Ozonation for Low-Load Greywater Treatment: A Review and Experimental Considerations for Small-Scale Systems
Abstract
:1. Introduction
2. Methodology in the Literature Review
2.1. Description of O3 and Its Applications in Water Treatment
- Its ability to corrode certain materials with prolonged exposure.
- The presence of bromide in raw water can lead to the formation of brominated organic by-products during ozonation, which can be carcinogenic at very high levels [49,59]. However, if the pH is below 8, hypobromite ions, which are precursors to bromate ions, can be avoided [39]. For their part, the WHO and the EPA have established a maximum limit of 10 μg/L for bromate in drinking water, and given that LGW comes directly from washbasins, it is highly likely that this condition is met. Moreover, if the concentration of bromide ions is below 20 μg/L, there is no significant risk. However, if it exceeds 50 μg/L, bromate formation must be evaluated [62].
- It is a rather costly process at an industrial scale due to the low solubility of O3 in water, which increases the need for O3 supply to treat the contaminant and requires a long contact time [63]. Due to the low transfer efficiency of O3, the residual gas must be removed when concentration levels exceed the limits established by OSHA. This removal can be achieved through thermal, catalytic, or activated carbon adsorption methods. The thermal method is the easiest to operate, while the latter is not recommended for large-scale plants due to safety concerns and high maintenance requirements [35].
2.2. Factors Affecting the Ozonation Process in Treatment
2.3. Considerations for the Design of GW Treatment Systems
2.4. Relevance of Bubble Size in the Reactor
2.5. Microorganism Inactivation with O3
2.6. Criterion in Inactivation with O3 Ct
2.7. Mass Transfer Parameters
- kLa: Volumetric mass transfer coefficient (s−1).
- V ≈ VL: Reactor volume and volume of liquid in the reactor (m3).
2.8. Reactor Configuration
3. Materials and Methods
3.1. System Configuration
3.2. Description of Equipment
- Free residual chlorine according to St. Methods Ed. 23rd 2017-4500 Cl G.
- Turbidity—Nephelometric according to St. Methods Ed. 23rd 2017-2130 B
- Fecal coliforms according to Chilean standard Nch 2313/22:1995 [101]
- BOD5 according to Chilean standard Nch 2313/5:2005 [102]
- Total suspended solids according to Chilean standard Nch 2313/3:1995 [103]
- pH according to Chilean standard Nch 2313/1:1995 [104]
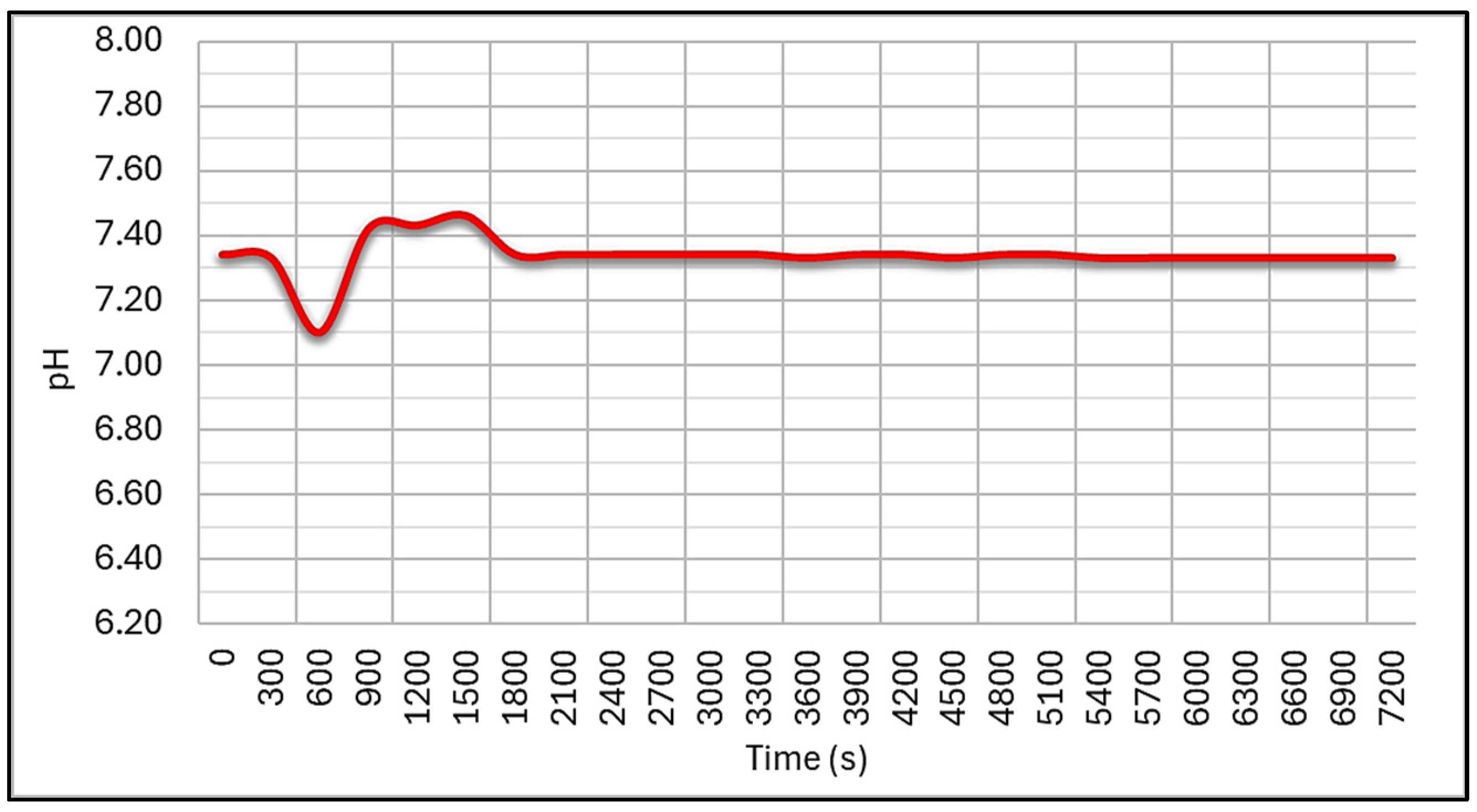
4. Discussion and Results
5. Conclusions
- Treatment of water with a higher contaminant load, such as water from showers, as it represents a considerable volume and lower contamination than HGW.
- Feasibility of scaling up the presented system to assess the possibility of treating larger volumes efficiently while complying with regulations.
- Testing the combination of physical systems such as sand and carbon filters together with higher-production O3 generators to evaluate the effectiveness in medium- and high-load systems without a significant increase in costs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aber, S.; Aguada, R.; Marasinghe, R.; Chow, C.W.K.; Rameezdeen, R.; Xing, K. A desktop assessment of ozone micro-nanobubble technology for algae and PFAS removal from surface water bodies using open-source water quality data. Sustainability 2024, 16, 668. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Ormad Melero, M.P.; Mosteo Abad, R. Inactivation of Escherichia coli in fresh water with advanced oxidation processes based on the combination of O3, H2O2, and TiO2. Kinetic modeling. Environ. Sci. Pollut. Res. 2015, 22, 10280–10290. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Yadav, D.; Singh, S.; Kumar, M.; Song, M. Insights into the Domestic Wastewater Treatment (DWWT) Regimes: A Review. Water 2022, 14, 3542. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Capodaglio, A.G.; Angelakis, A.N. Insights into Global Water Reuse Opportunities. Sustainability 2023, 15, 13007. [Google Scholar] [CrossRef]
- Jeong, H.; Broesicke, O.A.; Drew, B.; Crittenden, J.C. Life cycle assessment of small-scale greywater reclamation systems combined with conventional centralized water systems for the City of Atlanta, Georgia. J. Clean. Prod. 2018, 174, 333–342. [Google Scholar] [CrossRef]
- Lanchipa-Ale, T.; Cruz-Baltuano, A.; Molero-Yañez, N.; Chucuya, S.; Vera-Barrios, B.; Pino-Vargas, E. Assessment of Greywater Reuse in a University Building in a Hyper-Arid Region: Quantity, Quality, and Social Acceptance. Sustainability 2024, 16, 3088. [Google Scholar] [CrossRef]
- Azuma, T.; Hayashi, T. Disinfection of Antibiotic-resistant Bacteria in Sewage and Hospital Effluent by Ozonation. Ozone Sci. Eng. 2021, 43, 413–426. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Dunlop, P.S.M. Evaluation of ozone-based oxidation and solar advanced oxidation treatment of greywater. J. Environ. Chem. Eng. 2020, 8, 104309. [Google Scholar] [CrossRef]
- Ali, E.H.; Hussein, A.A.; Imran, N.J. Optimum conditions of the treatment of organic matters in gray water by Ozone. Revis Bionatura 2023, 8, 59. [Google Scholar] [CrossRef]
- Alrowais, R.; Yousef, R.S.; Ahmed, O.K.; Mahmoud-Aly, M.; Daiem, M.M.A.; Said, N. Enhanced detoxification methods for the safe reuse of treated olive mill wastewater in irrigation. Environ. Sci. Eur. 2023, 35, 95. [Google Scholar] [CrossRef]
- Díaz, M.A.; Decinti, A.; Blanco, D.; Vasquez, K. Metodología para la reutilización de aguas grises en viviendas ubicadas en áreas de estrés hídrico y estrés hídrico extremo—Caracterización, calidad y opciones de tratamiento para su reuso en Chile. Inf. Construcción 2021, 73, e408. [Google Scholar] [CrossRef]
- Khanam, K.; Patidar, S.K. Greywater characteristics in developed and developing countries. Mater. Today Proc. 2022, 57, 1494–1499. [Google Scholar] [CrossRef]
- AQUA España. Guía Técnica Española de Recomendaciones Para el Reciclaje de Aguas Grises en Edificios, Asociación Española de Empresas de Tratamiento y Control de Aguas. 2016. Available online: https://www.aquaespana.org/sites/default/files/documents/files/Guia.tecnica%20grises.pdf (accessed on 10 November 2024).
- Behin, J.; Farhadian, N. Response surface methodology for ozonation of trifluralin using advanced oxidation processes in an airlift photoreactor. Appl. Water Sci. 2017, 7, 3103–3112. [Google Scholar] [CrossRef]
- Vera-Puerto, I.; Valdés, H.; Bueno, M.; Correa, C.; Olave, J.; Carrasco-Benavides, M.; Schiappacasse, F.; Arias, C.A. Reclamation of Treated Wastewater for Irrigation in Chile: Perspectives of the Current State and Challenges. Water 2022, 14, 627. [Google Scholar] [CrossRef]
- Audino, F.; Arboleda, J.; Petrovic, M.; Cudinach, R.G.; Pérez, S.S. Pharmaceuticals Removal by Ozone and Electro-Oxidation in Combination with Biological Treatment. Water 2023, 15, 3180. [Google Scholar] [CrossRef]
- Koncagül, E.; Tran, M.; Connor, R.; Uhlenbrook, S.; CordeiroOrtigara, A. United Nations World Water Report, Facts and Figures, Wastewater, the Untapped Resource. 2017. Available online: https://digitallibrary.un.org/record/3905488?v=pdf#files (accessed on 10 November 2024).
- Noutsopoulos, C.; Andreadakis, A.; Kouris, N.; Charchousi, D.; Mendrinou, P.; Galani, A.; Mantziaras, I.; Koumaki, E. Greywater characterization and loadings–Physicochemical treatment to promote onsite reuse. J. Environ. Manag. 2018, 216, 337–346. [Google Scholar] [CrossRef] [PubMed]
- SISS. Superintendencia de Servicios Sanitarios, Informe de Coberturas Sanitarias. 2023. Available online: https://www.siss.gob.cl/586/articles-23290_recurso_1.pdf (accessed on 10 November 2024).
- Van de Walle, A.; Kim, M.; Alam, M.K.; Wang, X.; Wu, D.; Dash, S.R.; Rabaey, K.; Kim, J. Greywater reuse as a key enabler for improving urban wastewater management. Environ. Sci. Ecotechnol. 2023, 16, 100277. [Google Scholar] [CrossRef]
- México-Comisión Nacional del Agua (CONAGUA). Libro 35. Manual de Agua Potable, Alcantarillado y Saneamiento, Diseño de Plantas de Tratamiento de Aguas Residuales Municipales: Procesos Avanzados con Fines de Reúso; Secretaria de Medio Ambiente y Recursos Naturales: Tlalpan, Mexico, 2019; ISBN 978-607-626-032-6. Available online: https://files.conagua.gob.mx/conagua/mapas/SGAPDS-1-15-Libro35.pdf (accessed on 10 November 2024).
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Pal, P.; Joshi, A.; Anantharaman, H. Nanobubble ozonation for waterbody rejuvenation at different locations in India: A holistic and sustainable approach. Results Eng. 2022, 16, 100725. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Municipal Wastewater Disinfection Design Manual; EPA-625/1-86-021; U.S. Environmental Protection Agency (USEPA): Cincinnati, OH, USA, 1986. Available online: https://www.wbdg.org/FFC/EPA/EPACRIT/epa625_1_86_021.pdf (accessed on 10 November 2024).
- Hussain, K.; Khan, N.A.; Vambol, V.; Vambol, S.; Yeremenko, S.; Sydorenko, V. Advancement in Ozone base wastewater treatment technologies: Brief review. Ecol. Quest. 2022, 33, 7–19. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Wastewater Technology Fact Sheet; EPA 832-F-99-063; US EPA, Office Water: Washington, DC, USA, 1999. Available online: https://www3.epa.gov/npdes/pubs/ozon.pdf (accessed on 10 November 2024).
- U.S. Food and Drug Administration. (FDA). Secondary Direct Food Additives Permitted in Food for Human Consumption. Rules and Regulations, Federal Register Vol 66, no 123 Sec. 173.368 Ozone, 2001. Available online: https://www.federalregister.gov/d/01-15963 (accessed on 10 November 2024).
- Wang, B.; Shi, W.; Zhang, H.; Ren, H.; Xiong, M. Promoting the ozone-liquid mass transfer through external physical fields and their applications in wastewater treatment: A review. J. Environ. Chem. Eng. 2021, 9, 106115. [Google Scholar] [CrossRef]
- Manjunath, S.N.; Sakar, M.; Katapadi, M.; Geetha Balakrishna, R. Recent case studies on the use of ozone to combat coronavirus: Problems and perspectives. Environ. Technol. Innov. 2021, 21, 101313. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a Practical Method for Using Ozone Gas as a Virus Decontaminating Agent. Ozone Sci. Eng. 2009, 31, 216–223. [Google Scholar] [CrossRef]
- Megahed, A.; Aldridge, B.; Lowe, J. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS ONE 2018, 13, e0196555. [Google Scholar] [CrossRef]
- ISCO3. Potential Use of Ozone in SARS-CoV-2/COVID-19; International Scientific Committee of Ozone Therapy: Madrid, Spain, 2020; Available online: https://isco3.org/wp-content/uploads/2020/04/English-Covid-19-2.pdf (accessed on 10 November 2024).
- Gottschalk, C.; Libra, J.; Saupe, A. Ozonation of Water and Waste Water: A Practical Guide to Understanding Ozone and Its Application; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- México-Comisión Nacional del Agua (CONAGUA). Libro 23. Manual de Agua Potable, Alcantarillado y Saneamiento, Desinfección Para Sistemas de Agua Potable y Saneamiento; Secretaria de Medio Ambiente y Recursos Naturales: Tlalpan, Mexico, 2019; ISBN 978-607-626-023-4. Available online: https://files.conagua.gob.mx/conagua/mapas/SGAPDS-1-15-Libro23.pdf (accessed on 10 November 2024).
- Pulicharla, R.; Proulx, F.; Behmel, S.; Sérodes, J.-B.; Rodriguez, M.J. Trends in Ozonation Disinfection By-Products—Occurrence, Analysis and Toxicity of Carboxylic Acids. Water 2020, 12, 756. [Google Scholar] [CrossRef]
- Banach, J.L.; Sampers, I.; Van Haute, S.; van der Fels-Klerx, H.J. Effect of Disinfectants on Preventing the Cross-Contamination of Pathogens in Fresh Produce Washing Water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677. [Google Scholar] [CrossRef]
- Ratnawati, R.; Kusumaningtyas, D.A.; Suseno, P.; Prasetyaningrum, A. Mass Transfer Coefficient of Ozone in a Bubble Column. MATEC Web Conf. 2018, 156, 02015. [Google Scholar] [CrossRef]
- Bataller, M.; Fernández, L.; Veliz, E. Eficiencia y Sostenibilidad del Empleo del Ozono en la Gestión de los Recursos Hídricos. Revista Internacional de Contaminación Ambiental. 2010. Available online: https://www.researchgate.net/publication/47297235_EFICIENCIA_Y_SOSTENIBILIDAD_DEL_EMPLEO_DEL_OZONO_EN_LA_GESTION_DE_LOS_RECURSOS_HIDRICOS (accessed on 10 November 2024).
- Díaz, M.A.; Blanco, D.; Almendro-Candel, M.B.; Herrera, I.; Allende, I.; Pulgar Rubilar, P.; Lizana, M.; Pardo, F.; Perillán, L.; Tapia, C. Configurations for Four Urban Tree Species in the Santiago Metropolitan Region and Their Impact on the Environment According to CO2, PM2.5, Biogenic Volatile Organic Compounds and Water Resource Criteria. Buildings 2023, 13, 3052. [Google Scholar] [CrossRef]
- Leiva, E.; Rodríguez, C.; Sánchez, R.; Serrano, J. Light or Dark Greywater for Water Reuse? Economic Assessment of On-Site Greywater Treatment Systems in Rural Areas. Water 2021, 13, 3637. [Google Scholar] [CrossRef]
- Tiwari, G.; Bose, P. Determination of ozone mass transfer coefficient in a tall continuous flow counter-current bubble contactor. Chem. Eng. J. 2007, 132, 215–225. [Google Scholar] [CrossRef]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of COVID-19. Gases 2020, 1, 19–32. [Google Scholar] [CrossRef]
- Karamah, E.; Bismo, S.; Annasari, L.; Purwanto, W. Mass Transfer Study on Micro-Bubbles Ozonation in A Bubble Column. Int. J. Chem. Eng. Res. 2010, 2, 243–252. Available online: https://www.researchgate.net/publication/233386059_Mass_Transfer_Study_on_Micro-Bubbles_Ozonation_in_A_Bubble_Column#fullTextFileContent (accessed on 10 November 2024).
- Hu, L.; Xia, Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018, 342, 446–453. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. J. Water Process. Eng. 2020, 35, 101193. [Google Scholar] [CrossRef]
- Bataller, M.; Veliz, E.; Fernández García, A.; Hernández, C.; Fernández, I.; Alvarez, C.; Sanchez, E. Influencia de la Ozonización en el Tratamiento de un Efluente Secundario. 2005. Available online: https://www.researchgate.net/publication/238761597_Influencia_de_la_Ozonizacion_en_el_Tratamiento_de_un_Efluente_Secundario (accessed on 10 November 2024).
- Martínez, S.B.; Pérez-Parra, J.; Suay, R. Use of Ozone in Wastewater Treatment to Produce Water Suitable for Irrigation. Water Resour. Manag. 2011, 25, 2109–2124. [Google Scholar] [CrossRef]
- Avery, L.; Jarvis, P.; Macadam, J. Review of Literature to Determine the Uses for Ozone in the Treatment of Water and Wastewater. 2013. Available online: https://www.crew.ac.uk/publication/uses-ozone-treatment-water-and-wastewater (accessed on 10 November 2024).
- López Ramírez, M.A.; Castellanos Onorio, O.P.; Lango Reynoso, F.; Castañeda Chávez, M.d.R.; Montoya Mendoza, J.; Sosa Villalobos, C.A.; Ortiz Muñiz, B. Advanced Oxidation as an Alternative Treatment for Wastewater. A Review. Enfoque UTE 2021, 12, 76–87. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Cen, C.; Wu, X.; Mao, R.; Zheng, Y. Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Taylor, C.M.; King, W.; Chew, Y.M.J.; Wenk, J. Modelling of Ozone Mass-Transfer through Non-Porous Membranes for Water Treatment. Water 2017, 9, 452. [Google Scholar] [CrossRef]
- Kosowski, P.; Szostek, M.; Pieniążek, R.; Antos, P.; Skrobacz, K.; Piechowiak, T.; Żaczek, A.; Józefczyk, R.; Balawejder, M. New Approach for Sewage Sludge Stabilization with Ozone. Sustainability 2020, 12, 886. [Google Scholar] [CrossRef]
- Loeb, B.L. Ozone: Science & Engineering: Thirty-Three Years and Growing. Ozone Sci. Eng. 2011, 33, 329–342. [Google Scholar] [CrossRef]
- Dawley, C. Aqueous Ozone Inactivation of Viruses and Bacteria on Biotic and Abiotic Surfaces. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2018. Available online: https://scholarworks.uark.edu/etd/3022 (accessed on 10 November 2024).
- Oskin, S.; Tsokur, D.; Voloshin, S. Modeling process of water bubbling with ozone to obtain disinfectant solutions in beekeeping. Eng. Rural Dev. 2019, 22, 1210–1214. [Google Scholar] [CrossRef]
- Martinelli, M.; Giovannangeli, F.; Rotunno, S.; Trombetta, C.M.; Montomoli, E. Water and air ozone treatment as an alternative sanitizing technology. J. Prev. Med. Hyg. 2020, 58, E48–E52. [Google Scholar] [CrossRef]
- Doménech, J. Ozono frente a cloro, Desinfección y desinfectantes del agua para consumo humano. Offarm Farm. Soc. 2004, 23, 120–126. [Google Scholar]
- Loeb, B.L.; Thompson, C.M.; Drago, J.; Takahara, H.; Baig, S. Worldwide Ozone Capacity for Treatment of Drinking Water and Wastewater: A Review. Ozone Sci. Eng. 2012, 34, 64–77. [Google Scholar] [CrossRef]
- Orellana, M.; Menchaca, E.; Nava, J.F.; Nava, N.; Orellana, J.; Ponce, S. El Ozono Como una Alternativa Para Esterilizar Piezas de Mano y Fresas en Odontología. Revista Latinoamericana de Ortodoncia y Odontopediatria “Ortodoncia. ws” Edición Electrónica Junio. 2010. Available online: https://www.ortodoncia.ws/publicaciones/2010/art-13/ (accessed on 10 November 2024).
- Inagaki, H.; Saito, A.; Sudaryatma, P.E.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid Inactivation of SARS-CoV-2 with Ozonated Water. Ozone Sci. Eng. 2021, 43, 208–212. [Google Scholar] [CrossRef]
- Lu, H.; Li, Q.; Feng, W. Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water. Sustainability 2022, 14, 1556. [Google Scholar] [CrossRef]
- Lara-Ramos, J.A.; Diaz-Angulo, J.; Machuca-Martínez, F. Use of modified flotation cell as ozonation reactor to minimize mass transfer limitations. Chem. Eng. J. 2021, 405, 126978. [Google Scholar] [CrossRef]
- Gorito, A.M.; Pesqueira, J.F.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 105315. [Google Scholar] [CrossRef]
- Boano, F.; Caruso, A.; Costamagna, E.; Ridolfi, L.; Fiore, S.; Demichelis, F.; Galvão, A.; Pisoeiro, J.; Rizzo, A.; Masi, F. A review of nature-based solutions for greywater treatment: Applications, hydraulic design, and environmental benefits. Sci. Total Environ. 2020, 711, 134731. [Google Scholar] [CrossRef]
- Rajski, K.; Englart, S.; Sohani, A. Analysis of Greywater Recovery Systems in European Single-Family Buildings: Economic and Environmental Impacts. Sustainability 2024, 16, 4912. [Google Scholar] [CrossRef]
- do Couto, E.; Calijuri, M.; Peixoto, P.; da Fonseca, A.; Sampaio, L. Greywater treatment in airports using anaerobic filter followed by UV disinfection: An efficient and low cost alternative. J. Clean. Prod. 2015, 106, 372–379. [Google Scholar] [CrossRef]
- Ekeren, K.M.; Hodgson, B.A.; Sharvelle, S.E.; De Long, S.K. Investigation of pathogen disinfection and regrowth in a simple graywater recycling system for toilet flushing. Desalination Water Treat. 2016, 57, 26174–26186. [Google Scholar] [CrossRef]
- Mathur, M.; Choudhary, P.; Sujathan, S.; Naaz, F.; Trenado-Yuste, C.; Malik, A. A biologically driven model for rural wastewater management: Feasibility and efficiency of algal-bacterial biofilm reactors for combined treatment and algae farming. Front. Water 2024, 6, 1430900. [Google Scholar] [CrossRef]
- Chile-Ministerio de Obras Públicas (MOP). Ley Núm. 21.075 Regula la Recolección, Reutilización y Disposición de Aguas Grises, 15 de Febrero de 2018, Actualizada el 27 de Noviembre 2023. 2018. Available online: https://bcn.cl/2ficg (accessed on 10 November 2024).
- Chile-Ministerio de Salud (MINSAL). Decreto Núm. 40 Aprueba Reglamento Sobre Condiciones Sanitarias Básicas Para la Reutilización de Aguas Grises, 20 de Abril de 2022, Publicada el 09 de mayo 2024. 2024. Available online: https://bcn.cl/3jvf4 (accessed on 10 November 2024).
- Zheng, T.; Wang, Q.; Zhang, T.; Shi, Z.; Tian, Y.; Shi, S.; Smale, N.; Wang, J. Microbubble enhanced ozonation process for advanced treatment of wastewater produced in acrylic fiber manufacturing industry. J. Hazard. Mater. 2015, 287, 412–420. [Google Scholar] [CrossRef]
- Hong Kong-Water Supplies Department (WSD). Technical Specifications on Grey Water Reuse and Rainwater Harvesting, Special Administrative Region. 2015. Available online: https://www.wsd.gov.hk/filemanager/en/content_1177/technical_spec_grey_water_reuse_rainwater_harvest.pdf (accessed on 10 November 2024).
- U.K. Environment Agency. Greywater for Domestic Reuse: An Information Guide. 2011. Available online: https://sswm.info/sites/default/files/reference_attachments/ENVIRONMENT%20AGENCY%202011%20Greywater%20for%20Domestic%20Users.pdf (accessed on 10 November 2024).
- Ghaitidak, D.; Yadav, K. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef]
- Li, F.; Wichmann, K.; Otterpohl, R. Review of the technological approaches for greywater treatment and reuses. Sci. Total Environ. 2009, 407, 3439–3449. [Google Scholar] [CrossRef]
- España-Fundación Ecología y Desarrollo Barcelona. Ordenanza Tipo Para el Ahorro de Agua. Xarxa de Ciutats i Pobles cap a la Sostenibilitat. Grupo de trabajo Nueva Cultura del Agua, España. 2005. Available online: https://ecodes.org/docs/ordenanza-agua.pdf (accessed on 10 November 2024).
- U.S. Environmental Protection Agency (USEPA). Guidelines for Water Reuse; U.S. Environmental Protection Agency (USEPA): Washington, DC, USA, 2012. Available online: https://www3.epa.gov/region1/npdes/merrimackstation/pdfs/ar/AR-1530.pdf (accessed on 10 November 2024).
- Rakness, K.L.; Hunter, G.; Lew, J.; Mundy, B.; Wert, E.C. Design Considerations for Cost-Effective Ozone Mass Transfer in Sidestream Systems. Ozone Sci. Eng. 2018, 40, 159–172. [Google Scholar] [CrossRef]
- Batagoda, J.H.; Hewage, S.A.; Meegoda, J.N. Nano-ozone bubbles for drinking water treatment. J. Environ. Eng. Sci. 2019, 14, 57–66. [Google Scholar] [CrossRef]
- Hewage, S.A.; Batagoda, J.H.; Meegoda, J.N. In Situ Remediation of Sediments Contaminated with Organic Pollutants Using Ultrasound and Ozone Nanobubbles. Environ. Eng. Sci. 2020, 37, 521–534. [Google Scholar] [CrossRef]
- Martínez, R.; Arañó, M.; Custodio, J.; Garcia, J.; Monagas, P. Evidence of OH· radicals disinfecting indoor air and surfaces in a harmless for humans method. Int. J. Eng. Res. Sci. (IJOER) 2020, 6, 26–38. [Google Scholar] [CrossRef]
- Fernández-Cuadros, M.E.; Albaladejo-Florín, M.J.; Peña-Lora, D.; Álava-Rabasa, S.; Pérez-Moro, O.S. Ozone (O3) and SARS-CoV-2: Physiological Bases and Their Therapeutic Possibilities According to COVID-19 Evolutionary Stage. SN Compr. Clin. Med. 2020, 2, 1094–1102. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of RNA-dependent RNA polymerase from 2019-nCoV, a major antiviral drug target. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hernández, A.; Viñals, M.; Isidoro, T.; Vilás, F. Potential Role of Oxygen-Ozone Therapy in Treatment of COVID-19 Pneumonia. Am. J. Case Rep. 2020, 21, e925849. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.; Holbrook, M.; Gamble, A.; Williamson, B.; Tamin, A.; Harcourt, J.; Thornburg, N.; Gerber, S.; et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. medRxiv 2020. [Google Scholar] [CrossRef]
- Rosal, R. Ozonización. In Ecuaciones y Cálculos Para el Tratamiento de Aguas; Coord. por Mario Díaz; Editorial Paraninfo: Madrid, Spain, 2019; pp. 453–460. ISBN 9788428341523. Available online: https://www.researchgate.net/publication/330144584_Ozonizacion (accessed on 10 November 2024).
- Lage Filho, F.A. Ozone application in water sources: Effects of operational parameters and water quality variables on ozone residual profiles and decay rates. Braz. J. Chem. Eng. 2010, 27, 545–554. [Google Scholar] [CrossRef]
- Choi, J.M.; D’Souza, D.H. Inactivation of Tulane virus and feline calicivirus by aqueous ozone. J. Food Sci. 2023, 88, 4218–4229. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Alternative Disinfectants and Oxidants Guidance Manual; EPA-815-R-99-014, Office of Water (4607); U.S. Environmental Protection Agency (USEPA): Washington, DC, USA, 1999. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000229L.txt (accessed on 10 November 2024).
- Lim, M.Y.; Kim, J.; Lee, J.E.; Ko, G. Characterization of Ozone Disinfection of Murine Norovirus. Appl. Environ. Microbiol. 2010, 76, 1120–1124. [Google Scholar] [CrossRef]
- Hirai, K.; Ando, N.; Komada, H.; Sounai, A.; Murakami, M.; Nakayama, H. Investigation of the effective concentration of ozonated water for disinfection in the presence of protein contaminants. Biocontrol Sci. 2019, 24, 155–160. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Water Treatment Manual: Disinfection; U.S. Environmental Protection Agency (USEPA): Washington, DC, USA, 2011; ISBN 978-184095-421-0. Available online: https://www.epa.ie/publications/compliance--enforcement/drinking-water/advice--guidance/Disinfection2_web.pdf (accessed on 10 November 2024).
- Ferre Aracil, J. Diseño de Reactores de Burbujeo Para el Tratamiento de Aguas Residuales Mediante Ozono. Caracterización Física, Análisis Cinético y Optimización con Redes Neuronales Artificiales. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2017. [Google Scholar] [CrossRef]
- Yoldi, C.F.; Hidalgo, Ó.P.; Ramos, J.F.; Sánchez, R. Medida de la concentración del ozono en agua en dosis bajas. Ozone Ther. Glob. J. 2019, 9, 61–73. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=7306844 (accessed on 10 November 2024).
- Sander, R. Compilation of Henry’s law constants (version 5.0.0) for water as solvent. Atmos. Chem. Phys. 2023, 23, 10901–12440. [Google Scholar] [CrossRef]
- Levanov, A.V.; Kuskov, I.V.; Antipenko, E.E.; Lunin, V.V. The solubility of ozone and kinetics of its chemical reactions in aqueous solutions of sodium chloride. Russ. J. Phys. Chem. 2008, 82, 2045. [Google Scholar] [CrossRef]
- Sheffer, S.; Esterson, G.L. Mass transfer and reaction kinetics in the ozone/tap water system. Water Res. 1982, 16, 383–389. [Google Scholar] [CrossRef]
- Flores-Payán, V.; Herrera-López, E.J.; Navarro-Laboulais, J.; López-López, A. Parametric sensitivity analysis and ozone mass transfer modeling in a gas–liquid reactor for advanced water treatment. J. Ind. Eng. Chem. 2015, 21, 1270–1276. [Google Scholar] [CrossRef]
- Biń, A.K. Ozone dissolution in aqueous systems treatment of the experimental data. Exp. Therm. Fluid Sci. 2004, 28, 395–405. [Google Scholar] [CrossRef]
- NCh2313/22; Aguas Residuales—Métodos de Análisis—Parte 22: Determinación de Coliformes Fecales en Medio EC. Instituto Nacional de Normalización: Santiago, Chile, 1995.
- Nch2313/5; Aguas Residuales—Métodos de Análisis—Parte 5: Determinación de la Demanda Bioquímica de Oxígeno (DBO5). Instituto Nacional de Normalización: Santiago, Chile, 2005.
- Nch2313/3; Aguas Residuales—Métodos de Análisis—Parte 3: Determinación de Sólidos Suspendidos Totales Secados a 103 °C–105 °C. Instituto Nacional de Normalización: Santiago, Chile, 1995.
- Nch2313/1; Aguas Residuales—Métodos de Análisis—Parte 1: Determinación de pH. Instituto Nacional de Normalización: Santiago, Chile, 2021.
- Mohan, S.; Manthapuri, V.; Chitthaluri, S. Assessing factors influencing greywater characteristics around the world: A qualitative and quantitative approach with a short-review on greywater treatment technologies. Discov. Water 2024, 4, 37. [Google Scholar] [CrossRef]
- Mandičák, T.; Spišáková, M.; Mésároš, P. Sustainable Design and Building Information Modeling of Construction Project Management towards a Circular Economy. Sustainability 2024, 16, 4376. [Google Scholar] [CrossRef]
- Pekmez, H.; İbanoğlu, Ş. Effects of Ozonation on Thermal, Structure and Rheological Properties of Rice Starch in Aqueous Solution. Gıda Dergisi. 2013, 38, 63–70. Available online: https://www.researchgate.net/publication/312454837 (accessed on 10 November 2024).
- Asaithambi, P.; Saravanathamizhan, R.; Matheswaran, M. Comparison of treatment and energy efficiency of advanced oxidation processes for the distillery wastewater. Int. J. Environ. Sci. Technol. 2015, 12, 2213–2220. [Google Scholar] [CrossRef]
- Romanovski, V.; Paspelau, A.; Kamarou, M.; Likhavitski, V.; Korob, N.; Romanovskaia, E. Comparative Analysis of the Disinfection Efficiency of Steel and Polymer Surfaces with Aqueous Solutions of Ozone and Sodium Hypochlorite. Water 2024, 16, 793. [Google Scholar] [CrossRef]




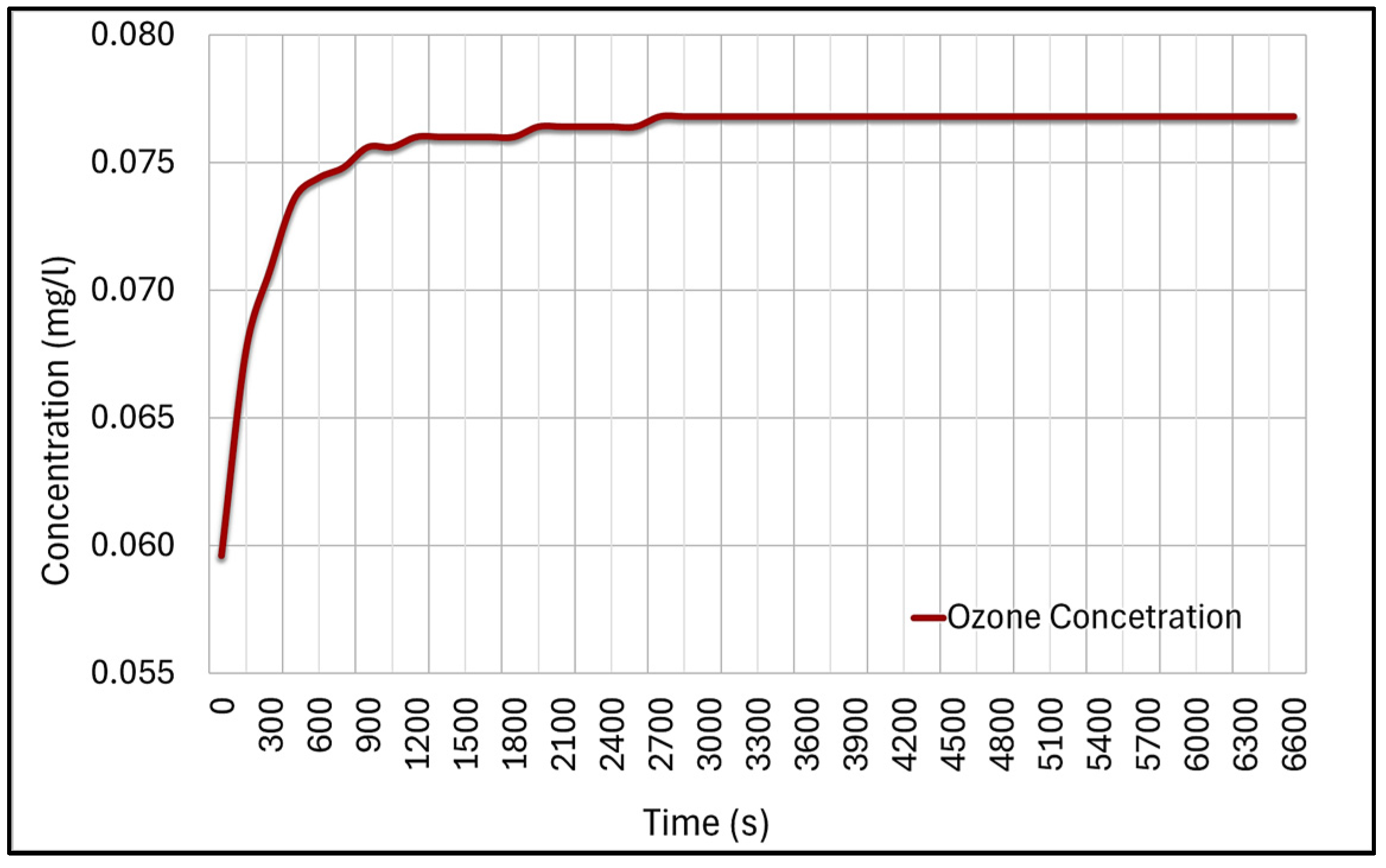
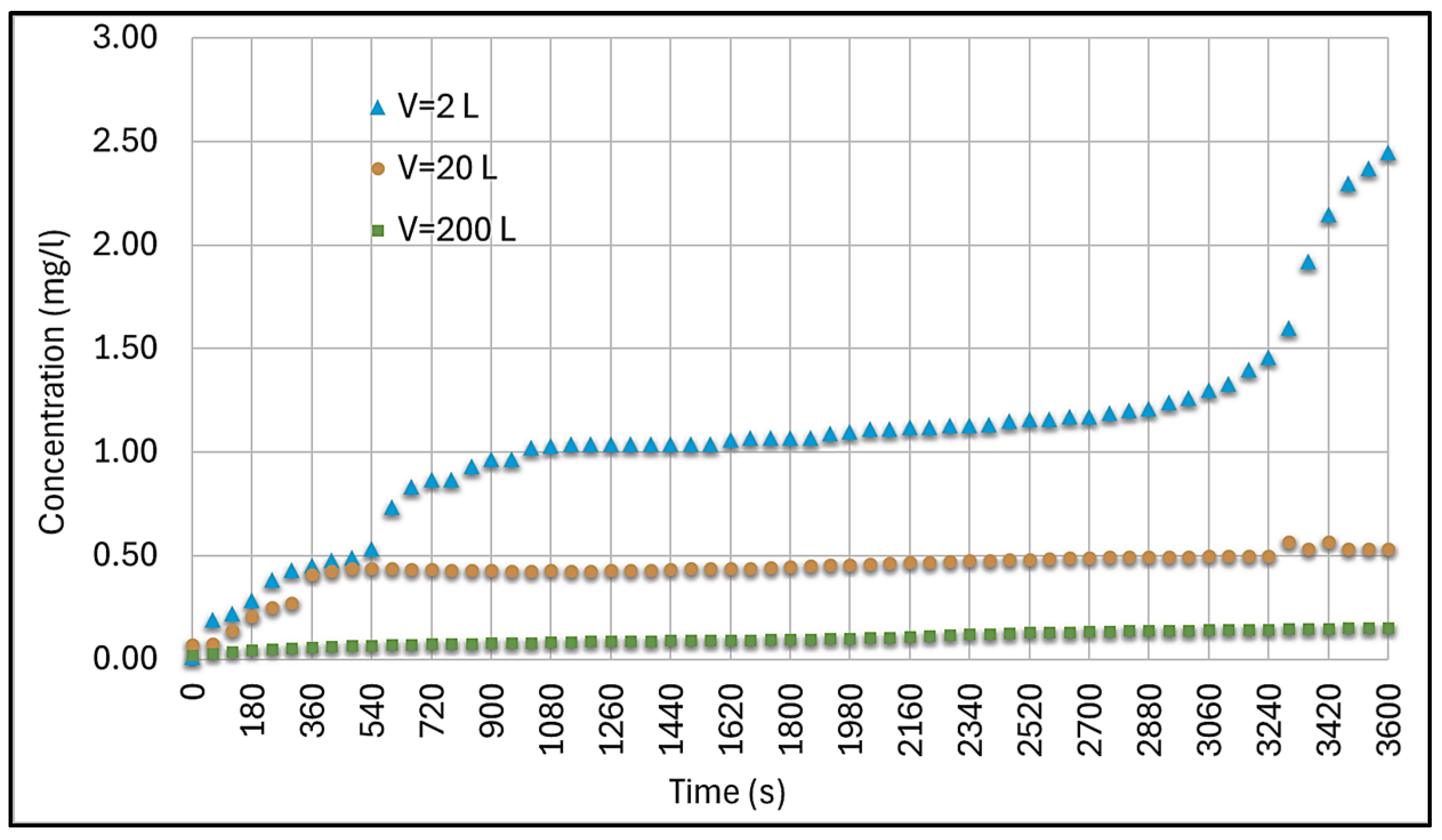
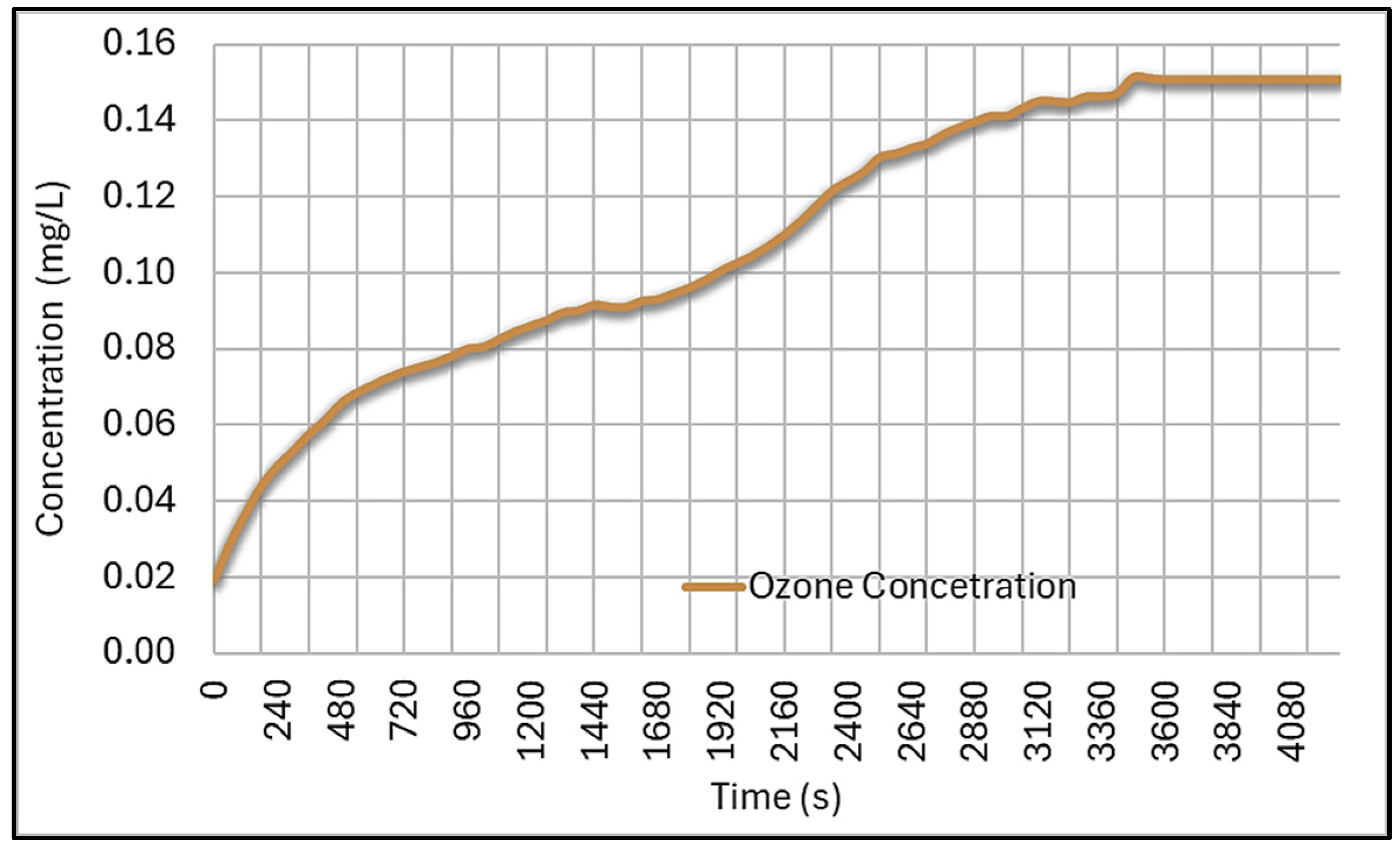
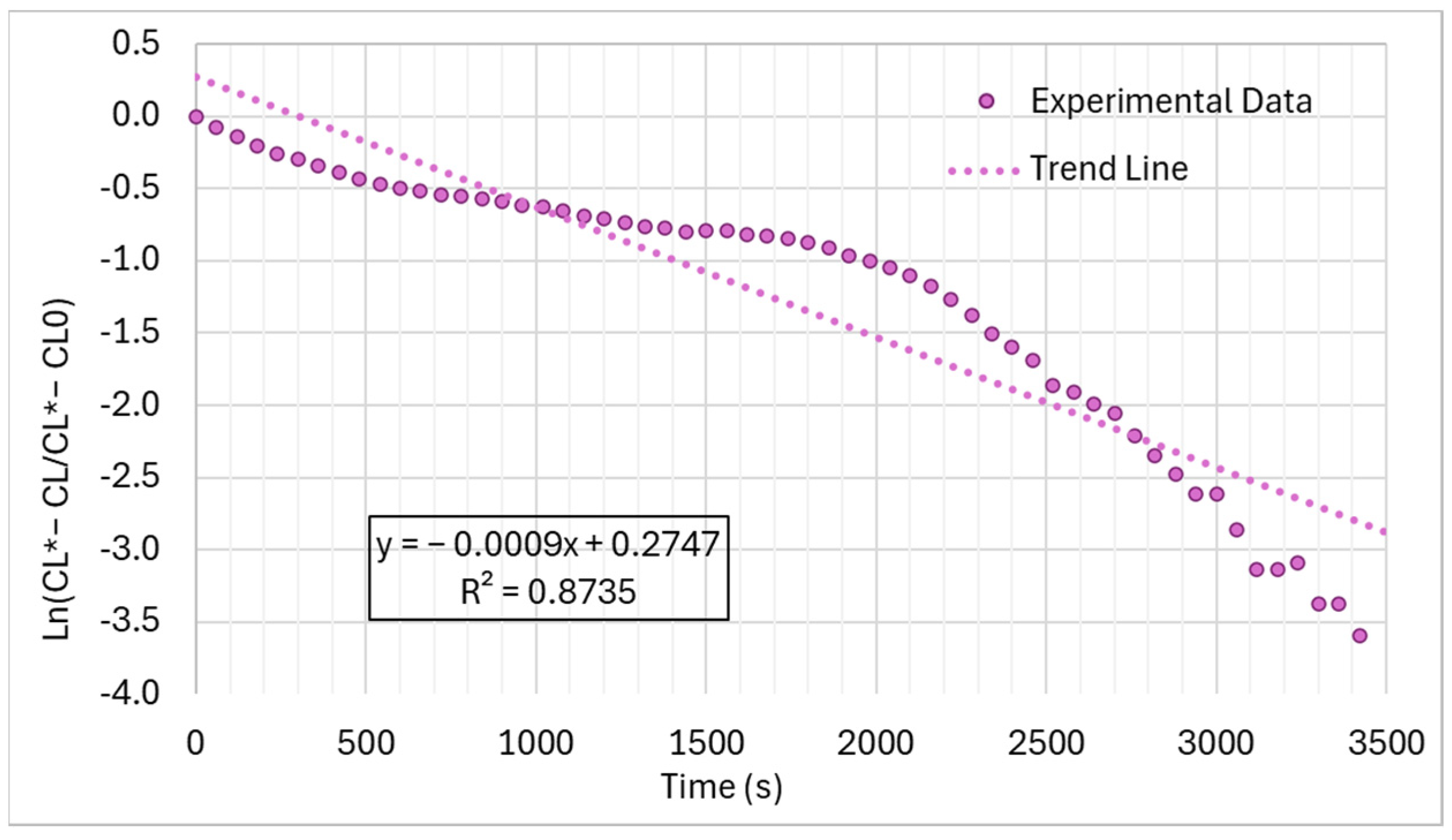

| Characteristic | Chlorine | Sodium Hypochlorite | Calcium Hypochlorite | Ozone | UV |
|---|---|---|---|---|---|
| Toxicity to microorganisms | High | High | High | High | High |
| Solubility | Slight | High | High | High | N.A. |
| Stability | Stable | Slightly stable | Relatively stable | Unstable | Must be generated upon use |
| Toxicity to higher life forms | High | Toxic | Toxic | Toxic | Toxic |
| Interaction with foreign matter | Oxidizes organic matter | Active oxidant | Active oxidant | Oxidizes organic matter | Moderate |
| Corrosion | Highly corrosive | Corrosive | Corrosive | Highly corrosive | N.A. |
| Deodorizing capacity | High | Moderate | Moderate | High | None |
| Toilet Flushing | [73] Hong Kong | [13] Spain | [13] R.D. 1620/2007 Spain | UK [74] | Ministry of Health Canada [67] | [75] | [76] China | [76] | Effluent Quality [70] |
|---|---|---|---|---|---|---|---|---|---|
| Suspended Solids (mg/L) | ≤5 | - | ≤20 | - | ≤20 | - | - | - | ≤10 |
| Turbidity (NTU) | ≤5 | ≤2 | ≤10 | <10 | <5 | ≤2 | <5 | <5 | ≤5 |
| BOD5 (mg/L) | ≤10 | - | ≤20 | ≤10 | ≤10 | ≤10 | ≤10 | ||
| Escherichia coli (UFC/100 mL) | No | No | No | 10 | <200 | No | 3 | ≤10 | |
| Fecal Coliforms (NMP/100 mL) | - | - | - | - | - | - | - | - | ≤10 |
| Free Residual Chlorine (mg/L) | ≥0.2 | 0.5–2.0 | - | <2 | 0.5–1.5 | ≤1 | >1 | ≤1 | 0.5–2.0 |
| Garden Irrigation | [77] Spain | [13] Spain | [13] R.D. 1620/2007 Spain | NSF350 [78] | UK [74] | [75] | [76] China | [76] | Effluent Quality [70] |
|---|---|---|---|---|---|---|---|---|---|
| Suspended Solids (mg/L) | <25 | - | ≤20 | ≤30 | - | - | - | ≤30 | ≤30 |
| Turbidity (NTU) | <5 | <10 | ≤10 | <5 | <10 | ≤2 | <20 | <5 | ≤10 |
| BOD5 (mg/L) | - | - | ≤25 | - | ≤30 | ≤20 | ≤30 | ≤30 | |
| Escherichia coli (UFC/100 mL) | <200 | <200 | ≤200 | ≤200 | 10 | ≤200 | 3 | ≤10 | |
| Fecal Coliforms (NMP/100mL) | - | - | - | - | - | - | - | - | ≤200 |
| Ct (mg/min/L) | ||||
|---|---|---|---|---|
| pH | 6–7 | 8–9 | 6–7 | 6–7 |
| Microorganism | Free Chlorine | Chloramine | Chlorine Dioxide | Ozone |
| E.coli | 0.034–0.05 | 95–180 | 0.4–0.75 | 0.02 |
| Poliovirus | 1.1–2.5 | 768–3740 | 0.2–6.7 | 0.1–0.2 |
| Rotavirus | 0.01–0.05 | 3800–6500 | 0.2–2.1 | 0.006–0.06 |
| Giardia lamblia (cysts) | 47–150 | 2200 | 26 | 0.5–0.6 |
| Giardia muris (cysts) | 30–630 | 1400 | 7.2–18.5 | 1.8–2.0 |
| Cryptosporidium parvum | 7200 | 7200 * | 78 * | 5–10 * |
| Cryptosporidium parvum (1 °C) | 200 | 10 | ||
| Cryptosporidium parvum (22 °C) | 120 ** | 7 ** |
| Log Inactivation | Temperature, °C | ||||
|---|---|---|---|---|---|
| ≤1 | 5 | 10 | 15 | 20 | |
| 2.0 | 0.90 | 0.60 | 0.50 | 0.30 | 0.25 |
| 3.0 | 1.40 | 0.90 | 0.80 | 0.50 | 0.40 |
| 4.0 | 1.80 | 1.20 | 1.00 | 0.60 | 0.50 |
| Application | Theoretical Ozone Dose (ppm) |
|---|---|
| Coxsackie Virus | 0.51 |
| Poliovirus | 0.012–0.015 |
| Porcine Pigina Virus | 0.024 |
| Mesophilic Aerobes UFC/mL | Total Coliforms NMP/100 mL | Fecal Coliforms NMP/100 mL | ||
|---|---|---|---|---|
| Before Ozonation | 4.6 × 102 | 7.6 × 103 | 5.2 × 103 | |
| After Ozonation (t = 5 min) | ||||
| Dose | Ct | |||
| (mg/L) | (mg·min/L) | |||
| 7 | 5 | 2.5 × 101 | negative | negative |
| 14 | 10 | 2.2 × 101 | negative | negative |
| 21 | 14 | 1.5 × 101 | negative | negative |
| Parameters | Raw Greywater | Treated Greywater | Reduction |
|---|---|---|---|
| pH | 7.61 | 7.69 | - |
| BOD5 mgO2/L | 880 | 372 | 58% |
| Total Suspended Solids mg/L | 193 | 52 | 73% |
| Turbidity UNT | 164 | 124 | 24% |
| Fecal Coliforms NMP/100 mL | <2 | <2 | - |
| mV-orp | ppm mg/L | mV-orp | ppm mg/L | mV-orp | ppm mg/L | mV-orp | ppm mg/L |
|---|---|---|---|---|---|---|---|
| 100 | 0.00 | 875 | 1.00 | 1125 | 3.50 | 1375 | 6.00 |
| 200 | 0.04 | 900 | 1.25 | 1150 | 3.75 | 1400 | 6.25 |
| 300 | 0.08 | 925 | 1.50 | 1175 | 4.00 | 1425 | 6.50 |
| 400 | 0.13 | 950 | 1.75 | 1200 | 4.25 | 1450 | 6.75 |
| 500 | 0.16 | 975 | 2.00 | 1225 | 4.50 | 1475 | 7.00 |
| 600 | 0.20 | 1000 | 2.25 | 1250 | 4.75 | 1500 | 7.25 |
| 700 | 0.22 | 1025 | 2.50 | 1275 | 5.00 | 1525 | 7.50 |
| 750 | 0.25 | 1050 | 2.75 | 1300 | 5.25 | 1550 | 7.75 |
| 800 | 0.39 | 1075 | 3.00 | 1325 | 5.50 | 1575 | 8.00 |
| 860 | 0.50 | 1100 | 3.25 | 1350 | 5.75 | 1600 | 8.25 |
| Parameters | Urban Use [71] | Irrigation for Recreational [71] | Afluent Greywater Quality | Efluent Greywater Quality | Reduction | |
|---|---|---|---|---|---|---|
| Irrigation or Toilets | Surface | Subsurface | ||||
| pH | - | - | - | 7.24 | 7.24 | - |
| BOD5 mgO2/L | 10 | 30 | 50 | 10 | <2 | 80% |
| Total Suspended Solids (mg/L) | 10 | 30 | 50 | 33 | <10 | 70% |
| Turbidity UNT | 5 | 10 | - | 4.8 | 1.7 | 65% |
| Fecal Coliforms NMP/100 mL | 10 | 200 | 1000 | 2 | <2 | 1 Log |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, M.A.; Blanco, D.; Chandia-Jaure, R.; Cataldo-Cunich, A.; Poblete, V.H.; Aguirre-Nuñez, C.; Almendro-Candel, M.B. Ozonation for Low-Load Greywater Treatment: A Review and Experimental Considerations for Small-Scale Systems. Water 2025, 17, 1195. https://doi.org/10.3390/w17081195
Díaz MA, Blanco D, Chandia-Jaure R, Cataldo-Cunich A, Poblete VH, Aguirre-Nuñez C, Almendro-Candel MB. Ozonation for Low-Load Greywater Treatment: A Review and Experimental Considerations for Small-Scale Systems. Water. 2025; 17(8):1195. https://doi.org/10.3390/w17081195
Chicago/Turabian StyleDíaz, Marco Antonio, David Blanco, Rosa Chandia-Jaure, Andrés Cataldo-Cunich, Victor H. Poblete, Carlos Aguirre-Nuñez, and María Belén Almendro-Candel. 2025. "Ozonation for Low-Load Greywater Treatment: A Review and Experimental Considerations for Small-Scale Systems" Water 17, no. 8: 1195. https://doi.org/10.3390/w17081195
APA StyleDíaz, M. A., Blanco, D., Chandia-Jaure, R., Cataldo-Cunich, A., Poblete, V. H., Aguirre-Nuñez, C., & Almendro-Candel, M. B. (2025). Ozonation for Low-Load Greywater Treatment: A Review and Experimental Considerations for Small-Scale Systems. Water, 17(8), 1195. https://doi.org/10.3390/w17081195









