Quantify Mercury Sulfide in Sediments for Bioavailability Assessment
Abstract
1. Introduction
2. Materials and Methods
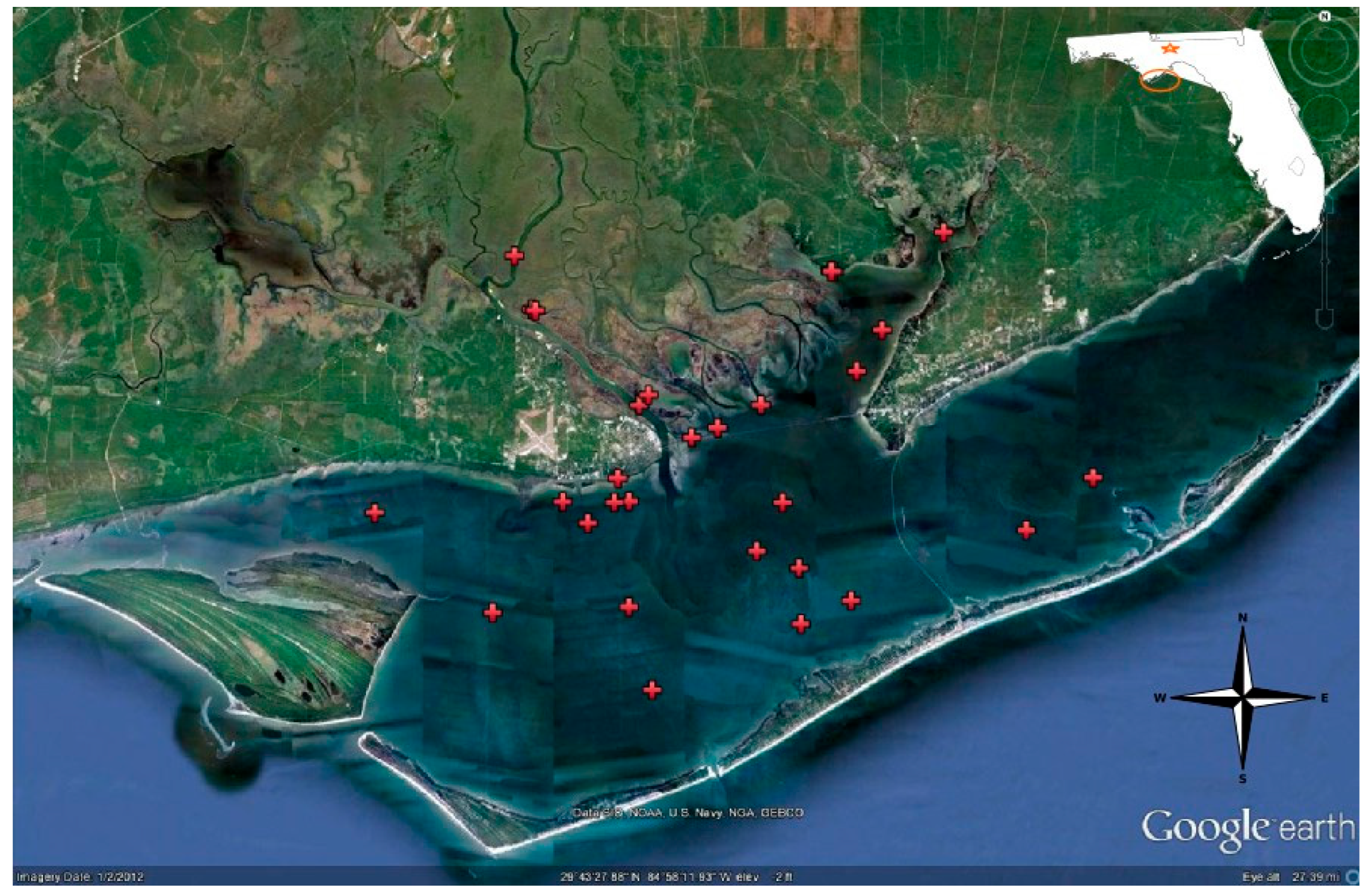
2.1. The Study Site
2.2. Sample Collection
2.3. Acid-Volatile-Sulfide (AVS) and Chromium-Reducible-Sulfide (CRS)
2.4. Acid-Extractable (AE-Hg) and Total Hg (HgT)
2.5. Bulk Density, Moisture Content, and Weight Loss-on-Ignition
2.6. Statistical Analysis
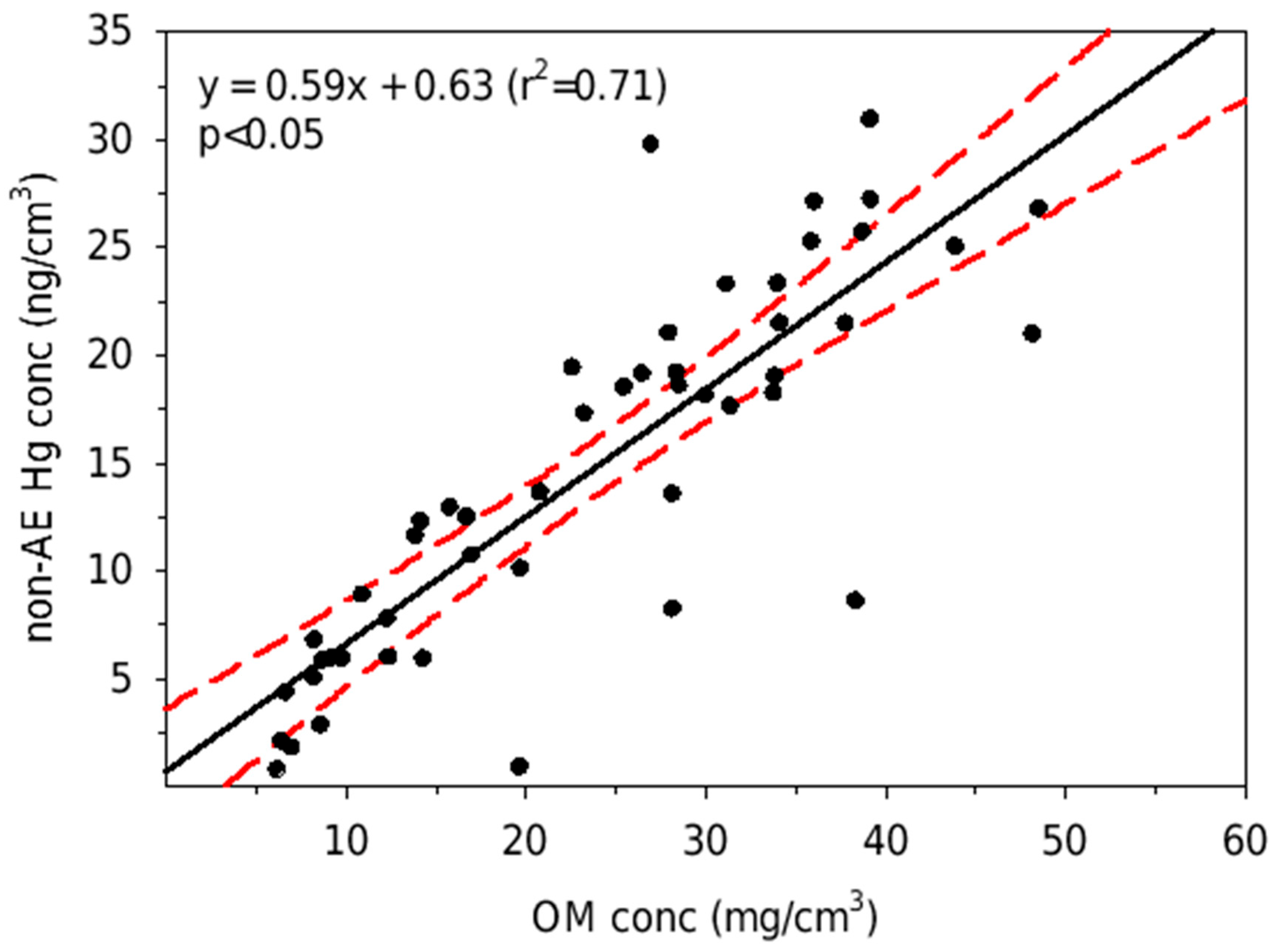
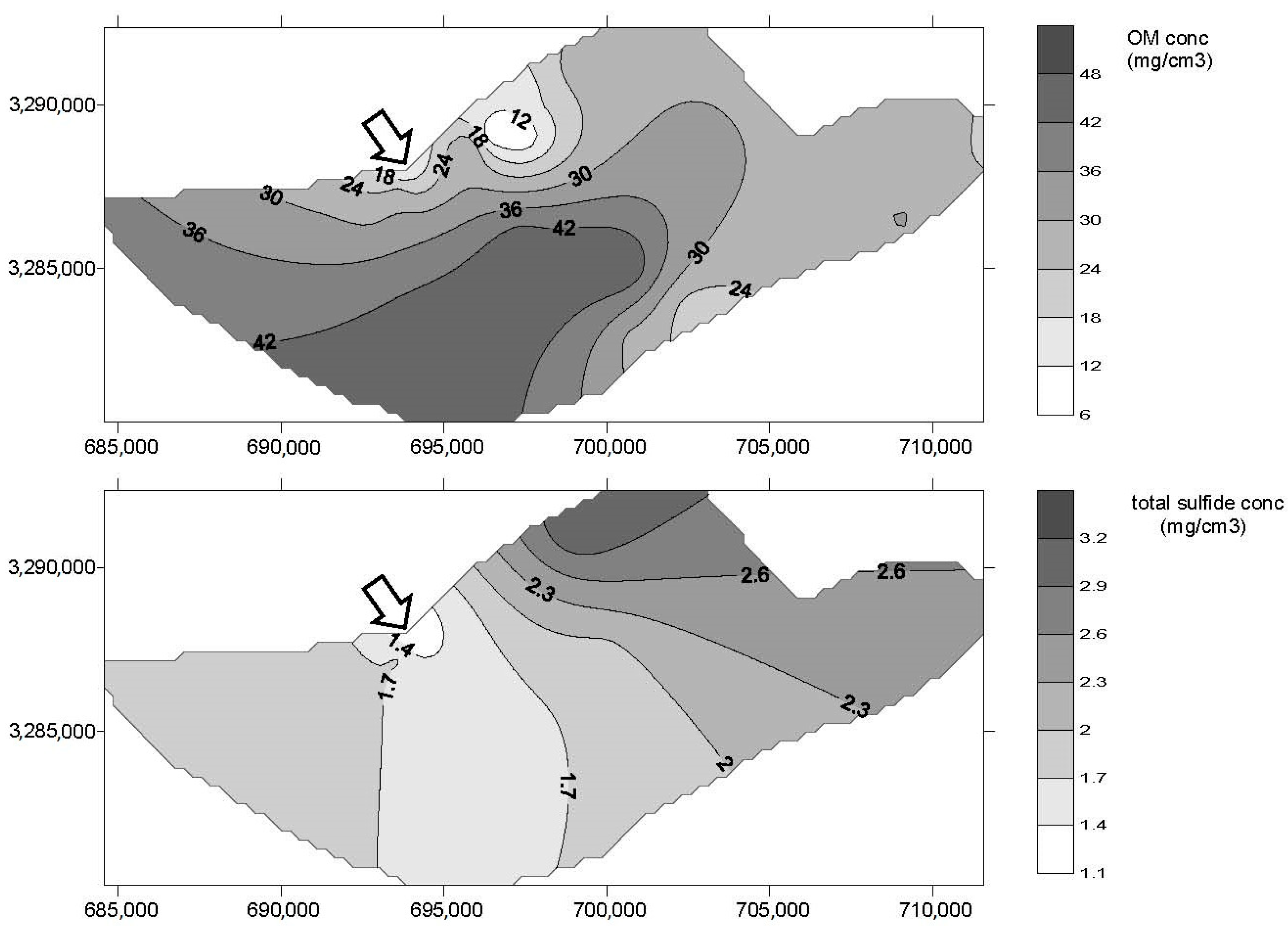
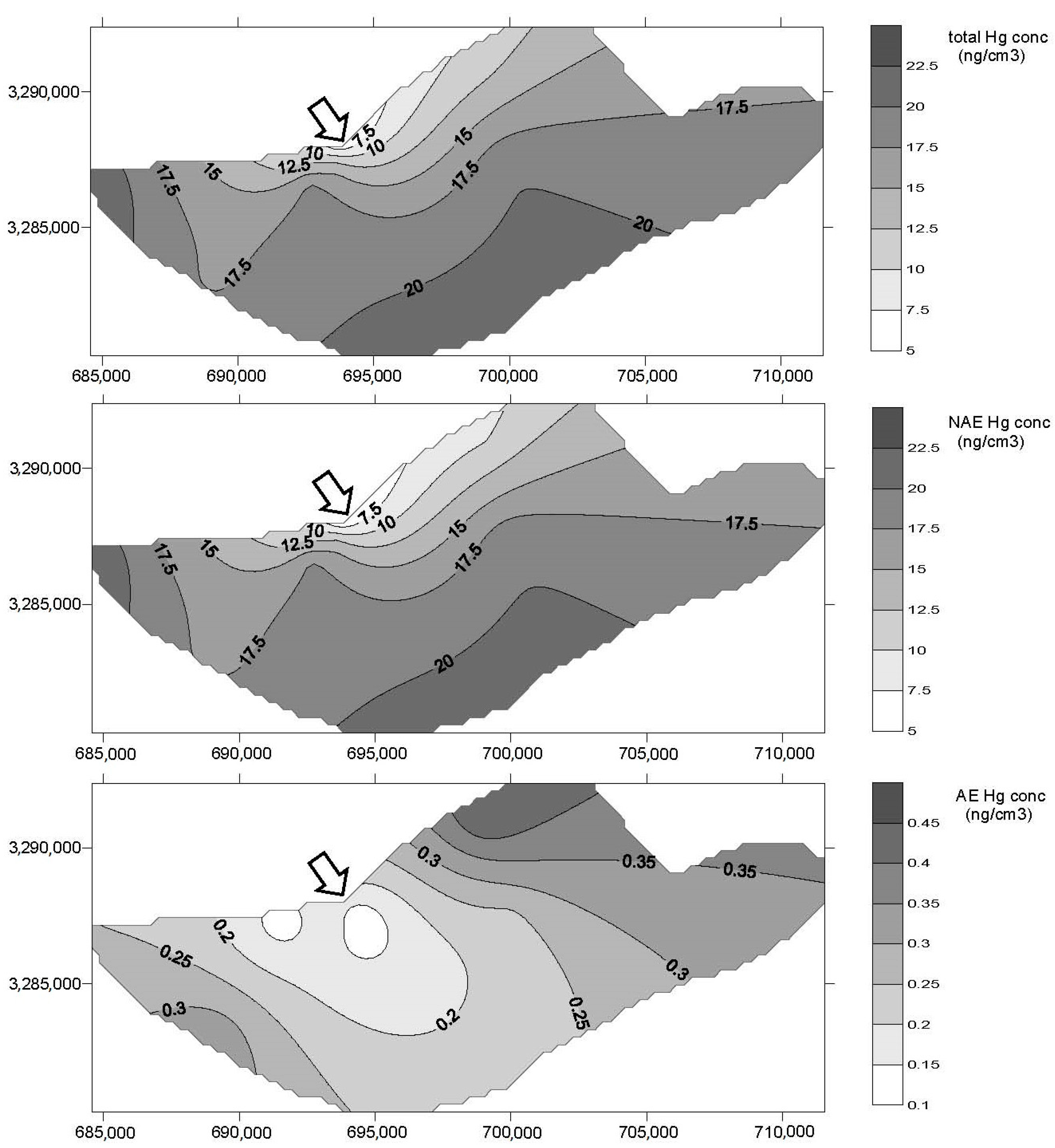
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, P.A.; Ferrari, R.G.; Santos, L.N.; Conte, C.A. Mercury in aquatic fauna contamination: A systematic review on its dynamics and potential health risks. J. Environ. Sci. 2019, 84, 2015–2018. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillete, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Kwasigroch, U.; Bełdowska, M.; Jędruch, A.; Lukawska-Matuszewska, K. Distribution and bioavailability of mercury in the surface sediments of the Baltic Sea. Environ. Sci. Pollut. Res. 2021, 28, 35690–35708. [Google Scholar] [CrossRef]
- Bloom, N.S. On the chemical form mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Lenanton, R.C.J. Mercury contamination in semi-enclosed marine embayment: Organic and inorganic mercury content of biota, and factors influencing mercury level in fish. Mar. Environ. Res. 1992, 33, 189–212. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P.; Heyes, A. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ. Sci. Technol. 1999, 33, 951–957. [Google Scholar] [CrossRef]
- Wallschläger, D.; Desai, M.V.M.; Spengler, M.; Wilken, R.D. Mercury Speciation in Floodplain Soils and Sediments along a Contaminated River Transect. J. Environ. Qual. 1998, 27, 1034–1044. [Google Scholar] [CrossRef]
- Beldowski, J.; Pempkowiak, J. Horizontal and vertical variabilities of mercury concentration and speciation in sediments of the Gdansk Basin, Southern Baltic Sea. Chemosphere 2003, 52, 645–654. [Google Scholar] [CrossRef]
- Sladeka, C.; Gustin, M.S. Evaluation of sequential and selective extraction methods for determination of mercury speciation and mobility in mine waste. Appl. Geochem. 2003, 18, 567–576. [Google Scholar] [CrossRef]
- Di Toro, D.M.; Mahony, J.D.; Hansen, D.J.; Scott, K.J.; Hicks, M.B.; Mayr, S.M.; Redmond, M.S. Toxicity of cadmium in sediments: The role of acid volatile sulfide. Environ. Sci. Technol. 1990, 9, 1487–1502. [Google Scholar] [CrossRef]
- Di Toro, D.M.; Mahony, J.D.; Hansen, D.J.; Scott, K.J.; Carlson, A.R.; Ankley, G.T. Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments. Environ. Sci. Technol. 1992, 26, 96–101. [Google Scholar] [CrossRef]
- Casas, A.M.; Crecelius, E.A. Relationship between acid volatile sulfide and the toxicity of zinc, lead, and copper in marine sediments. Environ. Toxicol. Chem. 1994, 13, 529–536. [Google Scholar] [CrossRef]
- Morse, J.W. Interactions of trace metals with authigenic sulfide minerals: Implications for their bioavailability. Mar. Chem. 1994, 46, 187–199. [Google Scholar] [CrossRef]
- Berry, W.J.; Hansen, D.J.; Mahony, J.D.; Robson, D.L.; Di Toro, D.M.; Shipley, B.P.; Rogers, B.; Corbin, J.M.; Boothman, W.S. Predicting the toxicity of metal-spiked laboratory sediments using acid-volatile sulfide and interstitial water concentrations. Environ. Toxicol. Chem. 1996, 15, 2067–2079. [Google Scholar] [CrossRef]
- Hansen, D.J.; Berry, W.J.; Mahony, J.D.; Boothman, W.S.; Di Toro, D.M.; Robson, D.L.; Ankley, G.T.; Ma, D.; Yan, Q.; Pesch, C.E. Predicting the toxicity of metal-contaminated field sediments using interstitial concentration of metals and acid-volatile sulfide normalizations. Environ. Toxicol. Chem. 1996, 15, 2080–2094. [Google Scholar] [CrossRef]
- Hsieh, Y.P. The chemical nature of mercury and copper sulfides and its implication for bioavailability assessment in sediments. Water 2024, 16, 70. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W. Modeling sediment resuspension and transport induced by storm wind in Apalachicola Bay, USA. Environ. Modell. Softw. 2009, 24, 1302–1313. [Google Scholar] [CrossRef]
- Joshi, I.D.; D’Sa, E.J.; Osburn, C.L.; Bianchi, T.S.; Ko, D.S.; Oviedo-Vargas, D.; Arellano, A.R.; Ward, N.D. Assessing chromophoric dissolved organic matter (CDOM) distribution, stocks, and fluxes in Apalachicola Bay using combined field, VIIRS ocean color, and model observations. Remote Sens. Environ. 2017, 191, 359–372. [Google Scholar] [CrossRef]
- Hallas, M.K.; Huettel, M. Bar-built estuary as a buffer for riverine silicate discharge to the coastal ocean. Cont. Shelf Res. 2013, 55, 76–85. [Google Scholar] [CrossRef]
- Chen, S.; Huang, W.; Chen, W.; Chen, X. An enhanced MODIS remote sensing model for detecting rainfall effects on sediment plume in the coastal waters of Apalachicola Bay. Mar. Environ. Res. 2011, 72, 265–272. [Google Scholar] [CrossRef]
- Huang, W.; Sun, H.; Nnaji, S.; Jones, W.K. Hydrodynamics in a multiple-inlet. J. Coast. Res. 2002, 18, 674–684. [Google Scholar]
- Northwest Florida Water Management District. Apalachicola River and Bay Surface Water Improvement and Management (SWIM) Plan; Northwest Florida Water Management District: Midway, FL, USA, 2017; 137p.
- Hsieh, Y.P.; Yang, C.H. Diffusion methods for the determination of reduced sulfur species in sediments. Limnol. Oceanogr. 1989, 34, 1126–1131. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Shieh, Y.N. Analysis of reduced inorganic sulfur by diffusion methods: Improved apparatus and evaluation for sulfur isotopic studies. Chem. Geol. 1997, 137, 255–261. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 14th ed.; American Public Health Association: Washington, DC, USA, 1976; p. 1193. [Google Scholar]
- SAS Institute, Inc. SAS User’s Guide: Statistics, Version 9; SAS Institute: Cary, NC, USA, 2005. [Google Scholar]
- Golden Software Inc. Surface Mapping System, Version 8.05; Golden Software, Inc.: Golden, CO, USA, 2004. [Google Scholar]
- Huerta-Diaz, M.A.; Morse, J.W. A quantitative method for determination of trace metal concentration in sedimentary pyrite. Mar. Chem. 1990, 29, 119–144. [Google Scholar] [CrossRef]
- Kamman, N.C.; Chalmers, A.; Clair, A.T.; Major, A.; Moore, R.B.; Norton, S.A.; Shanley, J.B. Factors Influencing Mercury in Freshwater Surface Sediments of Northeastern North America. Ecotoxicology 2005, 14, 101–111. [Google Scholar] [CrossRef]
- Andren, A.W.; Nriagu, J.O. The global cycle of mercury. In Topics in Environmental Health; The Biogeochemistry of Mercury in the Environment; Nriagu, J.O., Ed.; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1979; Volume 3, pp. 1–21. [Google Scholar]
- Shacklette, H.T.; Boerngen, J.G. Element concentrations in soils and other surficial materials of the conterminous United States. In U.S. Geological Survey Professional Paper 1270; U.S. Geological Survey: Alexandria, VA, USA, 1984; 105p. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 2nd ed.; John Wiley and Sons: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1981; 780p. [Google Scholar]
- Ravichandran, M.; Aiken, G.R.; Reddy, M.M.; Ryan, J.N. Enhanced dissolution of cinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida Everglades. Environ. Sci. Technol. 1998, 32, 3305–3311. [Google Scholar] [CrossRef]
- Ravichandran, M.; Aiken, G.R.; Ryan, J.N.; Reddy, M.M. Inhibition of precipitation and aggregation of metacinnabar (mercury sulfide) by dissolved organic matter isolated from the Florida Everglades. Environ. Sci. Technol. 1999, 33, 1418–1423. [Google Scholar] [CrossRef]
- Ravichandran, M. Interactions between mercury and dissolved organic matter—A review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef]
- Seelen, E.; Robert Mason, R.; Liem-Nguyen, V.; Wünsch, U.; Skyllberg, U.; Baumann, Z.; Björn, E. Dissolved organic matter thiol concentrations determine methylmercury bioavailability across the terrestrial-marine aquatic continuum. Nat. Commun. 2023, 14, 6728. [Google Scholar] [CrossRef] [PubMed]
| Depth | BD g/cm3 | OM mg/cm3 | AVS + CRS mg/cm3 | Total Hg ng/cm3 | non-AE-Hg ng/cm3 | non-AE-Hg % | AE Hg ng/cm3 |
|---|---|---|---|---|---|---|---|
| 0–20 cm | |||||||
| Min | 0.18 | 2.90 | 0.29 | 2.75 | 2.69 | 86.34 | 0.06 |
| Max | 0.99 | 48.53 | 4.95 | 28.22 | 28.01 | 99.47 | 1.14 |
| Mean | 0.46 | 25.42 | 2.35 | 12.64 | 11.60 | 96.94 | 0.26 |
| SD | 0.20 | 12.18 | 1.12 | 5.58 | 6.37 | 3.17 | 0.23 |
| n | 50 | 50 | 50 | 48 | 48 | 48 | 48 |
| 20–45 cm | |||||||
| Min | 0.30 | 10.91 | 0.31 | 5.33 | 5.08 | 93.38 | 0.10 |
| Max | 0.57 | 42.37 | 10.20 | 25.35 | 22.58 | 99.47 | 0.78 |
| Mean | 0.39 | 28.56 | 3.42 | 14.57 | 13.99 | 97.29 | 0.34 |
| SD | 0.09 | 9.36 | 2.19 | 5.05 | 5.41 | 1.85 | 0.20 |
| n | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-P.; Bugna, G. Quantify Mercury Sulfide in Sediments for Bioavailability Assessment. Water 2025, 17, 2759. https://doi.org/10.3390/w17182759
Hsieh Y-P, Bugna G. Quantify Mercury Sulfide in Sediments for Bioavailability Assessment. Water. 2025; 17(18):2759. https://doi.org/10.3390/w17182759
Chicago/Turabian StyleHsieh, Yuch-Ping, and Glynnis Bugna. 2025. "Quantify Mercury Sulfide in Sediments for Bioavailability Assessment" Water 17, no. 18: 2759. https://doi.org/10.3390/w17182759
APA StyleHsieh, Y.-P., & Bugna, G. (2025). Quantify Mercury Sulfide in Sediments for Bioavailability Assessment. Water, 17(18), 2759. https://doi.org/10.3390/w17182759






