Lanthanum and Sludge Extracellular Polymeric Substances Coprecipitation-Modified Ceramic for Treating Low Phosphorus-Bearing Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Adsorbent Preparation
2.3. Characterization of Adsorbent
2.4. Batch-Scale Adsorption Experiment and Kinetic Analysis
2.5. Continuous-Flow Adsorption Column Test
3. Results and Discussion
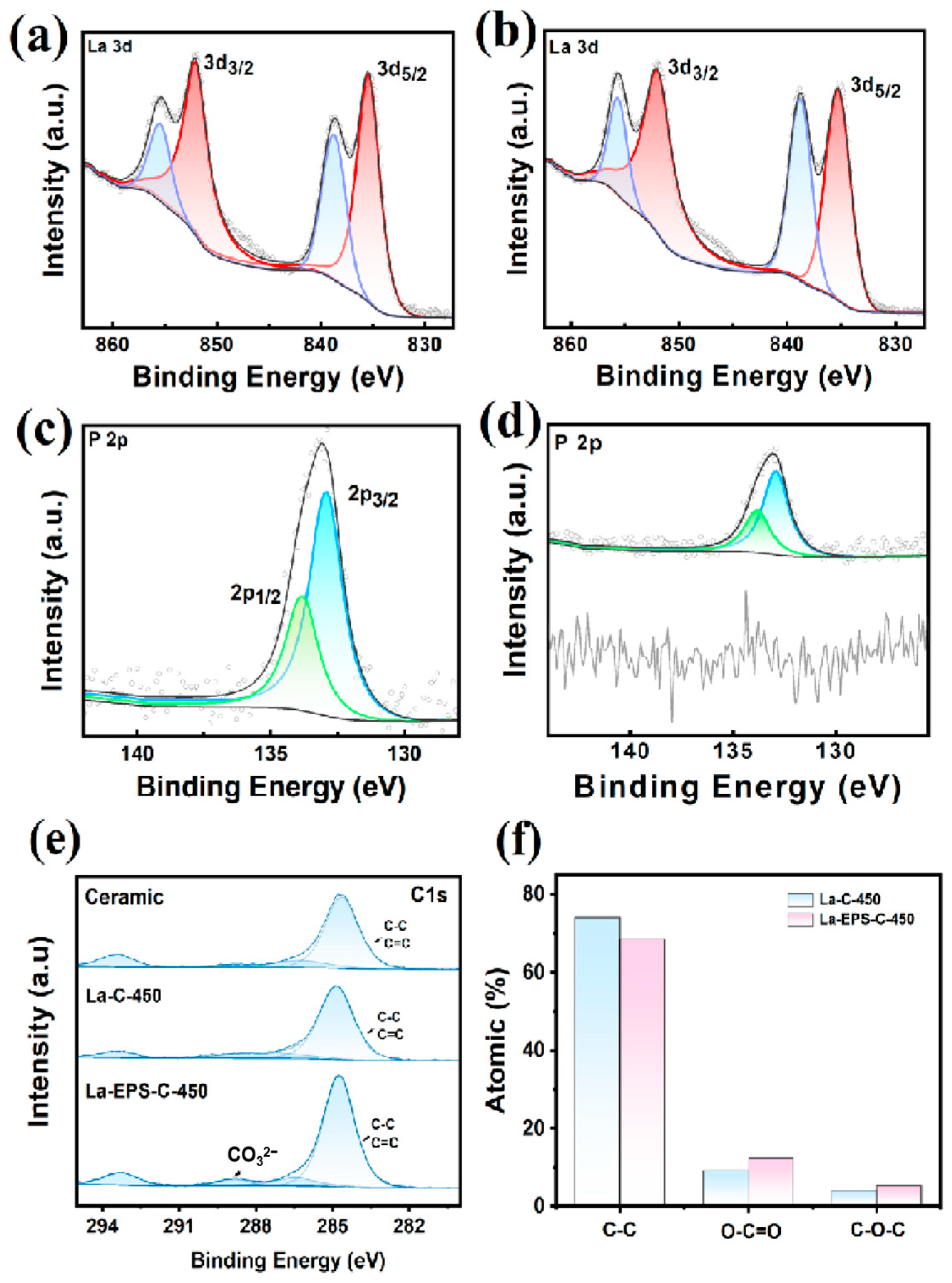
3.1. Synthesis and Structural Characterization of Adsorbent
3.2. Adsorption Kinetics and Thermodynamics
3.3. Adsorption Mechanism
3.4. Practical Application Potential of the Adsorbent
| Adsorption Material | Adsorption Capacity (mg P/g) | Fitted Adsorption Isotherm | Reference |
|---|---|---|---|
| La-Z | 17.20 | Langmuir | [26] |
| La0.5-PC | 32.40 | Langmuir | [28] |
| ACF-LaOH | 15.30 | Langmuir | [31] |
| LMB | 10.19 | Langmuir | [39] |
| SBP-La | 46.50 | Langmuir | [40] |
| GNS-LaOH | 41.96 | Langmuir | [41] |
| KLa | 24.42 | Langmuir–Freundlich | [42] |
| La-doped silica spheres | 47.89 | Freundlich | [43] |
| DSCT | 14.20 | Langmuir | [44] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gruau, G.; Legeas, M.; Riou, C.; Gallacier, E.; Martineau, F.; Hénin, O. The oxygen isotope composition of dissolved anthropogenic phosphates: A new tool for eutrophication research? Water Res. 2004, 39, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Tang, A.; Yu, Y.; Qu, X.; Chen, Z.; Wen, Q.; Li, A. Insight into the synthesis and adsorption mechanism of adsorbents for efficient phosphate removal: Exploration from synthesis to modification. Chem. Eng. J. 2022, 442, 136147. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 741311. [Google Scholar] [CrossRef]
- Liu, B.; Gai, S.; Lan, Y.; Cheng, K.; Yang, F. Metal-based adsorbents for water eutrophication remediation: A review of performances and mechanisms. Environ. Res. 2022, 212, 113353. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, Y.; Hao, C.; Chen, J.; Gao, H.; Han, S.; Shen, Y.; Wang, X. Emulsion synthesis of cellulose/lanthanum alginate /La(Ⅲ) composite microspheres for efficient and selective adsorption of phosphate. Chem. Eng. J. 2024, 488, 150949. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xia, D.W.; Cui, S.J.; Yu, W.P.; Wang, B.T.; Liu, H.Z. A high-capacity nanocellulose aerogel uniformly immobilized with a high loading of nano-La(OH)3 for phosphate removal. Chem. Eng. J. 2022, 433, 134439. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.H.; Shi, W.M.; Huang, X.L.; Ye, Y.Y.; Jiang, W.; Kang, J.X.; Liu, D.Q.; Ren, Y.Z.; Li, D.S. Preferable phosphate removal by nano-La(III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Ahmed, S.; Zheng, Z.; Liu, F.; Leung, C.-F.; Choy, T.-Y.; Kwok, Y.-T.; Pan, B.; Lo, I.M.C. Validation of pilot-scale phosphate polishing removal from surface water by lanthanum-based polymeric nanocomposite. Chem. Eng. J. 2021, 412, 128630. [Google Scholar] [CrossRef]
- Li, G.; Zhu, W.; Zhong, J.; Sun, J.; Wang, Y.; Mu, B.; Wang, X.; Xu, Y. Efficient phosphate recovery and treatment of high-phosphorus wastewater using sodium alginate-immobilized microspheres based on aluminum-rich water treatment plant sludge. Environ. Pollut. 2024, 363, 125139. [Google Scholar] [CrossRef]
- Wang, C.; Shan, S.; Yang, Z.; Xu, X.; Huang, X.; Li, X.; Liu, S.; Li, B.; Xu, Y.; Li, D. Synchronous sequestration of inorganic and organic phosphorus from eutrophic surface water and sediments via recoverable La-CaO2@HNTs/SA hydrogel beads. Chem. Eng. J. 2024, 499, 156593. [Google Scholar] [CrossRef]
- Feng, C.; Pan, X.; Lin, X.; Yang, Y.; Fan, F.; Jiang, C.; Mei, Y. Capacitive deionization exploiting La-based LDH composite electrode toward energy efficient and selective removal of phosphate. Desalination 2024, 594, 118259. [Google Scholar] [CrossRef]
- Fu, C.; Li, Y.; Zuo, Y.; Li, B.; Liu, C.; Liu, D.; Fu, Y.; Yin, Y. Fabrication of lanthanum/chitosan co-modified bentonite and phosphorus removal mechanism from low-concentration landscape water. Water Sci. Technol. 2022, 86, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Li, Q.; Zheng, X.; Chen, P.; Zhang, G.; Huang, Z. Lanthanum modified chitosan-attapulgite composite for phosphate removal from water: Performance, mechanisms and applicability. Int. J. Biol. Macromol. 2023, 224, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of pyrolysis temperature and feedstock on surface charge and functional group chemistry of biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef]
- Lian, J.; Yang, Y.; Qiu, W.; Huang, L.; Wang, C.; Chen, Q.; Ke, Q.; Wang, Q. Fluorescent characteristics and metal binding properties of different molecular weight fractions in stratified extracellular polymeric substances of activated sludge. Separations 2021, 8, 120. [Google Scholar] [CrossRef]
- Yan, P.; Xia, J.-S.; Chen, Y.-P.; Liu, Z.-P.; Guo, J.-S.; Shen, Y.; Zhang, C.-C.; Wang, J. Thermodynamics of binding interactions between extracellular polymeric substances and heavy metals by isothermal titration microcalorimetry. Bioresour. Technol. 2017, 232, 354–363. [Google Scholar] [CrossRef]
- Peng, S.; Hu, A.; Ai, J.; Zhang, W.; Wang, D. Changes in molecular structure of extracellular polymeric substances (EPS) with temperature in relation to sludge macro-physical properties. Water Res. 2021, 201, 117316. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Deng, D.; Li, R.; Guo, C.; Ma, J.; Chen, M. Investigation of extracellular polymeric substances (EPS) in four types of sludge: Factors influencing EPS properties and sludge granulation. J. Water Process. Eng. 2021, 40, 101924. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Krishna Veni, D.; Kannan, P.; Jebakumar Immanuel Edison, T.N.; Senthilkumar, A. Biochar from green waste for phosphate removal with subsequent disposal. Waste Manag. 2017, 68, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, H.; Lu, Y.-Y.; Ren, Z.-Q.; Gao, N.; Wang, J.-J.; Huang, B.-C.; Jin, R.-C. In-situ synthesis of lanthanum-coated sludge biochar for advanced phosphorus adsorption. J. Environ. Manag. 2024, 373, 13607. [Google Scholar] [CrossRef]
- Pan, B.; Han, F.; Nie, G.; Wu, B.; He, K.; Lu, L. New strategy to enhance phosphate removal from water by hydrous manganese oxide. Environ. Sci. Technol. 2014, 48, 5101–5107. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Zhu, R.; Liu, J.; Usman, M.; Chen, Q.; He, H. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated magnetite. J. Colloid Interf. Sci. 2018, 530, 704–713. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, H.; Dong, Y.; Wang, L. Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite: Characteristics and mechanism. Appl. Surf. Sci. 2017, 426, 995–1004. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Guo, J.; Zhang, L. Removal of phosphate from wastewater by lanthanum modified bio-ceramisite. J. Environ. Chem. Eng. 2021, 9, 106123. [Google Scholar] [CrossRef]
- Koilraj, P.; Sasaki, K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: Batch and column studies. Chem. Eng. J. 2017, 317, 1059–1086. [Google Scholar] [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]
- Xia, S.; Liang, S.; Qin, Y.; Chen, W.; Xue, B.; Zhang, B.; Xu, G. Significant improvement of adsorption for phosphate removal by lanthanum-loaded biochar. ACS Omega 2023, 8, 24853–24864. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber. Chem. Eng. J. 2012, 185–186, 160–167. [Google Scholar] [CrossRef]
- Lan, Y.; Gai, S.; Cheng, K.; Li, J.; Yang, F. Lanthanum carbonate hydroxide/magnetite nanoparticles functionalized porous biochar for phosphate adsorption and recovery: Advanced capacity and mechanisms study. Environ. Res. 2022, 214, 113783. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, P.; Zhou, J.; Liu, J.; Losic, D.; Wu, H.; Bu, H.; Tan, X.; Li, Z. Direct atomic-scale insight into the precipitation formation at the lanthanum hydroxide nanoparticle/solution interface. J. Phys. Chem. Lett. 2023, 14, 3995–4003. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liang, C.; Yu, J.; Zhang, Q.; Song, M.; Chen, F. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem. Eng. J. 2017, 315, 345–354. [Google Scholar] [CrossRef]
- Zhang, Q.; Teng, J.; Zou, G.; Peng, Q.; Du, Q.; Jiao, T.; Xiang, J. Efficient phosphate sequestration for water purification by unique sandwich-like MXene/magnetic iron oxide nanocomposites. Nanoscale 2016, 8, 7085–7093. [Google Scholar] [CrossRef]
- Qu, J.; Akindolie, M.S.; Feng, Y.; Jiang, Z.; Zhang, G.; Jiang, Q.; Deng, F.; Cao, B.; Zhang, Y. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration. Chem. Eng. J. 2020, 394, 124915. [Google Scholar] [CrossRef]
- Pap, S.; Zhao, Q.; Cakin, I.; Gaffney, P.P.J.; Gibb, S.W.; Taggart, M.A. Lanthanum and cerium functionalised forestry waste biochar for phosphate removal: Mechanisms and real-world applications. Chem. Eng. J. 2024, 494, 152848. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, C.; Zhang, G.; Wang, H.; Ji, Q.; Qu, J. Activation of Lattice Oxygen in LaFe(Oxy)hydroxides for Efficient Phosphorus Removal. Environ. Sci. Technol. 2019, 53, 9073–9080. [Google Scholar] [CrossRef]
- Haghseresht, F.; Wang, S.; Do, D.D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Pan, J.; Yang, H.; Liu, L.; Li, B.; Tang, X.; Wu, X.; Zhang, L.; Ying, G.-G. Sludge-based biochar with lanthanum modification for phosphate recovery from wastewater streams. Environ. Sci-Wat. Res. 2022, 8, 2873–2883. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Zhou, Q.; Kan, J.; Wang, Y. High-performance removal of phosphate from water by graphene nanosheets supported lanthanum hydroxide nanoparticles. Water Air Soil Pollut. 2014, 225, 1967. [Google Scholar] [CrossRef]
- Li, J.-R.; Wang, F.-K.; Xiao, H.; Xu, L.; Fu, M.-L. Layered chalcogenide modified by Lanthanum, calcium and magnesium for the removal of phosphate from water. Colloid Surface A 2018, 560, 306–314. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Tang, J. Lanthanum-doped ordered mesoporous hollow silica spheres as novel adsorbents for efficient phosphate removal. J. Mater. Chem. A 2014, 2, 8839–8848. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef]




| Adsorbent | Temperature | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| R12 | Qmax (mg/g-La) | KL (L/mg) | R22 | Kf (mg/g-La) | 1/n | ||
| La-EPS-C-450 | 35 °C | 0.86 | 74.42 | 0.23 | 0.98 | 25.23 | 0.39 |
| La-EPS-C-450 | 25 °C | 0.98 | 69.33 | 0.11 | 0.99 | 15.12 | 0.51 |
| La-EPS-C-450 | 15 °C | 0.95 | 45.82 | 0.19 | 0.99 | 14.37 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-Y.; Yang, C.-X.; Chen, K.-Y.; Wang, J.-J.; Huang, B.-C.; Jin, R.-C. Lanthanum and Sludge Extracellular Polymeric Substances Coprecipitation-Modified Ceramic for Treating Low Phosphorus-Bearing Wastewater. Water 2025, 17, 1237. https://doi.org/10.3390/w17081237
Lu Y-Y, Yang C-X, Chen K-Y, Wang J-J, Huang B-C, Jin R-C. Lanthanum and Sludge Extracellular Polymeric Substances Coprecipitation-Modified Ceramic for Treating Low Phosphorus-Bearing Wastewater. Water. 2025; 17(8):1237. https://doi.org/10.3390/w17081237
Chicago/Turabian StyleLu, Yao-Yao, Chao-Xi Yang, Ke-Yu Chen, Jiao-Jiao Wang, Bao-Cheng Huang, and Ren-Cun Jin. 2025. "Lanthanum and Sludge Extracellular Polymeric Substances Coprecipitation-Modified Ceramic for Treating Low Phosphorus-Bearing Wastewater" Water 17, no. 8: 1237. https://doi.org/10.3390/w17081237
APA StyleLu, Y.-Y., Yang, C.-X., Chen, K.-Y., Wang, J.-J., Huang, B.-C., & Jin, R.-C. (2025). Lanthanum and Sludge Extracellular Polymeric Substances Coprecipitation-Modified Ceramic for Treating Low Phosphorus-Bearing Wastewater. Water, 17(8), 1237. https://doi.org/10.3390/w17081237







