Recent Issues and Challenges in the Study of Inland Waters
Abstract
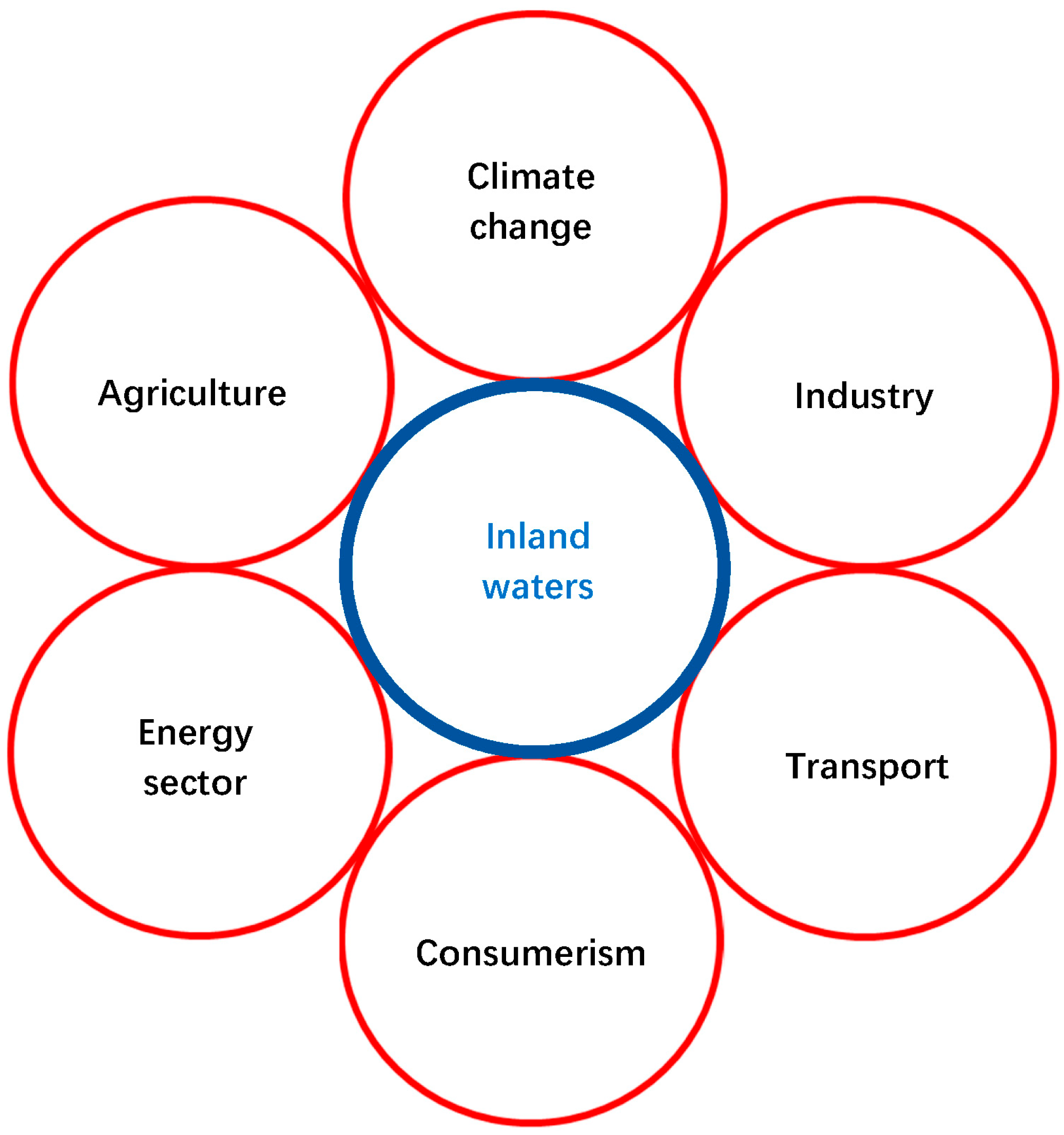
1. Introduction
2. Unmanned Aerial Vehicles in Inland Water Analyses
2.1. Non-Contact Solution
2.2. Contact Solutions
2.3. Sampling
3. The Role of Biodiversity
4. Chlorophyll a Fluorescence as a Tool for Monitoring the Development of Algae and Cyanobacteria
5. Impact of Coal Mine Waters on Aquatic Ecosystems
5.1. Brown Coal Mining and River Water Quality
5.2. Brown Coal Mining and River Sediments
5.3. Importance of the Length of Complete Mixing Zone
6. Microplastic—Development of Monitoring Methods
6.1. Particle Size
6.2. Collection, Extraction and Identification
6.3. Sampling
6.4. Extraction—Density Separation, Filtration and Etching
6.5. Visual Sorting, Visual Identification and Chemical Identification
6.6. Counting and Data Presenting
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | acid mine drainage |
| AOTF | acousto-optic tunable filter |
| AFM | atomic force microscopy |
| APLE | automatic pressure liquid extraction |
| ATR | attenuated total reflectance |
| BASEMAN | baselines and standards for microplastics analyses |
| CARS | fluorescence and coherent anti-Stokes Raman scattering |
| ChlF | chlorophyll a fluorescence |
| CLSM | confocal laser scanning microscopy |
| CMC | critical micelle concentration |
| CMP | complete mixing path |
| CPE | cloud-point extraction |
| DO | dissolved oxygen |
| DSC | differential scanning calorimetry |
| EDS | energy dispersive X-ray spectroscopy |
| FPA | focal plane array |
| FTIR | Fourier-transform infrared spectroscopy |
| GC | gas chromatography |
| HAB | harmful algal bloom |
| H-NMR | proton nuclear magnetic resonance |
| HPLC | high-performance liquid chromatography |
| NAP | non-algal particles |
| NMR | nuclear magnetic resonance |
| MP | microplastic |
| MPSS | microplastic–sediment separator |
| MW | mine waters |
| PAR | photosynthetically active radiation |
| PE | polyethylene |
| PET | polyethylene terephthalate |
| PP | polypropylene |
| PVC | polyvinyl chloride |
| Pyr-GC/MS | pyrolysis–gas chromatography–mass spectrometry |
| QAQC | quality assurance and quality control |
| RS | Raman Spectroscopy |
| SCS | Swiss Chemical Society |
| SEM | scanning electron microscope |
| SIF | Sun-Induced Chlorophyll Fluoresence |
| SOP | Standard Operating Procedure |
| SPE | Solid-phase extraction |
| TDS-GC/MS | thermodesorption gas chromatography with mass spectrometric detection |
| TED-GC/MS | automated thermal extraction—desorption gas chromatography mass spectrometry |
| TGA | thermal gravimetric analysis |
| UAV | unmanned aerial vehicle |
| UAS | unmanned aerial system |
References
- Behrenfeld, M.J.; Westberry, T.K.; Boss, E.S.; O’Malley, R.T.; Siegel, D.A.; Wiggert, J.D.; Franz, B.A.; McClain, C.R.; Feldman, G.C.; Doney, S.C.; et al. Satellite-Detected Fluorescence Reveals Global Physiology of Ocean Phytoplankton. Biogeosciences 2009, 6, 779–794. [Google Scholar] [CrossRef]
- Marszelewski, W.; Dembowska, E.A.; Napiórkowski, P.; Solarczyk, A. Understanding abiotic and biotic conditions in post-mining pit lakes for efficient management: A case study (Poland). Mine Water Environ. 2017, 36, 418–428. [Google Scholar] [CrossRef]
- Malea, L.; Nakou, K.; Papadimitriou, A.; Exadactylos, A.; Orfanidis, S. Physiological Responses of the Submerged Macrophyte Stuckenia pectinata to High Salinity and Irradiance Stress to Assess Eutrophication Management and Climatic Effects: An Integrative Approach. Water 2021, 13, 1706. [Google Scholar] [CrossRef]
- Ji, S.; Ma, S. The effects of industrial pollution on ecosystem service value: A case study in a heavy industrial area, China. Environ. Dev. Sustain. 2022, 24, 6804–6833. [Google Scholar] [CrossRef]
- Cheng, L.; Tan, X.; Yao, D.; Xu, W.; Wu, H.; Chen, Y. A Fishery Water Quality Monitoring and Prediction Evaluation System for Floating UAV Based on Time Series. Sensors 2021, 21, 4451. [Google Scholar] [CrossRef] [PubMed]
- Messyasz, B.; Pikosz, M.; Treska, E. Biology of freshwater macroalgae and their distribution. In Algae Biomass: Characteristics and Applications; Springer: Berlin/Heidelberg, Germany, 2018; Volume 8, pp. 17–31. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Staniszewski, R.; Niedzielski, P.; Sobczyński, T.; Sojka, M. Trace Elements in Sediments of Rivers Affected by Brown Coal Mining: A Potential Environmental Hazard. Energies 2022, 15, 2828. [Google Scholar] [CrossRef]
- Cheng, K.H.; Jiao, J.J.; Luo, X.; Yu, S. Effective Coastal Escherichia Coli Monitoring by Unmanned Aerial Vehicles (UAV) Thermal Infrared Images. Water Res. 2022, 222, 118900. [Google Scholar] [CrossRef] [PubMed]
- Duysens, L.N.M.; Sweers, H.E. Mechanisms of two photochemical reactions in algae as studied by means of fluorescence. In Studies on Microalgae and Photosynthetic Bacteria, Special Issue of Plant Cell Physiol; Mahlis, L., Ed.; Japanese Society of Plant Physiologists; University of Tokyo Press: Tokyo, Japan, 1963; pp. 353–372. [Google Scholar]
- Turnau, K.; Płachno, B.J.; Bień, P.; Świątek, P.; Dąbrowski, P.; Kalaji, H. Fungal symbionts impact cyanobacterial biofilm durability and photosynthetic efficiency. Curr. Biol. 2023, 33, 5257–5262.e3. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.; Wolkersdorfer, C. Renewed demands for mine water management. Mine Water Environ. 2012, 31, 147–158. [Google Scholar] [CrossRef]
- Staniszewski, R.; Diatta, J.B.; Andrzejewska, B. Impact of lignite mine waters from deep seated drainage on water quality of the Noteć River. J. Elem. 2014, 19, 749–758. [Google Scholar] [CrossRef]
- Akanle, O.; Adejare, G.S.; Adewusi, A.O.; Yusuf, Q.O. Farmers-Herders Conflicts and Development in Nigeria. Niger. J. Sociol. Anthropol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Talozi, S.; Altz-Stamm, A.; Hussein, H.; Reich, P. What constitutes an equitable water share? A reassessment of equitable apportionment in the Jordan–Israel water agreement 25 years later. Water Policy 2019, 21, 911–933. [Google Scholar] [CrossRef]
- Wheeler, K.G.; Hall, J.W.; Abdo, G.M.; Dadson, S.J.; Kasprzyk, J.R.; Smith, R.; Zagona, E.A. Exploring cooperative transboundary river management strategies for the Eastern Nile Basin. Water Resour. Res. 2018, 54, 9224–9254. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.G.; Hussein, H. Water research and nationalism in the post-truth era. Water Int. 2021, 46, 1216–1223. [Google Scholar] [CrossRef]
- Shelare, S.D.; Aglawe, K.R.; Waghmare, S.N.; Belkhode, P.N. Advances in Water Sample Collections with a Drone—A Review. Mater. Today Proc. 2021, 47, 4490–4494. [Google Scholar] [CrossRef]
- Guareschi, S.; Laini, A.; England, J.; Barrett, J.; Wood, P.J. Multiple co-occurrent alien invaders constrain aquatic biodiversity in rivers. Ecol. Appl. 2021, 31, e02385. [Google Scholar] [CrossRef]
- Dubelaar, G.B.; Geerders, P.J.; Jonker, R.R. High frequency monitoring reveals phytoplankton dynamics. J. Environ. Monit. 2004, 6, 946–952. [Google Scholar] [CrossRef]
- Srivastav, S.K.; Yadav, H.L.; Seervi, V.; Jamal, A. Assessment of water quality near vicinity of lignite mine region, Gujarat, India: A case study. Int. Adv. Res. J. Sci. Eng. Technol. 2017, 4, 42–47. [Google Scholar]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Guimarães, T.T.; Veronez, M.R.; Koste, E.C.; Souza, E.M.; Brum, D.; Gonzaga, L.; Mauad, F.F. Evaluation of Regression Analysis and Neural Networks to Predict Total Suspended Solids in Water Bodies from Unmanned Aerial Vehicle Images. Sustainability 2019, 11, 2580. [Google Scholar] [CrossRef]
- Wei, L.; Huang, C.; Zhong, Y.; Wang, Z.; Hu, X.; Lin, L. Inland Waters Suspended Solids Concentration Retrieval Based on PSO-LSSVM for UAV-Borne Hyperspectral Remote Sensing Imagery. Remote Sens. 2019, 11, 1455. [Google Scholar] [CrossRef]
- Chicuazuque, C.; Sarmiento, J.; Rodríguez, J.; Upegui, E. Total Suspended Solids (TSS) Estimation Over a Section of the Upper Bogota River Basin (Colombia) through Processing Multispectral Images Captured Using UAV. In Proceedings of the 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11–16 July 2021; pp. 8189–8192. [Google Scholar]
- Prior, E.M.; O’Donnell, F.C.; Brodbeck, C.; Donald, W.N.; Runion, G.B.; Shepherd, S.L. Measuring High Levels of Total Suspended Solids and Turbidity Using Small Unoccupied Aerial Systems (SUAS) Multispectral Imagery. Drones 2020, 4, 54. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, A.; Lv, R.; Zhang, Y.; Ma, J.; Li, T. Machine learning algorithm inversion experiment and pollution analysis of water quality parameters in urban small and medium-sized rivers based on UAV multispectral data. Environ. Sci. Pollut. Res. 2023, 30, 78913–78932. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Sun, Y.; Huang, C.; Li, M. Water Turbidity Retrieval Based on UAV Hyperspectral Remote Sensing. Water 2022, 14, 128. [Google Scholar] [CrossRef]
- Bussières, S.; Kinnard, C.; Clermont, M.; Campeau, S.; Dubé-Richard, D.; Bordeleau, P.-A.; Roy, A. Monitoring Water Turbidity in a Temperate Floodplain Using UAV: Potential and Challenges. Can. J. Remote Sens. 2022, 48, 565–574. [Google Scholar] [CrossRef]
- McEliece, R.; Hinz, S.; Guarini, J.-M.; Coston-Guarini, J. Evaluation of Nearshore and Offshore Water Quality Assessment Using UAV Multispectral Imagery. Remote Sens. 2020, 12, 2258. [Google Scholar] [CrossRef]
- Zhang, L.; Han, W.; Niu, Y.; Chávez, J.L.; Shao, G.; Zhang, H. Evaluating the Sensitivity of Water Stressed Maize Chlorophyll and Structure Based on UAV Derived Vegetation Indices. Comput. Electron. Agric. 2021, 185, 106174. [Google Scholar] [CrossRef]
- Gai, Y.; Yu, D.; Zhou, Y.; Yang, L.; Chen, C.; Chen, J. An Improved Model for Chlorophyll-a Concentration Retrieval in Coastal Waters Based on UAV-Borne Hyperspectral Imagery: A Case Study in Qingdao, China. Water 2020, 12, 2769. [Google Scholar] [CrossRef]
- Kim, E.-J.; Nam, S.-H.; Koo, J.-W.; Hwang, T.-M. Hybrid Approach of Unmanned Aerial Vehicle and Unmanned Surface Vehicle for Assessment of Chlorophyll-a Imagery Using Spectral Indices in Stream, South Korea. Water 2021, 13, 1930. [Google Scholar] [CrossRef]
- Guimarães, T.T.; Veronez, M.R.; Koste, E.C.; Gonzaga, L.; Bordin, F.; Inocencio, L.C.; Larocca, A.P.C.; De Oliveira, M.Z.; Vitti, D.C.; Mauad, F.F. An Alternative Method of Spatial Autocorrelation for Chlorophyll Detection in Water Bodies Using Remote Sensing. Sustainability 2017, 9, 416. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Chen, Y.; Qiao, X.; Qian, W. Water Chlorophyll a Estimation Using UAV-Based Multispectral Data and Machine Learning. Drones 2023, 7, 2. [Google Scholar] [CrossRef]
- Pyo, J.C.; Ligaray, M.; Kwon, Y.S.; Ahn, M.-H.; Kim, K.; Lee, H.; Kang, T.; Cho, S.B.; Park, Y.; Cho, K.H. High-Spatial Resolution Monitoring of Phycocyanin and Chlorophyll-a Using Airborne Hyperspectral Imagery. Remote Sens. 2018, 10, 1180. [Google Scholar] [CrossRef]
- Logan, R.D.; Torrey, M.A.; Feijó-Lima, R.; Colman, B.P.; Valett, H.M.; Shaw, J.A. UAV-Based Hyperspectral Imaging for River Algae Pigment Estimation. Remote Sens. 2023, 15, 3148. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Pyo, J.; Kwon, Y.-H.; Duan, H.; Cho, K.H.; Park, Y. Drone-Based Hyperspectral Remote Sensing of Cyanobacteria Using Vertical Cumulative Pigment Concentration in a Deep Reservoir. Remote Sens. Environ. 2020, 236, 111517. [Google Scholar] [CrossRef]
- Pyo, J.; Hong, S.M.; Jang, J.; Park, S.; Park, J.; Noh, J.H.; Cho, K.H. Drone-Borne Sensing of Major and Accessory Pigments in Algae Using Deep Learning Modeling. GIsci. Remote Sens. 2022, 59, 310–332. [Google Scholar] [CrossRef]
- Fernandez-Figueroa, E.G.; Wilson, A.E.; Rogers, S.R. Commercially Available Unoccupied Aerial Systems for Monitoring Harmful Algal Blooms: A Comparative Study. Limnol. Oceanogr. Methods 2022, 20, 146–158. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Y.; Yu, C.; Zhang, Z. Multispectral Remote Sensing for Estimating Water Quality Parameters: A Comparative Study of Inversion Methods Using Unmanned Aerial Vehicles (UAVs). Sustainability 2023, 15, 298. [Google Scholar] [CrossRef]
- Bartz, R.L.; Feiden, A. Water Transparency Analysis in Fish Farming Environment through Unmanned Aerial Vehicles. J. Appl. Res. Technol. 2023, 21, 912–920. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Xia, H.; Cao, L. Transparency Estimation of Narrow Rivers by UAV-Borne Hyperspectral Remote Sensing Imagery. IEEE Access 2020, 8, 168137–168153. [Google Scholar] [CrossRef]
- Qun’ou, J.; Lidan, X.; Siyang, S.; Meilin, W.; Huijie, X. Retrieval Model for Total Nitrogen Concentration Based on UAV Hyper Spectral Remote Sensing Data and Machine Learning Algorithms—A Case Study in the Miyun Reservoir, China. Ecol. Indic. 2021, 124, 107356. [Google Scholar] [CrossRef]
- Wang, J.; Shi, T.; Yu, D.; Teng, D.; Ge, X.; Zhang, Z.; Yang, X.; Wang, H.; Wu, G. Ensemble Machine-Learning-Based Framework for Estimating Total Nitrogen Concentration in Water Using Drone-Borne Hyperspectral Imagery of Emergent Plants: A Case Study in an Arid Oasis, NW China. Environ. Pollut. 2020, 266, 115412. [Google Scholar] [CrossRef] [PubMed]
- Seoung-Hyeon, K.; Byung-Hyun, M.; Bong-Geun, S.; Kyung-Hun, P. An Analysis on the Usability of Unmanned Aerial Vehicle(UAV) Image to Identify Water Quality Characteristics in Agricultural Streams. J. Korean Assoc. Geogr. Inf. Stud. 2019, 22, 10–20. [Google Scholar] [CrossRef]
- Liu, J.; Ding, J.; Ge, X.; Wang, J. Evaluation of Total Nitrogen in Water via Airborne Hyperspectral Data: Potential of Fractional Order Discretization Algorithm and Discrete Wavelet Transform Analysis. Remote Sens. 2021, 13, 4643. [Google Scholar] [CrossRef]
- Koparan, C.; Koc, A.B.; Privette, C.V.; Sawyer, C.B. Autonomous In Situ Measurements of Noncontaminant Water Quality Indicators and Sample Collection with a UAV. Water 2019, 11, 604. [Google Scholar] [CrossRef]
- Sandu, M.A.; Vîrsta, A.; Scăețeanu, G.V.; Iliescu, A.-I.; Ivan, I.; Nicolae, C.G.; Stoian, M.; Madjar, R.M. Water Quality Monitoring Of Moara Domnească Pond, Ilfov County, Using Uav-Based Rgb Imaging. Agrolife Sci. J. 2023, 12, 191–201. [Google Scholar] [CrossRef]
- Hu, W.; Liu, J.; Wang, H.; Miao, D.; Shao, D.; Gu, W. Retrieval of TP Concentration from UAV Multispectral Images Using IOA-ML Models in Small Inland Waterbodies. Remote Sens. 2023, 15, 1250. [Google Scholar] [CrossRef]
- Chen, B.; Mu, X.; Chen, P.; Wang, B.; Choi, J.; Park, H.; Xu, S.; Wu, Y.; Yang, H. Machine Learning-Based Inversion of Water Quality Parameters in Typical Reach of the Urban River by UAV Multispectral Data. Ecol. Indic. 2021, 133, 108434. [Google Scholar] [CrossRef]
- Arango, J.G.; Nairn, R.W. Prediction of Optical and Non-Optical Water Quality Parameters in Oligotrophic and Eutrophic Aquatic Systems Using a Small Unmanned Aerial System. Drones 2020, 4, 1. [Google Scholar] [CrossRef]
- Taddia, Y.; Russo, P.; Lovo, S.; Pellegrinelli, A. Multispectral UAV Monitoring of Submerged Seaweed in Shallow Water. Appl. Geomat. 2020, 12, 19–34. [Google Scholar] [CrossRef]
- Kislik, C.; Dronova, I.; Kelly, M. UAVs in Support of Algal Bloom Research: A Review of Current Applications and Future Opportunities. Drones 2018, 2, 35. [Google Scholar] [CrossRef]
- Becker, R.H.; Sayers, M.; Dehm, D.; Shuchman, R.; Quintero, K.; Bosse, K.; Sawtell, R. Unmanned Aerial System Based Spectroradiometer for Monitoring Harmful Algal Blooms: A New Paradigm in Water Quality Monitoring. J. Great Lakes Res. 2019, 45, 444–453. [Google Scholar] [CrossRef]
- Novković, M.; Cvijanović, D.; Minučer, M.; Pavić, D.; Drešković, N.; Milošević, Đ.; Anđelković, A.; Damnjanović, B.; Radulović, S. Towards UAV Assisted Monitoring of an Aquatic Vegetation within the Large Rivers—The Middle Danube. Carpathian J. Earth Environ. Sci. 2023, 18, 307–322. [Google Scholar] [CrossRef]
- Flynn, K.F.; Chapra, S.C. Remote Sensing of Submerged Aquatic Vegetation in a Shallow Non-Turbid River Using an Unmanned Aerial Vehicle. Remote Sens. 2014, 6, 12815–12836. [Google Scholar] [CrossRef]
- Chabot, D.; Dillon, C.; Shemrock, A.; Weissflog, N.; Sager, E.P.S. An Object-Based Image Analysis Workflow for Monitoring Shallow-Water Aquatic Vegetation in Multispectral Drone Imagery. ISPRS Int. J. Geoinf. 2018, 7, 294. [Google Scholar] [CrossRef]
- Song, B.; Park, K. Detection of Aquatic Plants Using Multispectral UAV Imagery and Vegetation Index. Remote Sens. 2020, 12, 387. [Google Scholar] [CrossRef]
- Husson, E.; Hagner, O.; Ecke, F. Unmanned Aircraft Systems Help to Map Aquatic Vegetation. Appl. Veg. Sci. 2014, 17, 567–577. [Google Scholar] [CrossRef]
- Zeng, Y.; Meng, X.; Zhang, Y.; Dai, W.; Fang, N.; Shi, Z. Estimation of the Volume of Sediment Deposited behind Check Dams Based on UAV Remote Sensing. J. Hydrol. 2022, 612, 128143. [Google Scholar] [CrossRef]
- Larson, M.D.; Simic Milas, A.; Vincent, R.K.; Evans, J.E. Multi-Depth Suspended Sediment Estimation Using High-Resolution Remote-Sensing UAV in Maumee River, Ohio. Int. J. Remote Sens. 2018, 39, 5472–5489. [Google Scholar] [CrossRef]
- Wei, L.; Huang, C.; Wang, Z.; Wang, Z.; Zhou, X.; Cao, L. Monitoring of Urban Black-Odor Water Based on Nemerow Index and Gradient Boosting Decision Tree Regression Using UAV-Borne Hyperspectral Imagery. Remote Sens. 2019, 11, 2402. [Google Scholar] [CrossRef]
- Wang, F.; Hu, H.; Luo, Y.; Lei, X.; Wu, D.; Jiang, J. Monitoring of Urban Black-Odor Water Using UAV Multispectral Data Based on Extreme Gradient Boosting. Water 2022, 14, 3354. [Google Scholar] [CrossRef]
- Sarigai; Yang, J.; Zhou, A.; Han, L.; Li, Y.; Xie, Y. Monitoring Urban Black-Odorous Water by Using Hyperspectral Data and Machine Learning. Environ. Pollut. 2021, 269, 116166. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jiang, J.; Wang, F.; Luo, Y.; Lei, X.; Lai, C.; Wu, X.; Xu, M. Retrieving Eutrophic Water in Highly Urbanized Area Coupling UAV Multispectral Data and Machine Learning Algorithms. Water 2023, 15, 354. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Hu, B.; Hou, X.; Zhang, Z.; Liu, X.; Liu, J.; Wang, X.; Zhong, J.; Tan, Z.; et al. UAV-Borne Hyperspectral Imaging Remote Sensing System Based on Acousto-Optic Tunable Filter for Water Quality Monitoring. Remote Sens. 2021, 13, 469. [Google Scholar] [CrossRef]
- Smith, B.; Beman, M.; Gravano, D.; Chen, Y. Development and Validation of a Microbe Detecting UAV Payload. In Proceedings of the 2015 Workshop on Research, Education and Development of Unmanned Aerial Systems (RED-UAS), Cancun, Mexico, 23–25 November 2015; pp. 258–264. [Google Scholar]
- Morgan, B.J.; Stocker, M.D.; Valdes-Abellan, J.; Kim, M.S.; Pachepsky, Y. Drone-Based Imaging to Assess the Microbial Water Quality in an Irrigation Pond: A Pilot Study. Sci. Total Environ. 2020, 716, 135757. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Hanlon, R.; Seifried, T.M.; Baloh, P.; Powers, C.W.; Grothe, H.; Schmale, D.G. Microorganisms Collected from the Surface of Freshwater Lakes Using a Drone Water Sampling System (DOWSE). Water 2019, 11, 157. [Google Scholar] [CrossRef]
- Cillero Castro, C.; Domínguez Gómez, J.A.; Delgado Martín, J.; Hinojo Sánchez, B.A.; Cereijo Arango, J.L.; Cheda Tuya, F.A.; Díaz-Varela, R. An UAV and Satellite Multispectral Data Approach to Monitor Water Quality in Small Reservoirs. Remote Sens. 2020, 12, 1514. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J.J. Hyperspectral Imaging: A Review on UAV-Based Sensors, Data Processing and Applications for Agriculture and Forestry. Remote Sens. 2017, 9, 110. [Google Scholar] [CrossRef]
- Ying, H.; Xia, K.; Huang, X.; Feng, H.; Yang, Y.; Du, X.; Huang, L. Evaluation of Water Quality Based on UAV Images and the IMP-MPP Algorithm. Ecol. Inform. 2021, 61, 101239. [Google Scholar] [CrossRef]
- Lu, Q.; Si, W.; Wei, L.; Li, Z.; Xia, Z.; Ye, S.; Xia, Y. Retrieval of Water Quality from UAV-Borne Hyperspectral Imagery: A Comparative Study of Machine Learning Algorithms. Remote Sens. 2021, 13, 3928. [Google Scholar] [CrossRef]
- Burdziakowski, P.; Zima, P.; Wielgat, P.; Kalinowska, D. Tracking Fluorescent Dye Dispersion from an Unmanned Aerial Vehicle. Sensors 2021, 21, 3905. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Hanlon, R.; Rypina, I.I.; Hodges, B.A.; Peacock, T.; Schmale, D.G. Tracking a Surrogate Hazardous Agent (Rhodamine Dye) in a Coastal Ocean Environment Using In Situ Measurements and Concentration Estimates Derived from Drone Images. Remote Sens. 2021, 13, 4415. [Google Scholar] [CrossRef]
- Baek, D.; Seo, I.W.; Kim, J.S.; Nelson, J.M. UAV-Based Measurements of Spatio-Temporal Concentration Distributions of Fluorescent Tracers in Open Channel Flows. Adv. Water Resour. 2019, 127, 76–88. [Google Scholar] [CrossRef]
- Powers, C.; Hanlon, R.; Schmale, D.G. Tracking of a Fluorescent Dye in a Freshwater Lake with an Unmanned Surface Vehicle and an Unmanned Aircraft System. Remote Sens. 2018, 10, 81. [Google Scholar] [CrossRef]
- Sharma, C.; Isha, I.; Vashisht, V. Water Quality Estimation Using Computer Vision in UAV. In Proceedings of the 2021 11th International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 28–29 January 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 448–453. [Google Scholar]
- Etikasari, B.; Husin; Kautsar, S.; Riskiawan, H.Y.; Setyohadi, D.P.S. Wireless Sensor Network Development in Unmanned Aerial Vehicle (UAV) for Water Quality Monitoring System. IOP Conf. Ser. Earth Environ. Sci. 2020, 411, 012061. [Google Scholar] [CrossRef]
- Koparan, C.; Koc, A.B.; Privette, C.V.; Sawyer, C.B. In Situ Water Quality Measurements Using an Unmanned Aerial Vehicle (UAV) System. Water 2018, 10, 264. [Google Scholar] [CrossRef]
- Koparan, C.; Koc, A.B.; Privette, C.V.; Sawyer, C.B.; Sharp, J.L. Evaluation of a UAV-assisted autonomous water sampling. Water 2018, 10, 655. [Google Scholar] [CrossRef]
- Koparan, C.; Koc, A.B. Unmanned Aerial Vehicle (UAV) Assisted Water Sampling. In Proceedings of the 2016 ASABE International Meeting, Orlando, FL, USA, 17–20 July 2016; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2016. [Google Scholar]
- Koparan, C.; Koc, A.B.; Privette, C.V.; Sawyer, C.B. Adaptive Water Sampling Device for Aerial Robots. Drones 2020, 4, 5. [Google Scholar] [CrossRef]
- Ore, J.-P.; Elbaum, S.; Burgin, A.; Detweiler, C. Autonomous Aerial Water Sampling. J. Field Robot. 2015, 32, 1095–1113. [Google Scholar] [CrossRef]
- Schwarzbach, M.; Laiacker, M.; Mulero-Pázmány, M.; Kondak, K. Remote Water Sampling Using Flying Robots. In Proceedings of the 2014 International Conference on Unmanned Aircraft Systems (ICUAS), Orlando, FL, USA, 27–30 May 2014; pp. 72–76. [Google Scholar]
- Koparan, C.; Koc, A.B.; Sawyer, C.; Privette, C. Temperature Profiling of Waterbodies with a UAV-Integrated Sensor Subsystem. Drones 2020, 4, 35. [Google Scholar] [CrossRef]
- Doi, H.; Akamatsu, Y.; Watanabe, Y.; Goto, M.; Inui, R.; Katano, I.; Nagano, M.; Takahara, T.; Minamoto, T. Water Sampling for Environmental DNA Surveys by Using an Unmanned Aerial Vehicle. Limnol. Oceanogr. Methods 2017, 15, 939–944. [Google Scholar] [CrossRef]
- Terada, A.; Morita, Y.; Hashimoto, T.; Mori, T.; Ohba, T.; Yaguchi, M.; Kanda, W. Water Sampling Using a Drone at Yugama Crater Lake, Kusatsu-Shirane Volcano, Japan. Earth Planets Space 2018, 70, 64. [Google Scholar] [CrossRef]
- Banerjee, B.P.; Raval, S.; Maslin, T.J.; Timms, W. Development of a UAV-Mounted System for Remotely Collecting Mine Water Samples. Int. J. Min. Reclam. Environ. 2020, 34, 385–396. [Google Scholar] [CrossRef]
- Moustakas, A.; Karakassis, I. How diverse is aquatic biodiversity research? Aqaut. Ecol. 2005, 39, 367–375. [Google Scholar] [CrossRef]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Hill, M.J.; Biggs, J.; Thornhill, I.; Briers, R.A.; Gledhill, D.G.; White, J.C.; Wood, P.J.; Hassall, C. Urban ponds as an aquatic biodiversity resource in modified landscapes. Glob. Chang. Biol. 2017, 23, 986–999. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Habitat mosaics and path analysis can improve biological conservation of aquatic biodiversity in ecosystems with low-head dams. Sci. Total Environ. 2018, 619, 221–231. [Google Scholar] [CrossRef]
- Sun, Z.H.; Brittain, J.E.; Sokolova, E.; Thygesen, H.; Saltveit, S.J.; Rauch, S.; Meland, S. Aquatic biodiversity in sedimentation ponds receiving road runoff—What are the key drivers? Sci. Total Environ. 2018, 610, 1527–1535. [Google Scholar] [CrossRef]
- Bernhardt, J.R.; O’Connor, M.I. Aquatic biodiversity enhances multiple nutritional benefits to humans. Proc. Natl. Acad. Sci. USA 2021, 118, e1917487118. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowrońska, B.; Kalinowska, R.; Skowroński, T. Cyanotoxin diversity and food web bioaccumulation in a reservoir with decreasing phosphorus concentrations and perennial cyanobacterial blooms. Harmful Algae 2013, 28, 118–125. [Google Scholar] [CrossRef]
- Shao, N.F.; Yang, S.T.; Sun, Y.; Gai, Y.; Zhao, C.S.; Wang, F.; Yin, X.; Dong, B. Assessing aquatic ecosystem health through the analysis of plankton biodiversity. Mar. Freshw. Res. 2019, 70, 647–655. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; De Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.; Matias, M.; Castillo-Escriva, A.; Messyasz, B.; Leoni, B. Microalgae colonization of different microplastic polymers in experimental mesocosms across an environmental gradient. Glob. Chang. Biol. 2021, 28, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Stockenreiter, M.; Isanta Navarro, J.; Buchberger, F.; Stibor, H. Community shifts from eukaryote to cyanobacteria dominated phytoplankton: The role of mixing depth and light quality. Freshw. Biol. 2021, 66, 2145–2157. [Google Scholar] [CrossRef]
- Donis, D.; Mantzouki, E.; McGinnis, D.F.; Vachon, D.; Gallego, I.; Grossart, H.; Domis, L.N.d.S.; Teurlincx, S.; Seelen, L.; Lürling, M.; et al. Stratification strength and light climate explain variation in chlorophyll a at the continental scale in a European multilake survey in a heatwave summer. Limnol. Oceanogr. 2021, 66, 4314–4333. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E.; Krztoń, W.; Budziak, M.; Walusiak, E.; Žutinič, P.; Gligora Udovič, M.; Koreivienė, J.; Karosienė, J.; Kasperovičienė, J.; Kobos, J.; et al. Harmful blooms across a longitudinal gradient in central Europe during heatwave: Cyanobacteria biomass, cyanotoxins, and nutrients. Ecol. Indic. 2024, 160, 111929. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Abingdon, UK, 2021; pp. 1–858. [Google Scholar]
- Rybak, A.; Messyasz, B.; Łęska, B. Freshwater Ulva (Chlorophyta) as a bioaccumulator of selected heavy metals (Cd, Ni and Pb) and alkaline earth metals (Ca and Mg). Chemosphere 2012, 89, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, J.; Lago, M.; Abhold, K.; Roeschel, L.; Kafyeke, T.; Klimmek, H.; Mattheiss, V. Protecting and Restoring Biodiversity across the Freshwater, Coastal and Marine Realms: Is the existing EU policy framework fit for purpose? Environ. Policy Gov. 2018, 28, 114–128. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Latimer, P.; Bannister, T.T.; Rabinowitch, E. Quantum yields of fluorescence of plant pigments. Science 1956, 124, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.S.; Rabinowitch, E. Excitation lifetime of photosynthetic pigments in vitro and in vivo. Science 1957, 125, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Bussotti, F.; Pollastrini, P.; Piekut, K.; Kowalik, W.; Wróbel, J.; Kalaji, H.M. Photosynthetic efficiency of Microcystis ssp. under salt stress. Environ. Exp. Bot. 2021, 186, 104459. [Google Scholar] [CrossRef]
- Millie, D.F.; Pigg, R.J.; Fahnenstiel, G.L.; Carrick, H.J. Algal chlorophylls: A synopsis of analytical methodologies. Am. Water Work. Assoc. Man. M 2010, 57, 93–122. [Google Scholar]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Hamilton, D.P. A systematic literature review of forecasting and predictive models for cyanobacteria blooms in freshwater lakes. Water Res. 2020, 182, 115959. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Beutler, M.; Wiltshire, K.H.; Arp, M.; Kruse, J.; Reineke, C.; Moldaenke, C.; Hansen, U.-P. A reduced model of the fluorescence from the cyanobacterial photosynthetic apparatus designed for the in situ detection of cyanobacteria. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2013, 1604, 33–46. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.A.; Rinke, K.; Hamilton, D.P. Light-induced fluorescence quenching leads to errors in sensor measurements of phytoplankton chlorophyll and phycocyanin. Water Res. 2021, 198, 117133. [Google Scholar] [CrossRef] [PubMed]
- Rosero-Lopez, D.; Walter, M.T.; Flecker, A.S.; Ontaneda, D.F.; Dangles, O. Standardization of instantaneous fluoroprobe measurements of benthic algal biomass and composition in streams. Ecol. Indic. 2021, 121, 107185. [Google Scholar] [CrossRef]
- Catherine, A.; Escoffier, N.; Belhocine, A.; Nasri, A.B.; Hamlaoui, S.; Yepremian, C.; Bernard, C.; Troussellier, M. On the use of the FluoroProbe®, a phytoplankton quantification method based on fluorescence excitation spectra for large-scale surveys of lakes and reservoirs. Water Res. 2012, 46, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Hodges, C.M.; Wood, S.A.; Puddick, J.; McBride, C.G.; Hamilton, D.P. Sensor manufacturer, temperature and cyanobacteria morphology affect phycocyanin fluorescence measurements. Environ. Sci. Pollut. Res. 2018, 25, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.W.; Beecraft, L.; Smith, R.E.H. Implications of irradiance exposure and non-photochemical quenching for multi-wavelength (bbe FluoroProbe) fluorometry. J. Photochem. Photobiol. B Biol. 2018, 189, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Maritorena, S.; Morel, A.; Gentili, B. Determination of the Fluorescence Quantum Yield by Oceanic Phytoplankton in Their Natural Habitat. Appl. Opt. 2000, 39, 6725–6737. [Google Scholar] [CrossRef] [PubMed]
- Huot, Y.; Babin, M.; Bruyant, F.; Grob, C.; Twardowski, M.S.; Claustre, H. Relationship between Photosynthetic Parameters and Different Proxies of Phytoplankton Biomass in the Subtropical Ocean. Biogeosciences 2007, 4, 853–868. [Google Scholar] [CrossRef]
- Lin, H.; Kuzminov, F.I.; Park, J.; Lee, S.; Falkowski, P.G.; Gorbunov, M.Y. The Fate of Photons Absorbed by Phytoplankton in the Global Ocean. Science 2016, 351, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Gilerson, A.; Zhou, J.; Hlaing, S.; Ioannou, I.; Schalles, J.; Gross, B.; Moshary, F.; Ahmed, S. Fluorescence Component in the Reflectance Spectra from Coastal Waters. Dependence on Water Composition. Opt. Express 2007, 15, 15702–15721. [Google Scholar] [CrossRef] [PubMed]
- Tenjo, C.; Ruiz-Verdú, A.; Van Wittenberghe, S.; Delegidom, J.; Moreno, J. A New Algorithm for the Retrieval of Sun Induced Chlorophyll Fluorescence of Water Bodies Exploiting the Detailed Spectral Shape of Water-Leaving Radiance. Remote Sens. 2021, 13, 329. [Google Scholar] [CrossRef]
- Downing, J. Limnology and oceanography: Two estranged twins reuniting by global change. Inland Waters 2014, 4, 215–232. [Google Scholar] [CrossRef]
- Soylak, M.; Divrikli, U.; Sarocoglu, S.; Elci, L. Monitoring trace metal levels in Yozgat-Turkey: Copper, iron, nickel, cobalt, lead, cadmium, manganese and chromium levels in stream sediments. Pol. J. Environ. Stud. 2002, 11, 7–51. [Google Scholar]
- Staniszewski, R. Heavy metals in waters and sediments of rivers affected by brown coal mine waters. Pol. J. Environ. Stud. 2014, 23, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Corral, A.M.; Val del Río, A.; Campos Gómez, J.L. Treatment and Valorisation of Saline Wastewater: Principles and Practice; IWA Publishing Republic: London, UK, 2021; 202p. [Google Scholar]
- Howell, D. Discharging Cooling Water from Power Plants into Rivers. J. Aquat. Pollut. Toxicol. 2021, 5, 29. [Google Scholar]
- Staniszewski, R.; Cais-Sokolińska, D.; Kaczyński, Ł.K.; Bielska, P. Use of Bioluminescence for Monitoring Brown Coal Mine Waters from Deep and Surface Drainage. Energies 2021, 14, 3558. [Google Scholar] [CrossRef]
- Skowysz, A. About using an empirical formulas for estimating the length of complete mixing waste waters discharged into the rivers and the channels. Przegląd Nauk.-Inżynieria I Kształtowanie Sr. 2011, 53, 237–246. [Google Scholar]
- Staniszewski, R.; Jusik, S. Impact of mine waters discharge from open-pit lignite mine on river water quality. Rocz. Ochr. Sr. 2013, 15, 2652–2665. [Google Scholar]
- European Commission 2010. Technical Guidelines for the Identification of Mixing Zones; Pursuant to Art. 4(4) of the Directive 2008/105/EC; European Commission: Brussels, Belgium, 2010; 63p. [Google Scholar]
- Shucksmith, J.; Boxall, J.; Guymer, I. Effects of emergent and submerged natural vegetation on longitudinal mixing in open channel flow. Water Resour. Res. 2010, 46, 1–14. [Google Scholar] [CrossRef]
- Jabłońska-Czapla, M.; Szopa, S.; Rosik-Dulewska, C. Impact of mining dump on the accumulation and mobility of metals in the Bytomka river sediments. Arch. Environ. Prot. 2014, 40, 3–19. [Google Scholar] [CrossRef]
- Das, P.A.; Swain, S.; Panda, S.; Pradhan, N.; Sukla, L.B. Reductive acid leaching of low grade manganese ores. Geomaterials 2012, 2, 70–72. [Google Scholar] [CrossRef]
- Siebecker, M.; Madison, A.S.; Luther, G.W., III. Reduction kinetics of polymeric (soluble) manganese (IV) oxide (MnO2) by ferrous iron (Fe2+). Aquat. Geochem. 2015, 21, 143–158. [Google Scholar] [CrossRef]
- Mercado-Garcia, D.; Wyseure, G.; Goethals, P. Freshwater ecosystem services in mining regions: Modelling options for policy development support. Water 2018, 10, 531. [Google Scholar] [CrossRef]
- Sonter, L.J.; Ali, S.H.; Watson, J.E.M. Mining and biodiversity: Key issues and research needs in conservation science. Proc. R. Soc. B 2018, 285, 20181926. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, M.; Sobczyński, T.; Zioła, A. The effect of grain size structure on the content of heavy metals in alluvial sediments of the Odra River. Pol. J. Environ. Stud. 2005, 14, 81. [Google Scholar]
- Rutherford, J.C. River Mixing; John Wiley & Sons: Hamilton, New Zealand, 1994; 362p. [Google Scholar]
- Ramezani, M.; Noori, R.; Hooshyaripor, F.; Deng, Z.; Sarang, A. Numerical modelling-based comparison of longitudinal dispersion coefficient formulas for solute transport in rivers. Hydrol. Sci. J. 2019, 64, 808–819. [Google Scholar] [CrossRef]
- Lin, S.; Boegman, L.; Jabbari, A.; Valipour, R.; Zhao, Y. Observation and parameterization of bottom shear stress and sediment resuspension in a large shallow lake. Earth Space Sci. 2023, 10, e2022EA002786. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Trzebiatowska, P.J.; Kadac-Czapska, K.; Grembecka, M. Microplastic—Sources, separation and identification techniques. Chem. News 2023, 77, 153–178. [Google Scholar]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Rands, M.R.W.; Adams, W.M.; Bennun, L.; Butchart, S.H.M.; Clements, A.; Coomes, D.; Entwistle, A.; Hodge, I.; Kapos, V.; Scharlemann, P.W.; et al. Biodiversity conservation: Challenges beyond 2010. Science 2010, 329, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the ‘Plastisphere’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.; Persaud, B.D.; Cowger, W.; Szigeti, K.; Roche, D.G.; Clary, E.; Slowinski, S.; Lei, B.; Abeynayaka, A.; Nyadjro, E.S.; et al. Current State of Microplastic Pollution Research Data: Trends in Availability and Sources of Open Data. Front. Environ. Sci. 2022, 10, 912107. [Google Scholar] [CrossRef]
- Wąsowski, J.; Bogdanowicz, A. Microplastics in the Aquatic Environment; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2020; 144p. (In Polish) [Google Scholar]
- Hanke, G.; Galgani, F.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.; Palatinus, A.; Van Franeker, J.; et al. Guidance on Monitoring of Marine Litter in European Union Seas; Leslie, H., Gago, J., Liebezeit, G., Eds.; EUR 26113; Publications Office of the European Union: Luxembourg, 2013; Volume 128, p. JRC83985. [Google Scholar] [CrossRef]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozode, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods, R. Soc. Chem. 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Gago, J.; Filgueiras, A.; Pedrotti, M.L.; João, F. Standardised Protocol for Monitoring Microplastics in Seawater; Deliverable 4.1; JPI-Oceans BASEMAN Project: Brussels, Belgium, 2019; 33p. [Google Scholar] [CrossRef]
- MSFD Technical Group on Marine Litter: Galgani, F.; Ruiz-Orejón, L.F.; Ronchi, F.; Tallec, K.; Fischer, E.K.; Matiddi, M.; Anastasopoulou, A.; Andresmaa, E.; Angiolillo, M.; Bakker Paiva, M.; et al. Guidance on the Monitoring of Marine Litter in European Seas an Update to Improve the Harmonised Monitoring of Marine Litter under the Marine Strategy Framework Directive; EUR 31539 EN; Publications Office of the European Union: Luxembourg, 2023; JRC133594; ISBN 978-92-68-04093-5. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Isobe, A.; Buenaventura, N.T.; Chastain, S.; Chavanich, S.; Cózar, A.; DeLorenzo, M.; Hagmann, P.; Hinata, H.; Kozlovskii, N.; Lusher, A.L.; et al. An interlaboratory comparison exercise for the determination of microplastics in standard sample bottles. Mar. Pollut. Bull. 2019, 146, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Phinikettou, V.; Papamichael, I.; Voukkali, I.; Zorpas, A.A. Comparison of Methodologies for Microplastic Isolation through Multicriteria Analysis (AHP). Microplastics 2024, 3, 184–204. [Google Scholar] [CrossRef]
- Imhof, H.K.; Schmid, J.; Niessner, R.; Ivleva, N.P.; Laforsch, C. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr.-Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Liebezeit, G.; Dubaish, F. Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 2012, 89, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, P.L.; Biesinger, M.C.; Grif, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Mohan, S.; Tatarchuk, T. Microplastics in water: Types, detection, and removal strategies. Environ. Sci. Pollut. Res. 2023, 30, 84933–84948. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Maximenko, N.; Thiel, M.; Cummins, A.; Lattin, G.; Wilson, S.; Hafner, J.; Zellers, A.; Rifman, S. Plastic pollution in the South Pacific subtropical gyre. Mar. Pollut. Bull. 2013, 68, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.J.; Watson, W.; Bowlin, N.M.; Sheavly, S.B. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific Ocean. Mar. Environ. Res. 2011, 71, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Jani, V.; Wu, S.; Venkiteshwaran, K. Advancements and Regulatory Situation in Microplastics Removal from Wastewater and Drinking Water: A Comprehensive Review. Microplastics 2024, 3, 98–123. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Advances in microplastics detection: A comprehensive review of methodologies and their effectiveness. Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Samsonowska, K.; Kaszuba, A. Microplastics in the natural environment. Polimery 2022, 1, 28–33. (In Polish) [Google Scholar] [CrossRef]
- Laglbauer, B.J.L.; Franco-Santos, R.M.; Cazenave, M.A.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880. [Google Scholar] [CrossRef] [PubMed]
- Liza, A.A.; Ashrafy, A.; Islam, M.N.; Billah, M.M.; Arafat, S.T.; Rahman, M.M.; Karim, M.R.; Hasan, M.M.; Promie, A.R.; Rahman, S.M. Microplastic pollution: A review of techniques to identify microplastics and their threats to the aquatic ecosystem. Environ. Monit. Assess. 2024, 196, 285. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Lannuzel, D.; Rodemann, T.; Meiners, K.M.; Auman, H.J. Microplastic contamination in east Antarctic sea ice. Mar. Pollut. Bull. 2020, 154, 111130. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Hefnawy, A.; Hazeem, L.J.; Rashdana, S.A.; Abd-Rabboh, H.S.M. Current perspectives, challenges, and future directions in the electrochemical detection of microplastics. RSC Adv. 2024, 14, 2134–2158. [Google Scholar] [CrossRef] [PubMed]
- Bordos, G.; Urbanyi, B.; Micsinai, A.; Kriszt, B.; Palotai, Z.; Szabo, I.; Hantosi, Z.; Szoboszlay, S. Identification of microplastics in fish ponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere 2019, 216, 110.e116. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; SpringerOpen: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Crawford, C.B.; Quinn, B. The interactions of microplastics and chemical pollutants. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–157. [Google Scholar]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef] [PubMed]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci.-Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Peez, N.; Rinesch, T.; Kolz, J.; Imhof, W. Applicable and cost-efficient microplastic analysis by quantitative 1H-NMR spectroscopy using benchtop NMR and NoD methods. Magn. Reson. Chem. 2022, 60, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Mauel, A.; Pötzschner, B.; Meides, N.; Siegel, R.; Strohriegl, P.; Senker, J. Quantification of photooxidative defects in weathered microplastics using 13C multiCP NMR spectroscopy. RSC Adv. 2022, 12, 10875–10885. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Földi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef] [PubMed]
- Adelugba, A.; Emenike, C. Comparative Review of Instrumental Techniques and Methods for the Analysis of Microplastics in Agricultural Matrices. Microplastics 2024, 3, 1–21. [Google Scholar] [CrossRef]
- Dümichen, E.; Barthel, A.-K.; Braun, U.; Bannick, C.G.; Brand, K.; Jekel, M.; Senz, R. Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res. 2015, 85, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Chialanza, M.R.; Sierra, I.; Parada, A.P.; Fornaro, L. Identification and quantitation of semi-crystalline microplastics using image analysis and differential scanning calorimetry; Environ. Sci. Pollut. Res. 2018, 25, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastics-benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Jagiello, Z.; Dylewski, Ł.; Szulkin, M. The plastic homes of hermit crabs in the Anthropocene. Sci. Total Environ. 2024, 913, 168959. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wan, H. Analytical methods for microplastics in the environment: A review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniszewski, R.; Messyasz, B.; Dąbrowski, P.; Burdziakowski, P.; Spychała, M. Recent Issues and Challenges in the Study of Inland Waters. Water 2024, 16, 1216. https://doi.org/10.3390/w16091216
Staniszewski R, Messyasz B, Dąbrowski P, Burdziakowski P, Spychała M. Recent Issues and Challenges in the Study of Inland Waters. Water. 2024; 16(9):1216. https://doi.org/10.3390/w16091216
Chicago/Turabian StyleStaniszewski, Ryszard, Beata Messyasz, Piotr Dąbrowski, Pawel Burdziakowski, and Marcin Spychała. 2024. "Recent Issues and Challenges in the Study of Inland Waters" Water 16, no. 9: 1216. https://doi.org/10.3390/w16091216
APA StyleStaniszewski, R., Messyasz, B., Dąbrowski, P., Burdziakowski, P., & Spychała, M. (2024). Recent Issues and Challenges in the Study of Inland Waters. Water, 16(9), 1216. https://doi.org/10.3390/w16091216









