A Critical Review of Nanobubble Flotation for Seawater Treatment Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objective
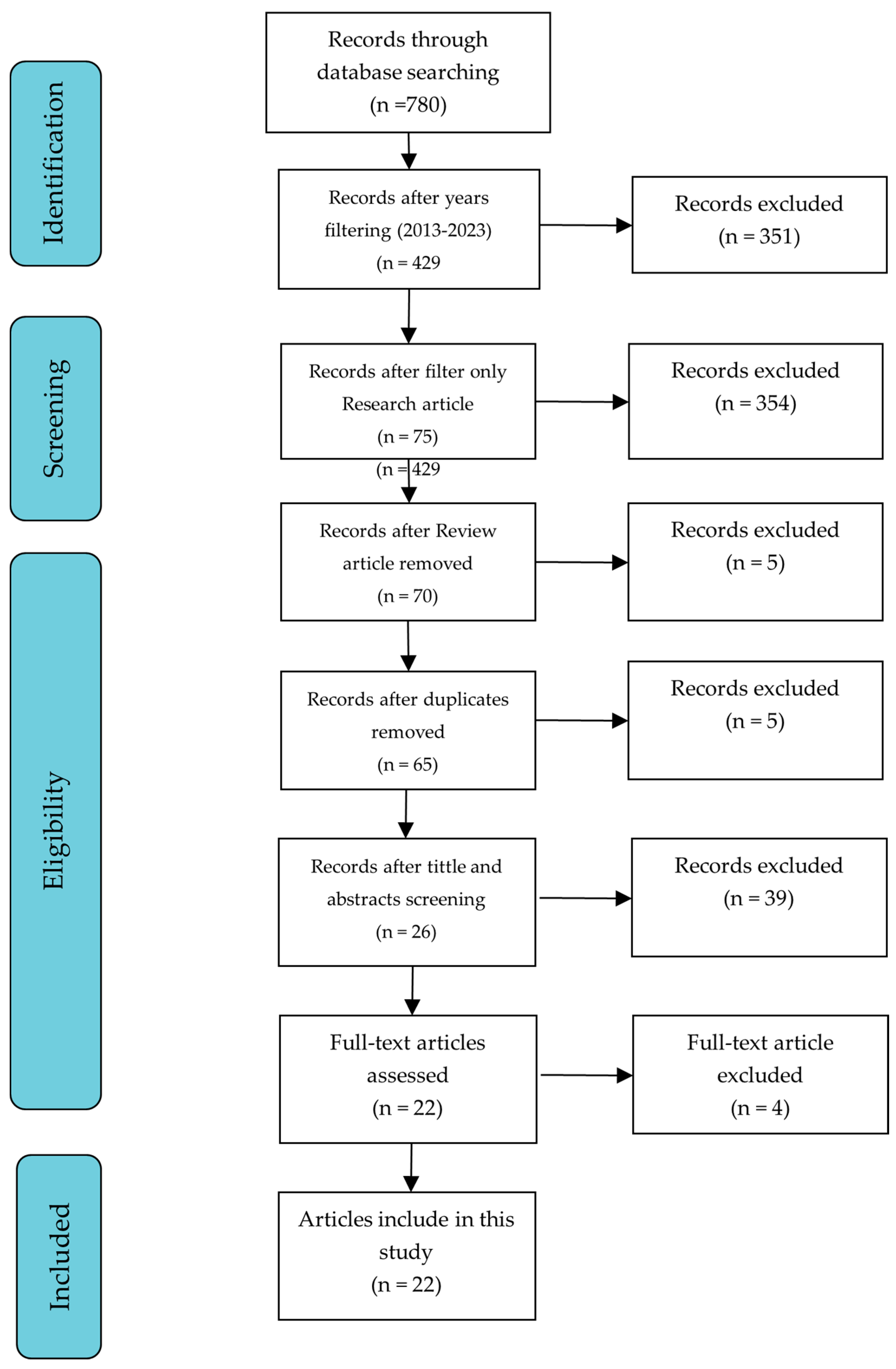
2.2. Screening
| Search Local | Search Expression | Search Result | Types of Documents |
|---|---|---|---|
| Science Direct | (Seawater! OR Seawater desalination!) AND (with nanobubbles) (Ion flotation! OR salt ion!) AND (with nanobubbles) | 246 |
|
| Google Scholar | (Seawater! OR Seawater treatment!) AND (with nanobubbles) (Ion flotation! OR salt ion!) AND (with nanobubbles) | 380 |
|
2.3. Eligibility
2.4. Inclusion
3. Types of Gas Nanobubbles and Flotation in the Seawater Desalination
3.1. Type of Gas Nanobubbles
3.2. Generation of Nanobubbles
- Hydrodynamics—system geometry-induced variation in the pressure of liquid flux [62].
- Acoustic—a sound made when ultrasound is applied to liquids [63].
- Particle—passing light photons with high intensity through liquids [64].
- Optical—lasers with short pulses focused on solutions with low absorption coefficients [65].
3.3. Generation and Production of Different Gas Nanobubbles
3.4. Nanobubble Application in the Water Treatment
3.4.1. Aeration Process
3.4.2. Flotatixon Process
3.4.3. Disinfection Process
4. Results & Discussion
4.1. Types of Flotation
4.2. Effect of the Nanobubble Flotation to Enhance the Water Treatment Process
4.3. Interactions of NBs and Seawater: Physical, Chemical, Electronic, and Mechanical Interactions
4.3.1. Physical Interactions
4.3.2. Chemical Interactions
4.3.3. Electronic Interactions
4.3.4. Mechanical Interactions
4.4. Nanobubbles Technology for Various Applications
4.4.1. Ion Separation in Seawater Desalination
4.4.2. Nanobubble Generations Methods
- (a)
- Mechanical Stirring Method
- (b) Nanoscale Pore Membrane Method
- (c) Microfluidic Method
- (d) Acoustic Cavitation Method
- (e) Hydrodynamics Cavitation Method
4.5. Effect of Gas Nanobubbles in the Water Treatment
4.6. Effect of Surfactant on the Ion Flotation for Seawater Desalination
5. Author Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Nicolai, A.; Sumpter, B.G.; Meunier, V. Tunable water desalination across graphene oxide framework membranes. Phys. Chem. Chem. Phys. 2014, 16, 8646–8654. [Google Scholar] [PubMed]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, S. Influence of hydraulic pressure on performance deterioration of direct contact membrane distillation (DCMD) process. Membranes 2019, 9, 37. [Google Scholar] [CrossRef]
- Rameshkumar, C.; Senthilkumar, G.; Subalakshmi, R. Purification of tap water to drinking water: Nanobubbles technology. Desalin. Water Treat. 2021, 233, 11–18. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Bae, J. Membrane processes and renewable energies. Renew. Sustain. Energy Rev. 2015, 43, 1343–1398. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, F.; Li, X.G.; Ma, J. Recent progress on adsorption and membrane separation for organic contaminants on multi-dimensional graphene. Mater. Today Chem. 2021, 22, 100603. [Google Scholar] [CrossRef]
- Tao, D. Recent advances in fundamentals and applications of nanobubble enhanced froth flotation: A review. Miner. Eng. 2022, 183, 107554. [Google Scholar] [CrossRef]
- Halali, M.A. Electrically Conductive Membranes for Water and Wastewater Treatment: Their Surface Properties, Antifouling Mechanisms, and Applications. 2021. Available online: https://macsphere.mcmaster.ca/handle/11375/26707 (accessed on 5 January 2021).
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitroupolus, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.-T.; Li, Q.; et al. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669. [Google Scholar] [CrossRef] [PubMed]
- Atab, M.S.; Smallbone, A.J.; Roskilly, A.P. A hybrid reverse osmosis/adsorption desalination plant for irrigation and drinking water. Desalination 2018, 444, 44–52. [Google Scholar] [CrossRef]
- ILevitsky; Tavor, D.; Gitis, V. Micro and nanobubbles in water and wastewater treatment: A state-of-the-art review. J. Water Process Eng. 2022, 47, 102688. [Google Scholar] [CrossRef]
- Zhan, X.; Gao, Z.; Ge, R.; Lu, J.; Li, J.; Wan, X. Rigid POSS intercalated graphene oxide membranes with hydrophilic/hydrophobic heterostructure for efficient pervaporation desalination. Desalination 2022, 543, 116106. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, D.; Zhang, X. Degradation Mechanism of Micro-Nanobubble Technology for Organic Pollutants in Aqueous Solutions. Nanomaterials 2022, 12, 2654. [Google Scholar] [CrossRef]
- Tyszer, M.; Tomaszewska, B.; Kabay, N. Desalination of geothermal wastewaters by membrane processes: Strategies for environmentally friendly use of retentate streams. Desalination 2021, 520, 115330. [Google Scholar] [CrossRef]
- Chen, Q.; Burhan, M.; Shahzad, M.W.; Ybyraiymkul, D.; Akhtar, F.H.; Li, Y.; Ng, K.C. A zero liquid discharge system integrating multi-effect distillation and evaporative crystallization for desalination brine treatment. Desalination 2021, 502, 114928. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Z.; Jiao, F.; Qin, W. Understanding bubble growth process under decompression and its effects on the flotation phenomena. Miner. Eng. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Mørch, K.A. Cavitation inception from bubble nuclei. Interface Focus 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 1–15. [Google Scholar] [CrossRef]
- Palani, G.; Arputhalatha, A.; Kannan, K.; Lakkaboyana, S.K.; Hanafiah, M.M.; Kumar, V.; Marella, R.K. Current Trends in the Application of Nanomaterials for the Removal of Pollutants from Industrial Wastewater Treatment—A Review. Molecules 2021, 26, 2799. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Flores, A.; Solongo, S.K.; Heyes, G.W.; Ilyas, S.; Kim, H. Bubble−particle interactions with hydrodynamics, XDLVO theory, and surface roughness for flotation in an agitated tank using CFD simulations. Miner. Eng. 2020, 152, 106368. [Google Scholar] [CrossRef]
- Xiong, Y.; Peng, F. Optimization of cavitation venturi tube design for pico and nano bubbles generation. Int. J. Min. Sci. Technol. 2015, 25, 523–529. [Google Scholar] [CrossRef]
- Nazari, S.; Zhou, S.; Hassanzadeh, A.; Li, J.; He, Y.; Bu, X.; Kowalczuk, P.B. Influence of operating parameters on nanobubble-assisted flotation of graphite. J. Mater. Res. Technol. 2022, 20, 3891–3904. [Google Scholar] [CrossRef]
- Rulyov, N.; Filippov, L.; Kravchenko, O. Combined microflotation of glass beads. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124810. [Google Scholar] [CrossRef]
- Han, B.M.; Dockko, S. Zeta potential measurement of bubbles in DAF process. Water Supply 1998, 2, 461–466. [Google Scholar]
- Swart, B.; Pihlajamäki, A.; Chew, Y.J.; Wenk, J. Microbubble-microplastic interactions in batch air flotation. Chem. Eng. J. 2022, 449, 137866. [Google Scholar] [CrossRef]
- Aaltonen, A.; Le, T.M.K.; Saari, E.; Dahl, O.; Musuku, B.; Lang, A.; Hiidenheimo, S.; Dixon, R. Improving Nickel Recovery in Froth Flotation by Purifying Concentrators Process Water Using Dissolved Air Flotation. Minerals 2023, 13, 319. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Rejeb, Z.B.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2022, 292, 133102. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Farid, M.U.; Kharraz, J.A.; Kumar, N.M.; Chopra, S.S.; Jang, A.; Chew, J.; Khanal, S.K.; Chen, G.; An, A.K. Nanobubbles in water and wastewater treatment systems: Small bubbles making big difference. Water Res. 2023, 245, 120613. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G. Mass transfer of nanobubble aeration and its effect on biofilm growth: Microbial activity and structural properties. Sci. Total Environ. 2020, 703, 134976. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Shkirin, A.V.; Penkov, N.V.; Goltayev, M.V.; Ignatiev, P.S.; Gudkov, S.V.; Izmailov, A.Y. Effect of Gas Type and Its Pressure on Nanobubble Generation. Front. Chem. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Dong, C.D. Advances in micro- and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729. [Google Scholar] [CrossRef]
- Han, Z.; Kurokawa, H.; Matsui, H.; He, C.; Wang, K.; Wei, Y.; Dodbiba, G.; Otsuki, A.; Fujita, T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials 2022, 12, 237. [Google Scholar] [CrossRef]
- Yang, X.; Nie, J.; Wang, D.; Zhao, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Lei, Z.; Zhang, Z.; Yang, X.; et al. Enhanced hydrolysis of waste activated sludge for methane production via anaerobic digestion under N2-nanobubble water addition. Sci. Total Environ. 2019, 693, 133524. [Google Scholar] [CrossRef]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Formation and Stability of Carbon Dioxide Nanobubbles for Potential Applications in Food Processing. Food Eng. Rev. 2021, 13, 3–14. [Google Scholar] [CrossRef]
- Azhin, M.; Popli, K.; Afacan, A.; Liu, Q.; Prasad, V. A dynamic framework for a three phase hybrid flotation column. Miner. Eng. 2021, 170, 107028. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Q.; Wang, J.; Liu, J.; Liu, G.; Xie, W.S.L.; Liu, Q.; Zeng, H. Nanomechanical insights into hydrophobic interactions of mineral surfaces in interfacial adsorption, aggregation and flotation processes. Chem. Eng. J. 2023, 455, 140642. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Hewage, S.A.; Batagoda, J.H. Stability of Nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Wu, C.; Nesset, K.; Masliyah, J.; Xu, Z. Generation and characterization of submicron size bubbles. Adv. Colloid Interface Sci. 2012, 179–182, 123–132. [Google Scholar] [CrossRef]
- Liu, H.; Cao, G. Effectiveness of the Young-Laplace equation at nanoscale. Sci. Rep. 2016, 6, 23936. [Google Scholar] [CrossRef]
- Farzanegan, A.; Khorasanizadeh, N.; Sheikhzadeh, G.A.; Khorasanizadeh, H. Laboratory and CFD investigations of the two-phase flow behavior in flotation columns equipped with vertical baffle. Int. J. Miner. Process. 2017, 166, 79–88. [Google Scholar] [CrossRef]
- Khan, P.; Zhu, W.; Huang, F.; Gao, W.; Khan, N.A. Micro-nanobubble technology and water-related application. Water Sci. Technol. Water Supply 2020, 20, 2021–2035. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Zhang, X. The existence and stability of bulk nanobubbles: A long-standing dispute on the experimentally observed mesoscopic inhomogeneities in aqueous solutions. Commun. Theor. Phys. 2020, 72, 037601. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, D.C.; Han, M. Average size and zeta potential of nanobubbles in different reagent solutions. J. Nanoparticle Res. 2019, 21, 173. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Wang, H.; Hu, B.; Lei, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Zhang, Z.; Guo, Z.; et al. Effects of nanobubble water on the growth of: Lactobacillus acidophilus 1028 and its lactic acid production. RSC Adv. 2019, 9, 30760–30767. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Han, M.; Kim, T. Verifying sub-micron (nano) bubbles generation and their fundamental characteristics. Environ. Eng. Res. 2019, in press. [Google Scholar]
- Atkinson, A.J.; Apul, O.G.; Schneider, O.; Garcia-Segura, S.; Westerhoff, P. Nanobubble Technologies Offer Opportunities to Improve Water Treatment. Acc. Chem. Res. 2019, 52, 1196–1205. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, Z.; Chen, H.; Pan, H.; Jiang, L.; Su, W.H.; Chen, Q.; Tang, Y.; Pan, J.; Yu, K. Full life circle of micro-nano bubbles: Generation, characterization and applications. Chem. Eng. J. 2023, 471, 144621. [Google Scholar] [CrossRef]
- Hanam, E.S.; Sofia, D.R.; Azhary, S.Y.; Panatarani, C.; Joni, I.M. Effect of Gas Sources on the Oxygen Transfer Efficiency Produced by Fine Bubbles Generator. J. Phys. Conf. Ser. 2022, 2376, 012004. [Google Scholar] [CrossRef]
- Kikuchi, K.; Nagata, S.; Tanaka, Y.; Saihara, Y.; Ogumi, Z. Characteristics of hydrogen nanobubbles in solutions obtained with water electrolysis. J. Electroanal. Chem. 2007, 600, 303–310. [Google Scholar] [CrossRef]
- Oliveira, H.; Azevedo, A.; Rubio, J. Nanobubbles generation in a high-rate hydrodynamic cavitation tube. Miner. Eng. 2018, 116, 32–34. [Google Scholar] [CrossRef]
- Xu, M.; Li, C.; Zhang, H.; Kupka, N.; Peuker, U.A.; Rudolph, M. A contribution to exploring the importance of surface air nucleation in froth flotation—The effects of dissolved air on graphite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127866. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical approach to nanobubble solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Nazari, S.; Hassanzadeh, A.; He, Y.; Khoshdast, H.; Kowalczuk, P.B. Recent Developments in Generation, Detection and Application of Nanobubbles in Flotation. Minerals 2022, 12, 462. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Kyzas, G.Z.; Mitrikas, G.; Mitropoulos, A.C.; Efthimiadou, E.K.; Favvas, E.P. Bulk nanobubbles: Production and investigation of their formation / stability mechanism. J. Colloid Interface Sci. 2020, 564, 371–380. [Google Scholar] [CrossRef]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Nanobubbles: Fundamental characteristics and applications in food processing. Trends Food Sci. Technol. 2020, 95, 118–130. [Google Scholar] [CrossRef]
- Nanobubbles: Fundamental characterisEl Arwadi, O.; Zuruzi, A.S. Towards Bulk Nanobubble Generation: Development of a Bulk Nanobubble Generator Based on Hydrodynamic Cavitation. Int. J. Recent Adv. Mech. Eng. 2022, 11, 2. [Google Scholar] [CrossRef]
- Folden, T.S.; Aschmoneit, F. A classification and review of cavitation models with an emphasis on physical aspects of cavitation. Phys. Fluids 2023, 35, 081301. [Google Scholar] [CrossRef]
- Ganguli, A.A.; Pandit, A.B. Hydrodynamics of liquid-liquid flows in micro channels and its influence on transport properties: A review. Energies 2021, 14, 6066. [Google Scholar] [CrossRef]
- Simon, J.C.; Sapozhnikov, O.A.; Khokhlova, V.A.; Crum, L.A.; Bailey, M.R. Ultrasonic atomization of liquids in drop-chain acoustic fountains. J. Fluid Mech. 2015, 766, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Sangal, M.; Keitel, C.H.; Tamburini, M. Observing light-by-light scattering in vacuum with an asymmetric photon collider. Phys. Rev. D 2021, 104, L111101. [Google Scholar] [CrossRef]
- Guzmán-Barraza, A.; Ortega-Mendoza, J.G.; Zaca-Morán, P.; Toto-Arellano, N.I.; Toxqui-Quitl, C.; Padilla-Martinez, J.P. Optical cavitation in non-absorbent solutions using a continuous-wave laser via optical fiber. Opt. Laser Technol. 2022, 154, 108330. [Google Scholar] [CrossRef]
- Tsuge, H. Micro- and Nanobubbles, Fundamental and Application; Jenny Stanford Publishing: New York, NY, USA, 2014. [Google Scholar]
- Lee, J.; Cho, W.-C.; Poo, K.-M.; Choi, S.; Kim, T.-N.; Son, E.-B.; Choi, Y.-J.; Kim, Y.M.; Chae, K.-J. Refractory oil wastewater treatment by dissolved air flotation, electrochemical advanced oxidation process, and magnetic biochar integrated system. J. Water Process Eng. 2020, 36, 1–11. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Boutilier, M.S.H.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef]
- Nazari, M.; Davoodabadi, A.; Huang, D.; Luo, T.; Ghasemi, H. On interfacial viscosity in nanochannels. Nanoscale 2020, 12, 14626–14635. [Google Scholar] [CrossRef] [PubMed]
- Kurita, Y.; Chiba, I.; Kijima, A. Physical eradication of small planktonic crustaceans from aquaculture tanks with cavitation treatment. Aquac. Int. 2017, 25, 2127–2133. [Google Scholar] [CrossRef]
- Azevedo, A.; Etchepare, R.; Calgaroto, S.; Rubio, J. Aqueous dispersions of nanobubbles: Generation, properties and features. Miner. Eng. 2016, 94, 29–37. [Google Scholar] [CrossRef]
- Mondal, S.; Acharjee, A.; Mandal, U.; Saha, B. Froth flotation process and its application. Vietnam J. Chem. 2021, 59, 417–425. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, W.; Liuv, R.; Qiu, J.; Wang, S. Water purification performance and energy consumption of gradient nanocomposite membranes. Compos. Part B Eng. 2020, 202, 108426. [Google Scholar] [CrossRef]
- Peng, W.; Chang, L.; Li, P.; Han, G.; Huang, Y.; Cao, Y. An overview on the surfactants used in ion flotation. J. Mol. Liq. 2019, 286, 110955. [Google Scholar] [CrossRef]
- Yan, T.; Hua, Z.; Deng, Y.; Guo, H.; Xu, W.; Xu, E.; Wang, W.; Ding, T.; Cao, Y.; Liu, Y.; et al. Air nanobubbles induced reversible self-assembly of 7S globulins isolated from pea (Pisum Sativum L.). Food Hydrocoll. 2022, 133, 107847. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Cen, C.; Wu, X.; Mao, R.; Zheng, Y. Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Qin, L.; Wang, D.; Zhang, Z.; Li, X.; Chai, G.; Lin, Y.; Liu, C.; Cao, R.; Song, Y.; Meng, H.; et al. Impact of Dissolved Oxygen on the Performance and Microbial Dynamics in Side-Stream Activated Sludge Hydrolysis Process. Water 2023, 15, 1977. [Google Scholar] [CrossRef]
- Zăbavă, B.-Ș.; Voicu, G.; Safta, V.-V.; Ungureanu, N. Types of Aerators Used in Wastewater Treatment Plants. In Proceedings of the 5th International Conference on Thermal Equipment, Renewable Energy and Rural Development, Golden Sands, Bulgaria, 2–4 June 2016; pp. 455–460. Available online: https://www.researchgate.net/publication/305325229_TYPES_OF_AERATORS_USED_IN_WASTEWATER_TREATMENT_PLANTS (accessed on 5 November 2024).
- Sharma, H.; Nirmalkar, N. Enhanced gas-liquid mass transfer coefficient by bulk nanobubbles in water. Mater. Today Proc. 2022, 57, 1838–1841. [Google Scholar] [CrossRef]
- Reichmann, F.; Herath, J.; Mensing, L.; Kockmann, N. Gas-liquid mass transfer intensification for bubble generation and breakup in micronozzles. J. Flow Chem. 2021, 11, 429–444. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L. Theoretical model for micro-nano-bubbles mass transfer during contaminant treatment. J. Environ. Eng. Sci. 2019, 14, 157–167. [Google Scholar] [CrossRef]
- Lee, J.I.; Han, J.-G.; Kim, J.-M. Formation and stability of bulk nanobubbles generated by gas-liquid mixing. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Hopper, S.H.; McCowen, M.C. A Flotation Process for Water Purification. J. AWWA 1952, 44, 719–726. [Google Scholar] [CrossRef]
- Haris, S.; Qiu, X.; Klammler, H.; Mohamed, M.M.A. The use of micro-nano bubbles in groundwater remediation: A comprehensive review. Groundw. Sustain. Dev. 2020, 11, 100463. [Google Scholar] [CrossRef]
- Ahmed, N.; Jameson, G.J. The effect of bubble size on the rate of flotation of fine particles. Int. J. Miner. Process. 1985, 14, 195–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Zhang, Y. Role of nanobubbles in the flotation of fine rutile particles. Miner. Eng. 2021, 172, 107140. [Google Scholar] [CrossRef]
- Collins, G.L.; Jameson, G.J. Experiments on the flotation of fine particles. The influence of particle size and charge. Chem. Eng. Sci. 1976, 31, 985–991. [Google Scholar] [CrossRef]
- Maeng, M.; Shahi, N.K.; Dockko, S. Enhanced flotation technology using low-density microhollow beads to remove algae from a drinking water source. J. Water Process Eng. 2021, 42, 102131. [Google Scholar] [CrossRef]
- Habib, S.; Weinman, S.T. A review on the synthesis of fully aromatic polyamide reverse osmosis membranes. Desalination 2021, 502, 114939. [Google Scholar] [CrossRef]
- Sumikura, M.; Hidaka, M.; Murakami, H.; Nobutomo, Y.; Murakami, T. Ozone micro-bubble disinfection method for wastewater reuse system. Water Sci. Technol. 2007, 56, 53–61. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Lu, Q.; Hu, W.; Yang, D.; Qiaob, C.; Peng, X.; Peng, Q.; Wang, T.; Sun, W.; et al. Surface interaction mechanisms in mineral flotation: Fundamentals, measurements, and perspectives. Adv. Colloid Interface Sci. 2021, 295, 102491. [Google Scholar] [CrossRef]
- Mezule, L.; Tsyfansky, S.; Yakushevich, V.; Juhna, T. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination 2009, 248, 152–159. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M. Nanobubbles effect on the mechanical flotation of phosphate ore fine particles. Physicochem. Probl. Miner. Process 2018, 54, 278–292. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z. Nanobubble generation and its applications in froth flotation (part IV): Mechanical cells and specially designed column flotation of coal. Min. Sci. Technol. (China) 2010, 20, 641–671. [Google Scholar] [CrossRef]
- Doyle, F.M. Ion flotation—Its potential for hydrometallurgical operations. Int. J. Miner. Process. 2003, 72, 387–399. [Google Scholar] [CrossRef]
- Asghar, F.; Shakoor, B.; Fatima, S.; Munir, S.; Razzaq, H.; Naheed, S.; Butler, I.S. Fabrication and prospective applications of graphene oxide-modified nanocomposites for wastewater remediation. RSC Adv. 2022, 12, 11750–11768. [Google Scholar] [CrossRef]
- Laskowski, J.; Castro, S.; Ramos, O. Effect of seawater main components on frothability in the flotation of Cu-Mo sulfide ore. Physicochem. Probl. Miner. Process. 2014, 50, 17–29. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.; Rubio, J. Bulk nanobubbles in the mineral and environmental areas: Updating research and applications. Adv. Colloid Interface Sci. 2019, 271, 101992. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, L.; Guo, J.; Liu, S.; Li, B. Adsorption and accumulation mechanism of N2 on groove-type rough surfaces: A molecular simulation study. J. Mol. Liq. 2022, 366, 120260. [Google Scholar] [CrossRef]
- Narayanan, T.N.; Liu, Z.; Lakshmy, P.R.; Gao, W.; Nagaoka, Y.; Kumar, D.S.; Lou, J.; Vajtai, R.; Ajayan, P.M. Synthesis of reduced graphene oxide-Fe3O4 multifunctional freestanding membranes and their temperature dependent electronic transport properties. Carbon NY 2012, 50, 1338–1345. [Google Scholar] [CrossRef]
- Senthilkumar, G.; Rameshkumar, C.; Nikhil, M.N.V.S.; Kumar, J.N.R. An investigation of nanobubbles in aqueous solutions for various applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 8, 91–97. [Google Scholar]
- Wang, W.; Huang, Y.; Zuo, J.; Kou, L.; Liu, B.; Sun, H.; Han, G. A feasible strategy for separating oxyanions-loaded microfine Fe-MOF adsorbents from solution by bubble flotation. Chem. Eng. J. 2023, 454, 140299. [Google Scholar] [CrossRef]
- Long, Q.; Wang, H.; Jiang, F.; Tan, W.; Chen, J.; Zhong, S.; Guo, X.; Wang, Q.; Xu, Z. Enhancing flotation separation of fine copper oxide from silica by microbubble assisted hydrophobic aggregation. Miner. Eng. 2022, 189, 107863. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.Y.; Xu, C.G.; Huang, Z.Y.; Li, X.S. Research progress on the effects of nanoparticles on gas hydrate formation. RSC Adv. 2022, 12, 20227–20238. [Google Scholar] [CrossRef] [PubMed]
- Kukizaki, M.; Goto, M. Size control of nanobubbles generated from Shirasu-porous-glass (SPG) membranes. J. Memb. Sci. 2006, 281, 386–396. [Google Scholar] [CrossRef]
- Kar, S.; Bindal, R.; Tewari, P. Carbon nanotube membranes for desalination and water purification: Challenges and opportunities. Nano Today 2012, 7, 385–389. [Google Scholar] [CrossRef]
- Polat, H.; Erdogan, D. Heavy metal removal from waste water by ion flotation. J. Hazard. Mater. 2007, 148, 267–273. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Madzokere, T.C.; Rusere, K.; Chiririwa, H. Nano-Silica based mineral flotation frother: Synthesis and flotation of Platinum Group Metals (PGMs). Miner. Eng. 2021, 166, 1–10. [Google Scholar] [CrossRef]
- Eisavi, R.; Ahmadi, F. Fe3O4@SiO2-PMA-Cu magnetic nanoparticles as a novel catalyst for green synthesis of β-thiol-1,4-disubstituted-1,2,3-triazoles. Sci. Rep. 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Yu, X.L.; He, Y. Development of a rapid and simple method for preparing tea-leaf saponins and investigation on their surface tension differences compared with tea-seed saponins. Molecules 2018, 23, 1796. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles effect on heavy metal ions adsorption by activated carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Senthilkumar, G.; Sankar, S.L. Implementation of Micro-Nanobubbles Technology for the Treatment of Domestic Wastewater: Experimental Study. Water Air Soil Pollut. 2023, 234, 1–9. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, M.; Li, J.; Kang, N.; Ahmed, A.; Zong, Y.; Tu, J.; Chen, Y.; Zhang, P.; Liu, X. Graphene-Based Membranes for Water Desalination: A Literature Review and Content Analysis. Polymers 2022, 14, 4246. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; He, J.; Lai, Y.; Li, Y.; Yan, W.; Zhou, Y.; Gao, C. Generation of Nano-Bubbles by NaHCO3 for Improving the FO Membrane Performance. Membranes 2023, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, Y.; Gao, J.; Liu, J.; Zhang, Y. Hydrotalcite/graphene oxide hybrid nanosheets functionalized nanofiltration membrane for desalination. Desalination 2019, 451, 209–218. [Google Scholar] [CrossRef]
- Eklund, F.; Alheshibri, M.; Swenson, J. Differentiating bulk nanobubbles from nanodroplets and nanoparticles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101427. [Google Scholar] [CrossRef]
- Rajapakse, N.; Zargar, M.; Sen, T.; Khiadani, M. Effects of influent physicochemical characteristics on air dissolution, bubble size and rise velocity in dissolved air flotation: A review. Sep. Purif. Technol. 2022, 289, 120772. [Google Scholar] [CrossRef]
- Ferrari, M.; Benedetti, A. Superhydrophobic surfaces for applications in seawater. Adv. Colloid Interface Sci. 2015, 222, 291–304. [Google Scholar] [CrossRef]
- Shen, W.; Mukherjee, D.; Koirala, N.; Hu, G.; Lee, K.; Zhao, M.; Li, J. Microbubble and nanobubble-based gas flotation for oily wastewater treatment: A review. Environ. Rev. 2022, 30, 359–379. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.A.; Rubio, J. Treatment and water reuse of lead-zinc sulphide ore mill wastewaters by high rate dissolved air flotation. Miner. Eng. 2018, 127, 114–121. [Google Scholar] [CrossRef]
- Tuziuti, T.; Yasui, K.; Kanematsu, W. Influence of addition of degassed water on bulk nanobubbles. Ultrason. Sonochem. 2018, 43, 272–274. [Google Scholar] [CrossRef]
- Hilares, R.T.; Singh, I.; Meza, K.T.; Andrade, G.J.C.; Tanaka, D.A.P. Alternative methods for cleaning membranes in water and wastewater treatment. Water Water Environ. Res. 2022, 94, e10708. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Ye, Z.; Chen, Y.; Lin, P. Robust seawater desalination and sewage purification enabled by the solar-thermal conversion of the Janus-type graphene oxide evaporator. Desalination 2022, 522, 115406. [Google Scholar] [CrossRef]
- Oikawa, M.; Takeuchi, H.; Chikyu, D.; Ohba, T.; Wang, Z.M.; Koura, S. Insight into the role of ionicity in the desalination and separation of a graphene oxide membrane. Desalination 2023, 552, 116433. [Google Scholar] [CrossRef]
- Taqieddin, A.; Allshouse, M.R.; Alshawabkeh, A.N. Editors’ Choice—Critical Review—Mathematical Formulations of Electrochemically Gas-Evolving Systems. J. Electrochem. Soc. 2018, 165, E694–E711. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Kim, D.; Yang, S.C.; Park, H. Numerical prediction of pressure drop and flow characteristics of activated carbon and salt-water for flow-electrode capacitive mixing (F-CapMix). Desalination 2023, 550, 116382. [Google Scholar] [CrossRef]
- MacHado, A.B.; Rodrigues, G.Z.P.; Feksa, L.R.; Berlese, D.B.; Tundisi, J.G. Applications of nanotechnology in water treatment. Rev. Conhecimento Online 2019, 1, 3–15. [Google Scholar] [CrossRef]
- Hilares, R.T.; Atoche-Garay, D.F.; Pagaza, D.A.P.; Ahmed, M.A.; Andrade, G.J.C.; Santos, J.C. Promising physicochemical technologies for poultry slaughterhouse wastewater treatment: A critical review. J. Environ. Chem. Eng. 2021, 9, 105174. [Google Scholar] [CrossRef]
- Tran, K.M.; Do, D.P.; Vu, H.N.; Nguyen, T.T.; Phan, B.T.; Thi, K.H.T.; Pham, K.N. Experimental combined theoretical study on chemical interactions of graphene oxide with chitosan and its resistive-switching effect. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114788. [Google Scholar] [CrossRef]
- Agarwal, A. An experimental study of nanobubbles on hydrophobic surfaces. Master Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005. [Google Scholar]
- Wang, L.; Zhang, J.; Cao, Z.; Zheng, Y.; Wang, Y.; Zhang, C.; Zuo, Y.; Jiao, F. Evaluation of Sulfonic Cellulose Membranes on Oil–Water Separation: Performance and Modeling of Flux. Ind. Eng. Chem. Res. 2021, 60, 13013–13022. [Google Scholar] [CrossRef]
- Agarwal, K.; Trivedi, M.; Nirmalkar, N. Does salting-out effect nucleate nanobubbles in water: Spontaneous nucleation? Ultrason. Sonochem. 2022, 82, 105860. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Hassanzadeh, A.; Legawiec, K.J.; Polowczyk, I.; Kowalczuk, P.B. Effect of ultrasound pre-treatment on carbonaceous copper-bearing shale flotation. Ultrason. Sonochem. 2022, 84, 105962. [Google Scholar] [CrossRef] [PubMed]
- Suenobu, T. Polymorphism of crystalline metal complexes affording luminochromism. Inorg. Chem. 2016. Available online: https://www.researchgate.net/profile/Vinoth-Kumar-Paramasivam/publication/333994170_Additive_Manufacturing_of_CFRP_Composites_Issues_and_Challenges/links/5d11a401299bf1547c7c7408/Additive-Manufacturing-of-CFRP-Composites-Issues-and-Challenges.pdf (accessed on 5 January 2021).
- Vlotman, D.; Key, D.; Cerff, B.; Bladergroen, B.J. Shear Enhanced Flotation Separation Technology in Winery Wastewater Treatment. Water 2023, 15, 2409. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, A.A.; Kerr, L.; Wong, D.A.; Kolios, M.C.; Tsai, S.S.H. Biomedical nanobubbles and opportunities for microfluidics. RSC Adv. 2021, 11, 32750–32774. [Google Scholar] [CrossRef]
- Pratopo, L.H.; Thoriq, A.; Sampurno, R.M.; Joni, I.M. Application of Fine Bubble Generator on the Hydroponic System of Nutrient Film Technique. Adv. Eng. Forum 2021, 41, 67–74. [Google Scholar] [CrossRef]
- Kumar, S. Smart and innovative nanotechnology applications for water purification. Hybrid Adv. 2023, 3, 100044. [Google Scholar] [CrossRef]
- Tang, C.; Bruening, M.L. Ion separations with membranes. J. Polym. Sci. 2020, 58, 2831–2856. [Google Scholar] [CrossRef]
- Li, F.; Fang, C.; Liu, W.; Yang, L.; Guo, B. High recovery, energy efficient wastewater desalination. J. Memb. Sci. 2021, 631, 119317. [Google Scholar] [CrossRef]
- Li, P.; Teng, K.; Guo, C.; Shi, H.; Li, B.; Pei, X.; Wang, W.; Xu, Z. Synergistic effect of polyvinyl alcohol sub-layer and graphene oxide condiment from active layer on desalination behavior of forward osmosis membrane. J. Taiwan Inst. Chem. Eng. 2020, 112, 366–376. [Google Scholar] [CrossRef]
- Labarre, L.A.; Saint-Jalmes, A.; Vigolo, D. Microfluidics investigation of the effect of bulk nanobubbles on surfactant-stabilised foams. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130169. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Peng, C.; Feng, Y.; Fan, X.; Yang, X.; Gao, W.; Zhang, Q. Air nanobubble simultaneously enhances hydrolysis and methane yield of sludge temperature phased-anaerobic digestion. Bioresour. Technol. 2025, 419, 132084. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M.; Nguyen, A.; Chelgani, S.C. Proving the existence of nanobubbles produced by hydrodynamic cavitation and their significant effects in powder flotation. Adv. Powder Technol. 2021, 32, 1810–1818. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Meng, Y.T.; Zeng, G.M.; Fang, Y.Y.; Shi, J.G. Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 256–261. [Google Scholar] [CrossRef]
- Santander, M.; Valderrama, L.; Guevara, M.; Rubio, J. Adsorbing colloidal flotation removing metals ions in a modified jet cell. Miner. Eng. 2011, 24, 1010–1015. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Matis, K.A. Various flotation techniques for metal ions removal. J. Mol. Liq. 2017, 225, 260–264. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Hernández-Expósito, A.; Chimenos, J.M.; Fernández, A.I.; Font, O.; Querol, X.; Coca, P.; Peña, F.G. Ion flotation of germanium from fly ash aqueous leachates. Chem. Eng. J. 2006, 118, 69–75. [Google Scholar] [CrossRef]
- Menezes, C.T.B.; Barros, E.C.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Replacing synthetic with microbial surfactants as collectors in the treatment of aqueous effluent produced by acid mine drainage, using the dissolved air flotation technique. Appl. Biochem. Biotechnol. 2011, 163, 540–546. [Google Scholar] [CrossRef]
- Kim, D.; Han, J. Remediation of Copper Contaminated Soils Using Water Containing Hydrogen Nanobubbles. Appl. Sci. 2020, 10, 2185. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, S.; Liu, J.; Zhu, Y.; Han, Y.; Zhang, F. Statistical analysis and optimization of reverse anionic hematite flotation integrated with nanobubbles. J. Mater. Res. Technol. 2022, 293, 106799. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environment 2022, 9, 141. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Rudolph, M.; Butt, H.-J.; Kappl, M. The role of surface forces in mineral flotation. Curr. Opin. Colloid Interface Sci. 2019, 44, 143–152. [Google Scholar] [CrossRef]

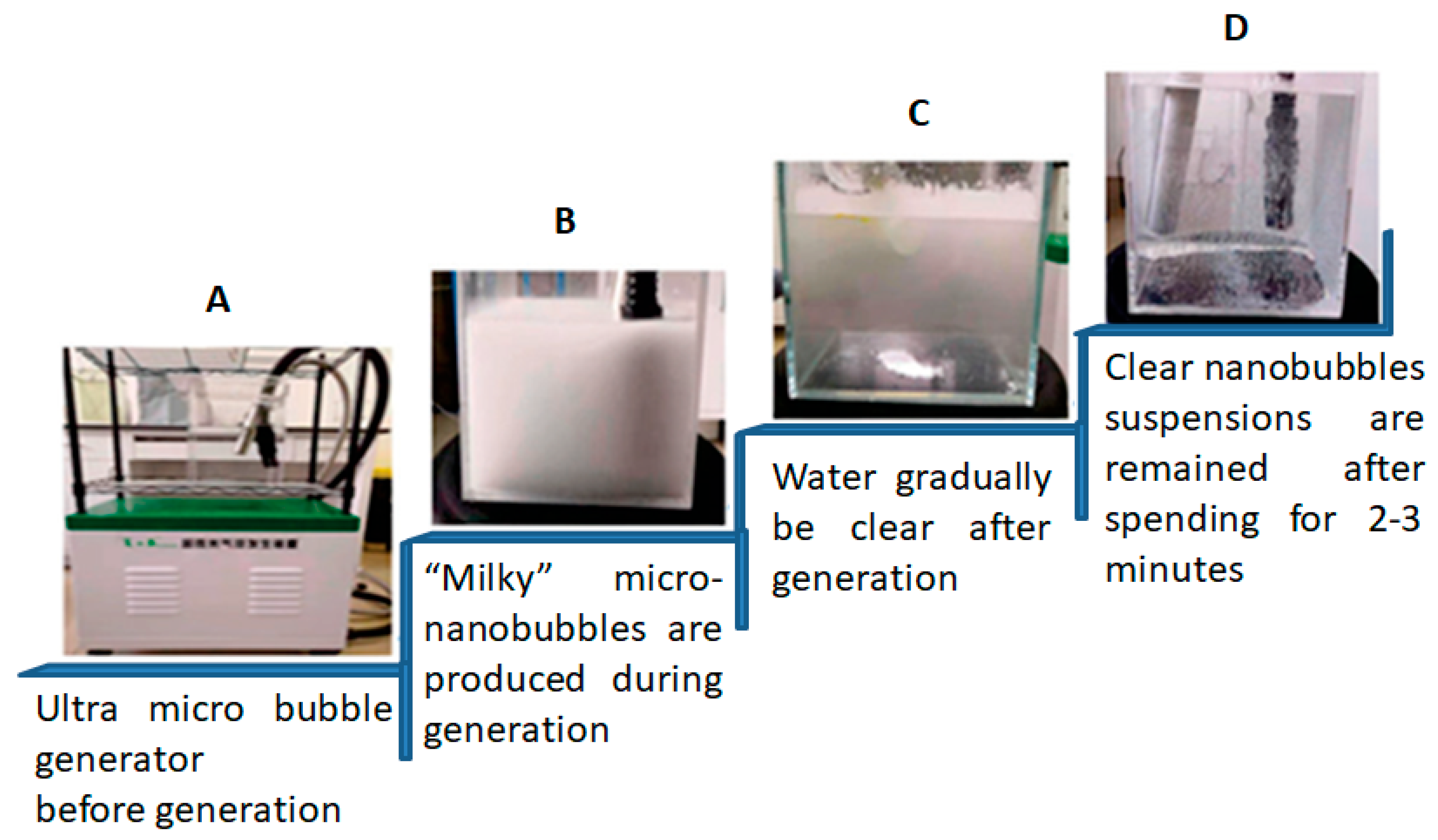
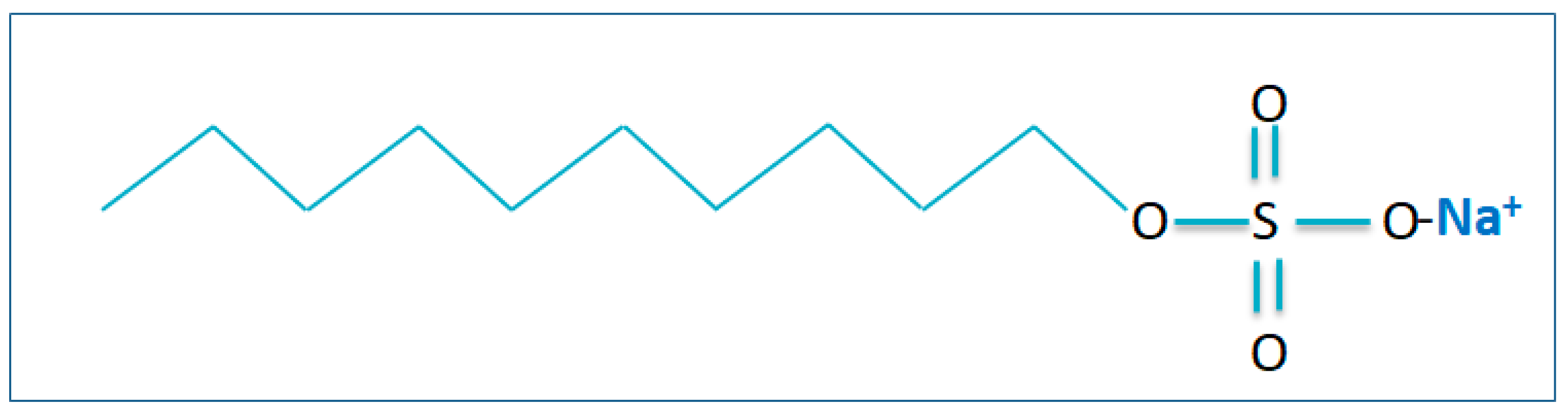
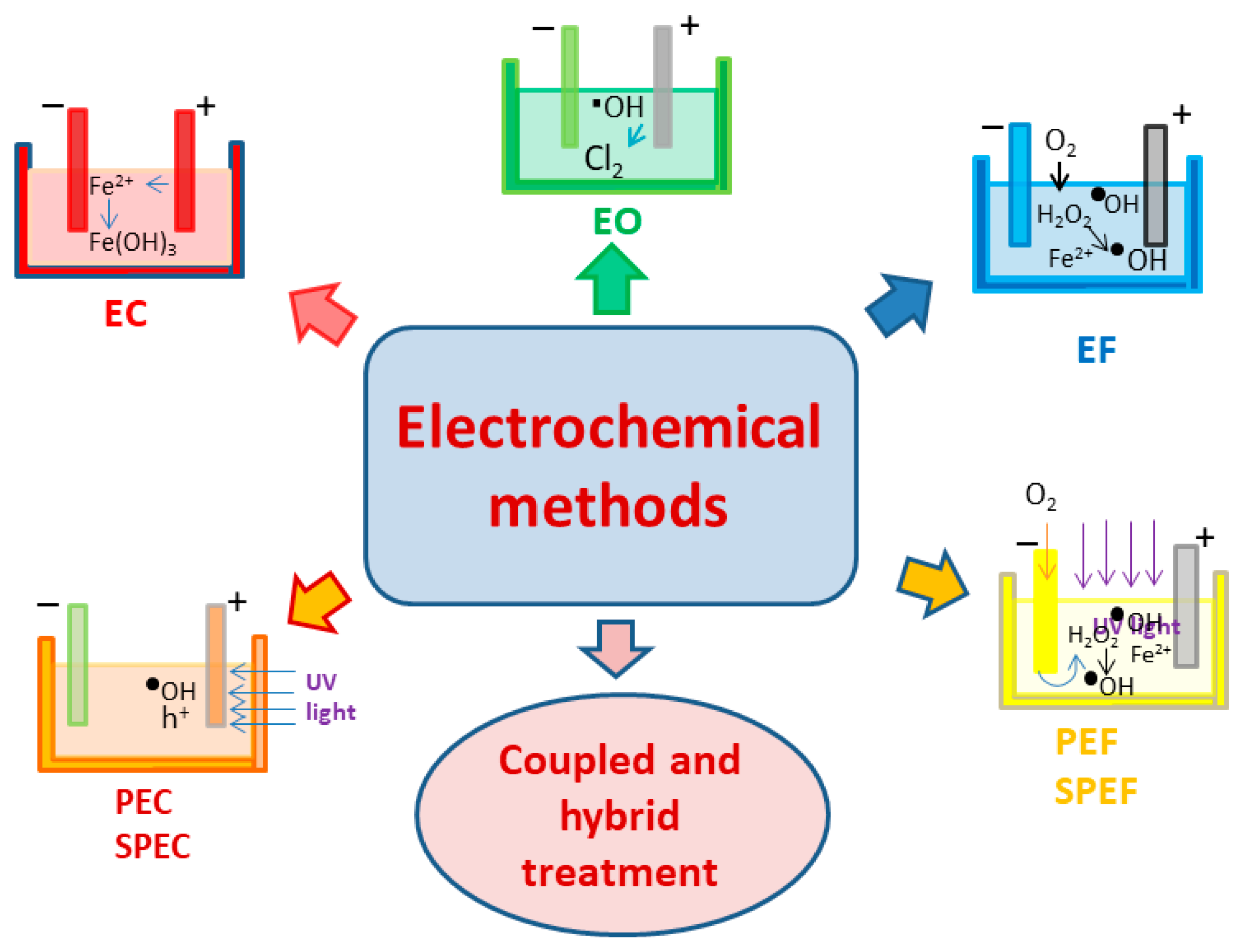
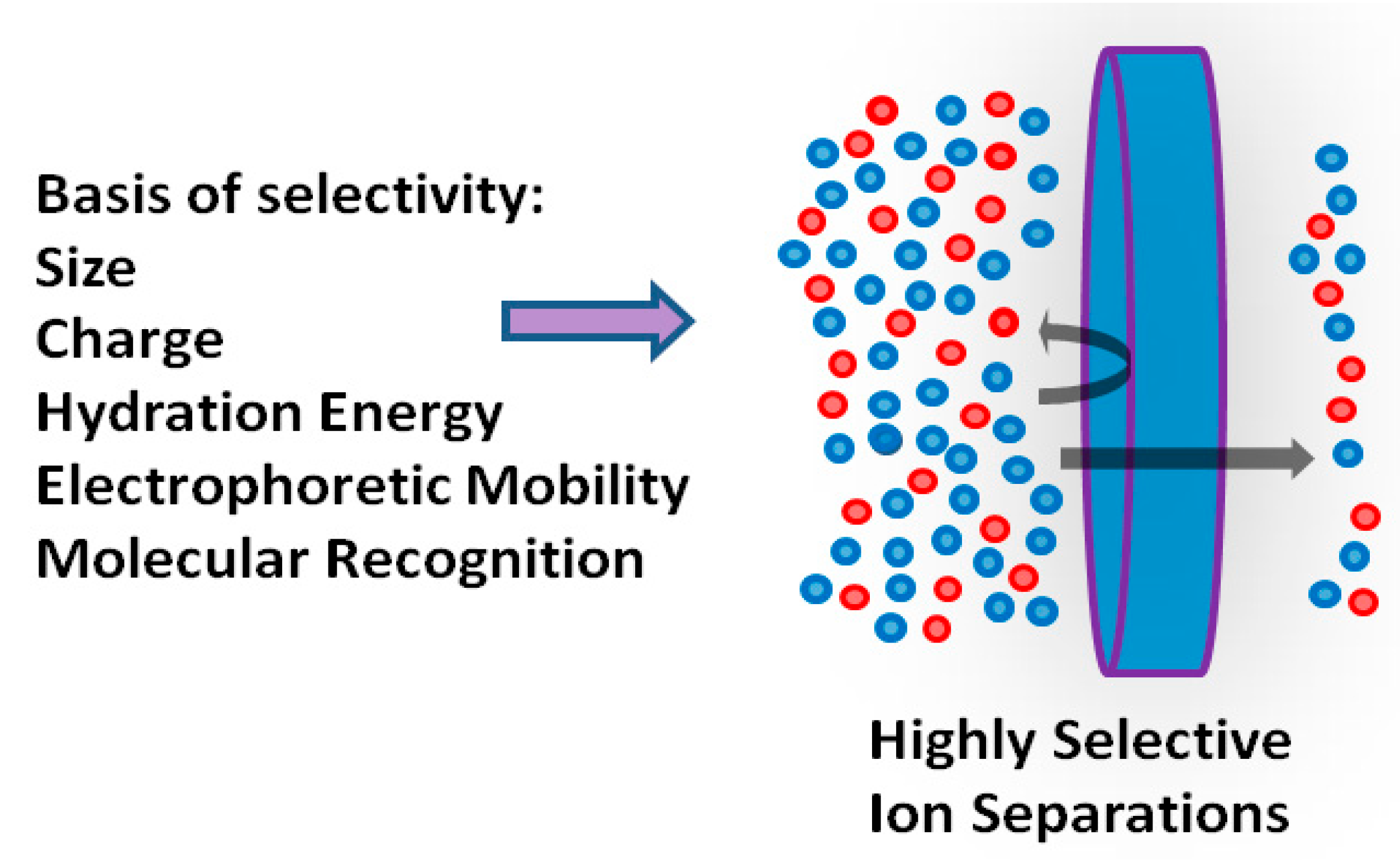


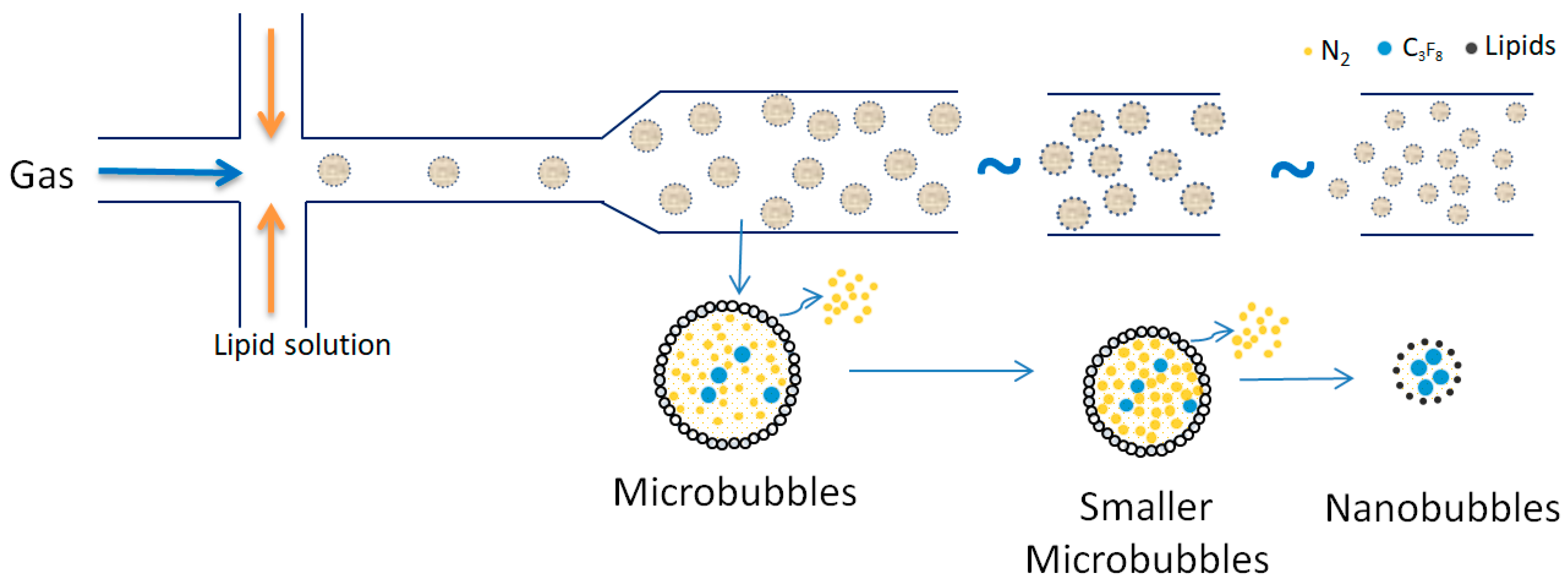


| No | Flotation Type | Types of Mineral Separation | Application(s) |
|---|---|---|---|
| 1 | Flotation of two types of graphite: lithium-ion batteries graphite (LIBG) and natural ore graphite (NOG) | Lithium-ion batteries, graphite (LIBG), and natural ore graphite (NOG) | The flotation efficiency should be examined under two conditions: without nanobubbles (NBs) and with their presence [94] |
| 2 | Dissolved air flotation (DAF): DAF combines with the other flotation to do the material separation | Fine Minerals | Removal of sulfate ions; Zeta potential measurement of bubbles size; Improving Nickel Recovery in Froth Flotation by Purifying Concentrators Process Water [27] |
| 3 | Cyclonic-Static Micro-Bubble Flotation Column (FCSMC) | Incorporated and industrialized for all flotation steps circuits in the mineral separation in China [95] | |
| 4 | Ion flotation | Ion particles such as Carbonate (CO3) | Iron, selenium, and gold ions can be removed, and the ion-flotation process can selectively remove specific ions from mixed ion solutions [96]. |
| Surfactant | Pollutants | Condition | Results of Ion Flotation | Removal (%) | Ref. |
|---|---|---|---|---|---|
| Sodium Dodecyl Sulphides (SDS) | Zn (II), Mn (II), Cu (II) | Cmetal:CSDC:Caxillary ligand = 1:5:5; pH = 4 | Water needs to be purified with the acids | 90.5, 99.8, 73.4 | [107] |
| Sodium Dodecyl Sulphides (SDS) | Cr (III) | Cmetal:CSDC = 2:1; pH = 8 | Water must be infused with the oxygen | 91.6 | [108] |
| Sodium Dodecyl Sulphides (SDS) | Cu (II), Pb (II), Ni (II), Cd (II), Zn (II) | Cmetal:CSDC = 1:1; pH = 9 | Water must be infused with the oxygen | 97.5, 87.5, 87, 83, 92.5 | [98,99] |
| Sodium Dodecyl Sulphides (SDS) | Cd (II) | CSDC:CCd = 3:1; pH = 4 | Water needs to be added to the distribution of nanobubbles | 94 | [109] |
| Sodium Dodecyl Sulphides (SDS) | Ni (II), Zn (II) | Cmetal:CSDC = 1:13.5; pH = 9.7 | Water must be infused with the oxygen | 99.8, 90.4 | [110] |
| Sodium Dodecyl Sulphides (SDS) | Cd (II) | Cmetal:CSDC = 1:2; pH = 10 | Water must be infused with the oxygen | 99.8 | [109] |
| Tea Saponin | Cu (II) | Csurfactant:Cmetal = 3:1; pH = 4 | Should added to the nanobubbles | 81 | [111] |
| Tea Saponin | Cd (II) | Csurfactant:Cmetal = 11:1; pH = 7.5 | Need to add the oxygen through the infused nanobubbles | 8 | [112] |
| Tea Saponin | Pb (II) | Csurfactant:Cmetal = 11:1; pH = 4.8 | Need to infuse the higher concentration of nanobubbles into it. | 12 | [113] |
| Type of Process Water Treatment Method | Application | Selectivity | Membranes | Installation of Water Treatment Technology |
|---|---|---|---|---|
| Reverse Osmosis (RO) | Water desalination | Salt removal | TFC membrane, cellulose acetate membrane | 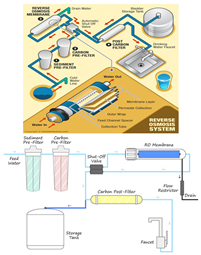 |
| Nanofiltration | Water softening, food processing | Polyvalent ion removal, organic matter removal | Polyamide TFC membranes, cellulose acetate membranes, poly(piperazine-amide) membranes | 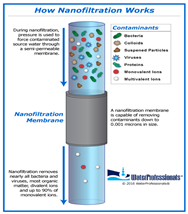 |
| Ultrafiltration | Water treatment, dairy processing | Removal of particulates and macromolecules when protein retention | Poly(vinylidene fluoride) hollow fiber membranes, polyether sulfone membranes, polyamide TFC membranes [144] | 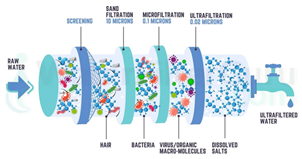 |
| Reverse electrodialysis Electrodialysis | Energy conversion Water desalination | Swollen gel-type ion-exchange membranes that carry positive or negative charges, fluorinated ion- exchange membranes with sulfonic acid side groups |  | |
| Gas separation | N2 production, waste gas stream treatment | N2 separation from air, CO2 capture from flue gas or natural gas | polymers: Polydimethylsiloxane, ethylene oxide/propylene oxide-amide copolymers |  |
| Methods of BNBs | Advantages | Disadvantages | Recommendation |
|---|---|---|---|
| Mechanical Stirring method | The principle is simple and easy to implement | Only a tiny number of nanobubbles can be prepared | Using stirring motors to produce a tiny number of nanobubbles |
| The nanoscale pore membrane method | Enables control over bubble size and distribution | Requires specialized membranes with accurate pore sizes. Potential blockage or fouling of pores may reduce efficiency over time. | Reconstruct the blockage or fouling of pores to do the process time efficiently |
| Microfluidic method | Enables precise control of bubble size and distribution. Offers a high degree of automation and integration with other processes | Requires complex microfluidic devices and fabrication techniques | Make a simulation and model for the complex microfluidic devices and the fabrication techniques |
| Acoustic cavitation method | Efficient and rapid generation of nanobubbles | Requires specialized equipment and ultrasound sources. Control over bubble size and distribution may be limited. | Using the special tool for producing the ultrasound sources to cover and control the distribution bubble size |
| Hydrodynamic cavitation method | High energy efficiency, low cost, and scalability | Efficiency can be influenced by factors such as the flow rate and pressure. | Doing the variations of pressure and flow rate through the change of geometry factors to produce the nanobubbles |
| Dissolved gas release method | Easy and straightforward to implement. Low cost | Limited control over bubble size and distribution. This may result in larger bubble sizes compared to other methods | Make various or combinations of the methods to control the production of the bubble size distribution |
| Periodic pressure variation method | A more uniform bubble can be prepared, and the pressure and period to control the bubble size. | Only a tiny number of nanobubbles can be | This method has the same recommendation as the first type of BNB method |
| Hydraulic air compression method | Nanobubbles can be produced on a large scale at low cost and with high efficiency. | Limited control over bubble size and distribution | Control the bubble size by using the tools to measure the bubble size, and do another process to change the bubble size |
| Contaminants | Inlet Gas | As (ppm) After 30 min | Removal (%) After 30 min | As (ppm) After 60 min | Removal (%) After 60 min | Ref. |
|---|---|---|---|---|---|---|
| Arsenic | Air | 0.137 | 97.3 | 0.006 | 99.9 | [50] |
| Arsenic | Nitrogen (N2) | 0.032 | 99.4 | 0.029 | 99.4 | |
| Mercury | Air | 0.024 | 99.5 | 0.020 | 99.6 | |
| Mercury | Nitrogen (N2) | 0.022 | 99.6 | 0.002 | 99.9 | [100] |
| Lead | Air | 0.399 | 92.0 | 0.0467 | 99.1 | [102] |
| Lead | Nitrogen (N2) | 0.257 | 94.9 | 0.032 | 99.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobai, J.A.; Joni, I.M.; Panatarani, C.; Faizal, F. A Critical Review of Nanobubble Flotation for Seawater Treatment Process. Water 2025, 17, 1054. https://doi.org/10.3390/w17071054
Gobai JA, Joni IM, Panatarani C, Faizal F. A Critical Review of Nanobubble Flotation for Seawater Treatment Process. Water. 2025; 17(7):1054. https://doi.org/10.3390/w17071054
Chicago/Turabian StyleGobai, John Alezander, I Made Joni, Camellia Panatarani, and Ferry Faizal. 2025. "A Critical Review of Nanobubble Flotation for Seawater Treatment Process" Water 17, no. 7: 1054. https://doi.org/10.3390/w17071054
APA StyleGobai, J. A., Joni, I. M., Panatarani, C., & Faizal, F. (2025). A Critical Review of Nanobubble Flotation for Seawater Treatment Process. Water, 17(7), 1054. https://doi.org/10.3390/w17071054



.jpg)



