 30 |
| C-1 | 11.5 | 46.6 | 15 |
| C-2 | 11.5 | 36.6 | 15 |
| C-3 | 11.5 | 26.6 | 15 |
| C-4 | 11.5 | 16.6 | 15 |
| C-5 | 11.5 | 6.6 | 15 |
| Sample | pH | The Content of Inorganic Phase, % w/w | ||||
|---|---|---|---|---|---|---|
| Thermal Activation | AMF Activation | |||||
| δ-FeOOH | Fe3O4 | γ-Fe2O3 | δ-FeOOH | Fe3O4 | ||
| A-1 | 8.5 | 75.4 | 24.6 | – | 61.1 | 38.9 |
| A-2 | 9.5 | 35.3 | 64.7 | – | 37.8 | 62.2 |
| A-3 | 10.5 | 2.7 | 97.3 | – | 9.1 | 90.9 |
| A-4 | 11.5 | – | 81.1 | 18.9 | – | 100 |
| Sample | The Time of the Ferritization Process, min | The Content of Inorganic Phase, % w/w | ||||||
|---|---|---|---|---|---|---|---|---|
| Thermal Activation | AMF Activation | |||||||
| δ-FeOOH | Fe3O4 | γ-Fe2O3 | γ-FeOOH | δ-FeOOH | Fe3O4 | γ-Fe2O3 | ||
| B-1 | 5 | 16.6 | 83.4 | – | 27.7 | 16.6 | 55.7 | – |
| B-2 | 10 | 2.9 | 97.1 | – | – | 28.6 | 71.4 | – |
| B-3 | 15 | – | 81.1 | 18.9 | – | – | 100 | – |
| B-4 | 20 | – | 34.6 | 65.4 | – | – | 97.1 | 2.9 |
| B-5 | 25 | – | 8.2 | 91.8 | – | – | 89.6 | 10.4 |
| B-6 | 30 | – | – | 100 | – | – | 74.6 | 25.4 |
| Sample | Initial Concentration Fe(total), g/dm3 | The Content of Inorganic Phase, % w/w | |||||
|---|---|---|---|---|---|---|---|
| Thermal Activation | AMF Activation | ||||||
| δ-FeOOH | Fe3O4 | γ-Fe2O3 | δ-FeOOH | Fe3O4 | γ-Fe2O3 | ||
| C-1 | 46.6 | 29.2 | 70.8 | – | 55.8 | 44.2 | – |
| C-2 | 36.6 | 14.1 | 85.9 | – | 35.5 | 64.5 | – |
| C-3 | 26.6 | – | 100 | – | 10.9 | 89.1 | – |
| C-4 | 16.6 | – | 81.1 | 18.9 | – | 100 | – |
| C-5 | 6.6 | – | – | 100 | – | 50.8 | 49.2 |
| The Method of Activation | Particle Size, μm | |||
|---|---|---|---|---|
| Average Value | D10,3 | D50,3 | D90,3 | |
| Thermal | 29.62 | 2.03 | 27.36 | 61.55 |
| AMF | 27.69 | 1.90 | 23.62 | 59.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samchenko, D.; Kochetov, G.; Trach, Y.; Chernyshev, D.; Kravchuk, A. Influence of Technological Factors on the Formation and Transformation of Iron-Containing Phases in the Process of Ferritization of Exhausted Etching Solutions. Water 2024, 16, 1085. https://doi.org/10.3390/w16081085
Samchenko D, Kochetov G, Trach Y, Chernyshev D, Kravchuk A. Influence of Technological Factors on the Formation and Transformation of Iron-Containing Phases in the Process of Ferritization of Exhausted Etching Solutions. Water. 2024; 16(8):1085. https://doi.org/10.3390/w16081085
Chicago/Turabian StyleSamchenko, Dmitry, Gennadii Kochetov, Yuliia Trach, Denys Chernyshev, and Andriy Kravchuk. 2024. "Influence of Technological Factors on the Formation and Transformation of Iron-Containing Phases in the Process of Ferritization of Exhausted Etching Solutions" Water 16, no. 8: 1085. https://doi.org/10.3390/w16081085
APA StyleSamchenko, D., Kochetov, G., Trach, Y., Chernyshev, D., & Kravchuk, A. (2024). Influence of Technological Factors on the Formation and Transformation of Iron-Containing Phases in the Process of Ferritization of Exhausted Etching Solutions. Water, 16(8), 1085. https://doi.org/10.3390/w16081085







