Abstract
Mine waste constitutes one of the biggest environmental and management problems, both due to its quantity and its danger when they are rich in toxic elements. There is a wide variety of waste from the oxidation of ores in metal mining areas, both metal sulphide and other minerals. These residues may be enriched in potentially toxic elements that can spread and contaminate ecosystems, farmland and villages. This study has focused on the characterization and evaluation of residues derived from metal-bearing mining waste in abandoned mining areas. Mineralogy and geochemical characteristics were determined by XRD, WDXRF and TG-MS techniques. In addition, DIN 38414-S4 leaching tests were carried out to assess the risk and mobility of potentially toxic elements. Silicates and oxides were found as the main mineral groups, followed by sulphates. These tailings were particularly enriched in Zn, Pb, As, Sb and Cd, while their leachates had high or extreme metal content. Consequently, these mining wastes are considered toxic and hazardous, even for landfills. Sulphides, as the primary source, and sulphates from their oxidation, were the main sources of these pollutants. Sulphates, As, Zn, Cd and Cu determined the specific environmental impact of the different tailing types, which were grouped into different clusters according to their mineralogy and geochemistry. These results provided a better understanding of the environmental hazards associated with the different types of metal mining waste in the area studied.
1. Introduction
A wide variety of anthropogenic activities have negative impacts on the environmental quality of their surroundings. Ore, gangue, industrial minerals, metal(loid)s, rock, loose sediments, metallurgical slag and waste, roasted ore, ash and processing chemicals are all components of metal mining waste. On average, for every tonne of metal ore mined, one tonne of waste is generated [1]. Globally, it is estimated that mining activities generate several billion tonnes of mining waste per year, a quantity comparable to the amount of regolith and other materials mobilised annually by geological processes [2]. In this sense, it is recognized that metal mining presents potential risks to environmental health [3]. Moreover, mining waste has become one of today’s major environmental concerns, constituting a serious threat to ecosystems and the health of nearby populations [2,4,5].
For many centuries, handling and management of mining liabilities have been a minor issue, as they were often dumped in landfills close to the mine [6]. Due to the depletion of mineral resources, profitability and/or environmental concerns, in most cases, companies have abandoned mining sites leaving a degraded area with few environmental restoration measures [5]. Other significant impacts of metal mining waste come from acid mine drainage (AMD), which can affect both surface water bodies, through surface runoff, and groundwater bodies. In these cases, the concentrations of sulphate and metal(loid) increase, and there is a significant reduction in their pH values [7].
On countless occasions, the rehabilitation of old mining facilities has been incomplete or inefficient, especially in the case of metal mining [8]. This represents a permanent potential risk for the population and the environment, insofar as its waste is rich in potentially toxic elements (PTEs). At the European level, legislation has been introduced to minimise these risks. In this context, the European Parliament and the Council drafted Directive 2006/21/EC [9] on the management of waste from extractive industries. This Directive obliges Member States to carry out an inventory of decommissioned and/or abandoned waste facilities for their restoration and minimize the risks associated with this waste.
Both locally and globally, waste generation by the extractive industry is not only an environmental concern but also an economic one [10]. Recent research in this field proposes to eliminate these risks, in addition to the structural risks posed by waste facilities, by storing pre-treated waste in underground landfills [11]. Nevertheless, due to the enrichment of metal mining waste in certain chemical elements of interest, in some cases, this waste could be reused as a source of raw materials for further uses, e.g., in nanomedicine [12]. The revalorization of metal mining waste using new technologies is a promising strategy for its quantitative reduction [13], as well as for the effective recovery of some elements contained in these tailings [12]. In this sense, the goal of reusing industrial waste and moving towards waste-free production is a trend that is likely to become the norm in the medium to long term, both because of the need for mineral raw materials and the depletion of their main deposits [10,14].
In response to this growing interest in this serious environmental problem, numerous studies with different approaches have been undertaken. Some of them have focused on assessing the environmental impacts of metal mining waste from different perspectives, such as innovative approaches [15,16,17], dispersion mechanisms, etc. [18,19,20]. However, the typology of mining waste generated is not usually considered when the risk assessment is carried out in abandoned mining areas. It is known that each type of waste has a different mineralogy and geochemistry [18]. They can contain high amounts of metals and metalloids that tend to spread and contaminate the surrounding soil, water and atmosphere [21]. Even so, studies on the environmental impact and risks associated with the different types of waste present in Mediterranean metal mining areas are scarce.
The oxidation of these metal mining wastes can generate intermediate species as a result of the dissolution and recrystallisation of metal salts present in mine tailings [22] and acid mine drainage [23] in the form of secondary or supergene minerals. These secondary minerals consist of sulphate salts and oxides, clays and various hydrated salts, carbonates, phosphates, arsenates and native elements [24]. Such supergene minerals are of great environmental relevance in environmental studies due to their role in the dynamics of potentially toxic elements (PTEs) in metal mining areas [23] and their function as a stable sink for metal(loid) [1,25]. Depending on the formation processes followed for the formation of secondary minerals [22,25], up to three groups of these supergene minerals can be differentiated [26]. The first group would be those formed by precipitation from acid sulphate waters rich in metallic salts from mining waters. A second group would be formed as a consequence of the filtration of solutions enriched in these metallic salts in mining waste, which give rise to consolidated crusts and hardpans. Additionally, a third group would correspond to saline efflorescences that appear because of the deposition on the surface of mining waste, rocks and soils of the metallic salts contained in the mine water and which are mobilised, either by evaporation during dry periods or by the exothermic heat released during the oxidation of sulphides.
When addressing the management, restoration and risk reduction associated with the mining environment liabilities is essential to assess the different types of waste and their associated risks. The vast majority of mining waste is disposed of via land-based strategies, although a significant amount is dumped directly into the sea [27], either intentionally [28] or accidentally [29]. Traditionally, the degree of soil contamination has been determined by measuring the total concentration of PTEs and comparing it with the respective geochemical background of the elements in the natural soils of an area [30]. However, the mobility and toxicity of these PTEs depend on several factors such as their origin, the mineralogy of the secondary minerals and the chemical fraction to which these elements are associated, soil properties and organic matter, to name a few [5,31]. Therefore, it may be of interest to analyse some of these properties and estimate the mobile fraction present in their leachates.
The aim of this research is to characterise mining waste for a studied area, but also for the first time a typification of the different types of tailings in a Mediterranean metal mining area. It includes both the materials generated by the extractive activity itself and the waste generated after the metallurgical treatment of the extracted mining materials deposited both on land and at sea. In order to carry out this typification, several mineralogical, geochemical and environmental aspects of each type of mining waste have been evaluated. In the case of the former mining region of Cartagena–La Unión, located in SE Spain, this discharge into the sea was very frequent [32], both in the coastal lagoon of the Mar Menor to the West [33] and in the Mediterranean Sea to the East [32,34]. It should be noted that most of the environmental problems caused by these materials arise from the dissolution of their pollutants in the AMD, so the study of their leachates is essential. In addition to the mineralogical and geochemical studies, an attempt has been also made to determine the origin of potentially toxic elements and the possible correlations between some parameters associated with these residues. Knowledge of the behaviour and risks associated with the PTEs of each different waste found in an abandoned metal mining area is necessary to predict contaminant pathways and select the most appropriate management practices. Lessons learned from this study provide a new understanding of the environmental impact and risks associated with metal mining waste and its metal(loid) contamination on surrounding populations and ecosystems.
2. Materials and Methods
2.1. Study Area and Sampling
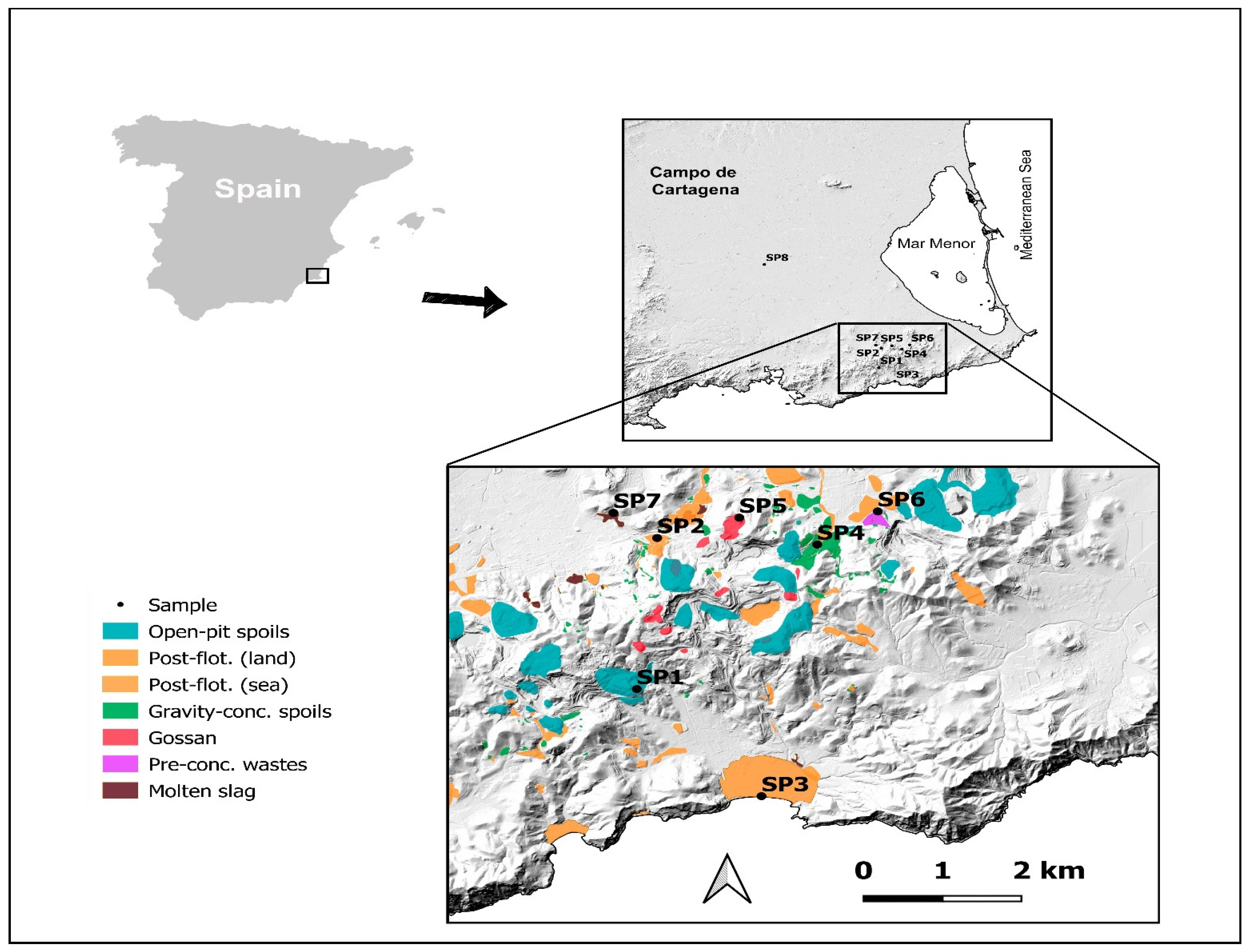
The study area was an abandoned mining sited in the district of Cartagena-La Unión (Region of Murcia (SE Spain) with an overall extension of about 50 km2 (Figure 1). The mining area studied is extended to the Betic Cordillera from the NE and f, within a coastal mountain system lying in the SE. It is located between the Mediterranean Sea to the south, the Mar Menor coastal lagoon to the north and the agricultural plain of “Campo de Cartagena” to the north and west. Geologically speaking, the inner domain is the Betic Cordillera which has two superimposed geological units formed by thick layers of carbonate interlayers interbedded with detritic series [35,36]. These units show an increasing degree of metamorphism from top to bottom with units undergoing strong tectonic folding between the Late Oligocene-Early Miocene and a subsequent extensional collapse during the Middle-Late Miocene [37,38]. The oldest and deepest unit is the Nevado-Filábride complex, consisting of a series of medium-grade metamorphic rocks. On the other hand, the most superficial one is the Alpujárride unit, whose materials show a lower degree of metamorphism than that of the lower layer.
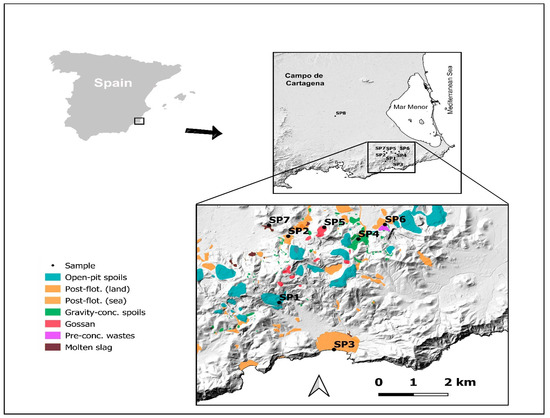
Figure 1.
Sampling points (SP) sited in the study area (SE Spain). SP1–SP7: within the mining area; SP8: in the control area.
During the Upper Miocene, this area suffered a series of calc-alkaline and shoshonitic volcanic episodes that generated powerful hydrothermal processes, where the water from deep areas with dissolved minerals was subjected to strong pressures and high temperatures. Thus, the minerals precipitated in faults and breccias, giving rise to veins enriched in metallic minerals [35]. This resulted in volcanic rocks of andesite, dacite and rhyolite type, generating important deposits of Pb and Ag (galena), Zn (sphalerite) and Sn, with a very important presence of pyrite (Fe) [39], while in the adjacent marine sedimentary basins, there was strong sedimentation [40]. The mineral complexes present in these mountains also include quartzites, mica schists, schists and limestone rocks [18,36].
The climate in this area is described as “steppe or sub-desert Mediterranean” according to the Köppen climate system. It is characterised by average annual temperatures above 18 °C, winters with mild average temperatures above 10 °C and hot summers with average temperatures above 26 °C [41]. On the other hand, the average annual rainfall is less than 300 mm, with rainfall basically concentrated in autumn and spring [41].
However, the most relevant fact is that these mountains are rich in metallic sulphide deposits, mainly Fe, Pb and Zn, which have been exploited for almost 2500 years [35]. The dispersion of the neglected materials led to a massive mobilisation of different metal(loid)s. This prolonged mining activity has generated a large volume of different types of mining waste, approximately 360 million tons of rocks were mobilized [18], with approximately 175 Mm3 of waste deposited on land (onshore) occupying an effective surface area of approximately 9 km2 [18]. In the study area, part of the mining waste was deposited in Portman Bay, in the Mediterranean Sea. Additionally, about 60 Mm3 were discharged into the Mediterranean Sea (offshore) [32,34] and an equivalent amount into the coastal lagoon of the Mar Menor (offshore) [33]. Portman Bay is considered one of the hottest spots for metal pollution both in the Mediterranean [28] and worldwide [29,33].
To obtain a representative sample of the different types of mining waste in this area, seven mining sites were considered that cover the most characteristic waste of this mining area [18]. In addition, a control area was selected in an agricultural area located 15 km away from the metal mining area (Figure 2). From a soil science point of view, all these mining materials would correspond to spolic Technosols developed on mining and metallurgical wastes, while the control soil could be classified as a typic calcaric Regosol or haplic Calcisol [42]. In this study, the dominant type of waste typology in each of the sampling points (SP) was the following: SP1: open-pit tailings; SP2: post-flotation tailings (land-based); SP3: post-flotation tailings (sea-based); SP4: gravity concentration tailings, SP5: gossan/alteration zone, SP6: pre-concentration tailings, SP7: molten slag zone and SP8: control soil. This control area corresponded to agricultural soil located 15 km from the mining area.
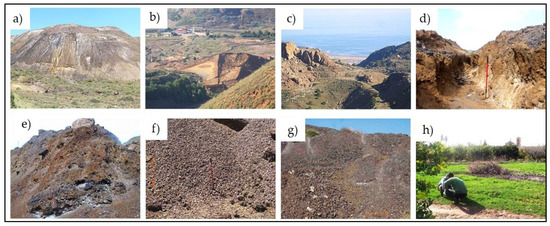
Figure 2.
Images of the different sampling points (SP) considered in this study: (a) open-pit tailings (SP1), (b) post-flotation tailings (land-based) (SP2), (c) post-flotation tailings (sea-based) (SP3), (d) gravity concentration tailings (SP4), (e) gossan/alteration zone (SP5), (f) pre-concentration tailings (SP6), (g) molten slag zone (SP7) and (h) control soil (SP8).
Sampling consisted of collecting the top layer of surface residues from the surface of different mining tailings and the soil of the control area. At each of the eight sampling points (Figure 2), 5 kg of composite samples of mining waste or soils were collected because of mixing and homogenising 10 sub-samples per sample. These samples were transported to the lab in plastic bags for storage. Once in the lab, 500 g of the homogenised samples were crushed to reduce the size of the waste or soil aggregates, which were finally ground with a disc mill to obtain 100 g of fine samples. Using a mortar and pestle, materials were fully ground by hand until they had a final particle size of less than 100 μm after being air-dried for 24 h to prevent peak broadening and amorphousness in geochemical and mineralogical analyses.
2.2. Analysis of the Mineralogy of the Soils and Waste Studied
The mineralogy of the powder from the waste and soil samples was investigated using a Bruker D8 Advance instrument (Bruker Corporation, Billerica, MA, USA). This device has a 2° window with CuK radiation generated at 40 kV and 30 mA, and a one-dimensional detector. To minimise possible peak broadening and amorphousness, the soil and residue samples were gently crushed by hand after air-drying with a pestle and mortar. Powder samples were rotated at 30 rpm and stepped scanned from 5 to 70° in 2θ at 0.05° stepping intervals. A plastic sample container with a rear loading mechanism was used to hold the prepared samples. The samples were dried at room temperature for 24 h to prevent peak broadening and amorphousness, and then meticulously hand-pulverized in a mortar until the final particle size was less than 100 μm [1]. The primary crystalline phases of the waste and soils were identified using XRD patterns (DIFFRAC.EVA 3.0, Bruker AXS, 2012; PDF-4 + powder diffraction file database, ICDD, 2013).
To estimate the stability, H2O content, organic matter content, SO3 content and the presence of non-mineral phases in these soils and wastes, a thermogravimetry coupled with mass spectrometry (TG-MS) analysis was performed. A Mettler Toledo TGA/DSC 1HT (Mettler Toledo, Küsnacht, Switzerland) was used for these analyses under oxygen gas. The loss steps that occurred were investigated by thermogravimetry coupled with mass spectrometry while heating this powder from 30 to 1075 °C. For each soil and/or mine waste, the sum of the decomposition profiles of each of its component elements provided its final thermogram. Since the diffraction powder of the prevailing crystalline states varied in each case, a computed amorphousness is also presented.
2.3. Elemental Analysis of Waste and Soils Studied
Following a procedure similar to the one explained in the previous section, the samples were processed for wavelength-dispersive X-ray fluorescence spectrometry (WDXRF), although this technique is only effective for elements with Z > 8. Since there is a lack of satisfactory certified reference materials with metal concentrations in the same range as the residues and soils investigated in this research, a non-standard approach was used to evaluate the reported spectra. For the elemental study of these samples, 10 g of soil or waste was taken to form granules by pressing. Due to the difficulty in aggregating the samples, about 0.8 g of a binding agent, made by taking 4 mL of a solution of n-butylmethacrylate synthetic resin diluted in acetone in a w/v ratio of 1:5 (Elvacite 2044, Lucite International, Tenn., USA), was added to each sample. Elemental analysis of the samples was performed using a commercial X-ray fluorescence spectrometer, the Bruker S4 Pioneer (Bruker AXS GmbH, Karlsruhe, Germany). The analyses of the obtained spectra were carried out using the commercial Bruker-AXS and Socabim SpectraPLUS software, EVA 1.7 (Bruker AXS GmbH, Karlsruhe, Germany, 2006).
In the meantime, to carry out a semi-quantitative evaluation, a previous assessment of the CO2 and H2O content was performed using the loss on ignition (LOI) method coupled with TG-MS analysis, complemented with CHN analysis (LECO 628 Series, LECO Corp., St. Joseph, MI), which made it possible to obtain an estimate of the H, N and C values in these samples.
Additionally, the enrichment of some of the PTEs present in mining waste was analysed according to the index known as the enrichment factor (EF) [43,44]:
EF= [metal(loid) in soil/residue mg/kg]/[metal(loid) soil background mg/kg]
2.4. Leaching Tests of the Waste and Soils under Study
Once the mineralogical and elemental characteristics of the soil and waste samples had been analysed, these same samples were processed by a DIN 38414-S4 leaching test [45]. From them, some physicochemical variables such as total dissolved solids (TDS), electrical conductivity (EC) and pH were measured, as well as the concentration of the most relevant metal(loid)s (Zn, Cd, As, Pb, Cu, Sb and Ni) and ions (Ca2+, Mg2+, K+, Na+, SO42−, NO3− and Cl−) by ICP-MS (Agilent 7900 ICP-MS, Agilent Technologies, Japan) and ion chromatography (Metrohm 850 Professional IC, Metrohm, Switzerland), respectively. For the leaching tests, 5 g of soil/waste were taken and dissolved by shaking in 50 mL of purified water in polypropylene tubes for 1 full day. After this process, the sample was processed for 24 h using a membrane with a pore size of 0.45 μm.
Furthermore, the leachates were studied using a Ficklin diagram [46] to analyse their chemical variability and origins [47]. For this purpose, the most common PTEs (Pb, Zn, Cd, As, Cu and Ni) in these studied materials were considered. In any case, it is to be expected that the different geological substrates will show distinct geochemical signatures of their own. These signatures are composed of the relative concentrations of each of these elements, in such a way as to assist in their characterization.
2.5. Statistical Analyses
This statistical study was applied to seven mining wastes plus one agricultural soil used as a control and its leachates. The parameters included the PTEs evaluated by WDXRF analysis (Pb, Zn, Cd, As, Cu and Ni) and the major groups of minerals detected by XRD in these materials (silicates, oxides, carbonates, sulphates, sulphides). In addition, several parameters associated with the mining waste and control soils and their leachates have been considered, including H2O, O.M., pH, EC, Cl− and SO42−. The statistical application IBM SPSS version 26 (IBM, NY, USA) was used to perform a multivariate analysis of the collected data.
The Kolmogorov–Smirnov test was used to determine whether the data were normally distributed. Given the non-normality of the data, Spearman’s rank correlation coefficient analysis was developed for each pair of variables, with statistical significance at the 0.01 and 0.05 levels. Consequently, non-parametric tests were used to analyse these data [48]. Additionally, to differentiate between the human and natural origins of the materials studied, and to understand the relationships between variables, a principal component analysis (PCA) with a Varimax rotation and a z-transformation was carried out. As a preliminary step, an analysis of the adequacy of the data matrix was carried out by analysing the communality values, Bartlett’s test of sphericity and the Kaiser–Meyer–Olkin (KMO) test [49,50]. After the elimination of the minor variables, a varimax rotation and z-transformation were performed to reduce the dimensions of the standardised variables to other principal components [49]. Finally, an agglomerative cluster analysis was carried out to establish a hierarchy between the different types of waste based on the available dataset.
3. Results and Discussion
3.1. Mineralogy of the Waste and Soils Studied
The mineral phases and crystalline percentage of the samples, in soils and metal mining waste, were studied and identified by XRD and relative intensity ratio (RIR) approach, respectively (Table 1). Additional studies by TG-MS provided semi-quantitative values for H2O, O.M., SO3 and residue content (Table 1). The main mineral categories found in soils and waste were silicates (24–83%, average 53%), oxides (0–62%, averaging 36%), sulphates (1–20% in mining waste and 0% in control soil), sulphides (weakly present in four mining waste samples and absent in agricultural soils) and carbonates (present only in one waste sample (11%) and control soil (29%)). The most common minerals were chlorite (silicate) and quartz (oxides). The measured amorphousness ranged from 15 to 40%, with an average of 23%.

Table 1.
Mineral phases identified by XRD in soils and mining waste. Semi-quantitative values (% by weight). SP1–SP8: different sampling points. n.d.: not detected.
This analysis revealed a different mineral composition for each type of waste and soil. In this sense, silicates were the main mineral component of the land-based post-flotation residues (83%), the molten slag zone (82%) and the sea-based post-flotation residues (67%). In addition, in the open pit and pre-concentration tailings, the majority consisted of equal parts silicates (51 and 50%, respectively) and oxides (49% in both cases). In the case of gravity concentration and gossan/alteration zone tailings, the most important mineral component was oxides (62 and 49%, respectively), followed by silicates (33 and 31%, respectively) and sulphates (5 and 20%, respectively). In the case of residues from the molten slag zone, sulphates also contributed a major mineral component (12%), while sulphides accounted for 6% of the total mineral components. To complete this description, the post-flotation residues (offshore) comprised 11% carbonates and 3% sulphides. Finally, in the control soil, the most frequent mineral groups were oxides, which contributed about 47% of the total minerals. Likewise, silicates and carbonates, with 24% and 29%, respectively, were the other major mineral groups. Taking into account the different mineral species found in the soils and tailings, the most common mineral in the post-flotation tailings and soil-based pre-concentration tailings was chlorite, where this mineral represented up to 61% and 46% of the total, respectively. Another important mineral was quartz, which accounted for 56, 48 and 48% of the gravity concentration tailings, open-pit tailings and pre-concentration tailings, respectively. These results are consistent with the geological background described for this area [7,18,35].
Regarding the control or agricultural soil, the most relevant minerals were quartz (47%), followed by calcite (24%), confirming a different pattern from that shown by mineral residues from the mining area.
Furthermore, when performing a thermogravimetric analysis coupled to mass spectrometry (Table 2), the whole mixture of minerals that make up each of the studied soil and waste samples was subjected to heating from 30 to 1075 °C. In carrying out this process, the different stages of loss were examined so that the sum of the decomposition profile of each component formed the final thermogram. It should be noted that these geological materials were composed of silicates, oxides, carbonates, sulphates and sulphides, as well as organic matter.

Table 2.
Thermogravimetric analysis coupled with mass spectrometry of the different types of soils and mining waste samples. O.M.: organic matter; Inor. CO2: inorganic CO2. H2O, organic matter, inorganic CO2, SO3 and residue are given in %, while the temperature range is given in °C.
After heating the samples, three main effluent gases appeared. Depending on the availability of water (free, crystalline and constituent) and organic matter, water loss (dehydration) took place mainly in the thermal range of 30–717 °C in different stages. According to the presence of organic matter and carbonates, the loss of carbon dioxide (decarbonisation) occurred mainly in the thermal range 176–984 °C in various phases. Finally, the loss of sulphur trioxide (desulphation) happened in the thermal range of 658–1075 °C and even higher, showing the existence of metal sulphides and sulphates. The values of the losses for H2O, organic matter and SO3 as well as the residual content are given in Table 2.
The average water content of the samples was 7.56%, ranging from 2.02 to 13.25%. The temperature range of the dewatering process varied between 30 and 717 °C in the case of the mining waste, reaching up to 1075 °C for the natural soil sample. Organic matter was found in all samples, except SP1 and SP3, and ranged from 0.13 to 1.82%, with an average of 0.11% in the mining waste samples, which contrasts with the value of 1.82 for the agricultural soil. In this case, the temperature range varied between 176 and 687 °C for the mining waste samples and between 237 and 569 °C for the control.
Regarding inorganic CO2, this fraction was only found in samples SP1, SP3, SP6 and SP8. Their values ranged from 0 to 6.05%, with an average of 1% for the mining waste samples, a value that contrasts with the 10.6% of the agricultural soil studied. Despite the average value for the mining waste samples being 1%, most of them presented inorganic CO2 values close to zero or almost non-existent. These results are not consistent with the large amount of inorganic carbon found in mining waste deposited in the sea, where the high concentration of carbonate-enriched minerals (Table 1) probably distorted this average. The temperature range of the inorganic CO2 removal process varied between 554 and 1075 °C for the samples considered.
Subsequently, the loss of sulphur trioxide (desulphation) was analysed. Five of the mining waste samples had SO3 concentrations ranging between 0.16 and 8.89%, with an average of 2.11% for mining waste samples as a whole. The temperature range of the desulphation process, or SO3 removal, ranged from 658 to 1075 °C for the mining waste samples studied.
Finally, the total calcination of the samples left a residue that ranged from 77.69 to 97.96%, with an average of 89.03% for mining waste samples and 81.25% for control soils. The composition of this residue is mainly, oxides and silicates. In this sense, the waste that reached the highest residual value was molten slag probably due to many sulphides, mostly galena, found in the waste products (Table 1).
It is important to note that these mining and metallurgical wastes contain sulphides, sulphates (found in all types of waste), oxides and hydroxides, in varying amounts depending on the meteorization level of the mined minerals and the materials discarded at each landfill site. This high diversity of minerals in the soils and mine waste is consistent with the results shown by other authors for other mining areas [1,51]. In summary, the mining waste samples can be distinguished from the control soil by their low presence of organic matter, inorganic CO2 and sulphur trioxide, largely confirmed by XRD tests.
3.2. Results of the Elemental Analysis of the Waste and Soils Studied
Besides the mineralogy, the elemental composition of the soil and waste samples studied was analysed (Table 3). For this, the ignition weight loss was evaluated by TG-MS to obtain a more precise evaluation of element concentration. When analysing the WDXRF results, only those values with intensities that were three times higher than the statistical background of the sample were considered.

Table 3.
Geochemistry of the soils and waste studied by WDXRF and TG-MS (wt.%). SP1–SP8: different sampling points. n.d.: not detected.
Following previous research [20], the elements present in the minerals of the mine waste samples can be divided into three different element fractions in the soil and mine waste samples. Without considering basic elements such as O, H and C, a major fraction values ranged between 10 and 100%, a minor one had concentrations of its elements between 0.1 and 10%, and finally a micro-fraction with minimum concentrations in the range of 1 to 1000 ppm. Within the major fraction, Si and Fe stood out, with concentrations ranging between 10.8 and 36.5% and 5.5 and 29.5%, respectively. The minor fraction included the following elements P (0–0.1%), As (0–0.2%), Ti (0.1–0.4%), Mn (0.1–1%), K (0.1–1.1%), Na (0.1–1.3%), Zn (0.1–1.5%), Cl (0–1.7%), Pb (0–2.8%), Ca (0.1–3.3%), S (0.3–4.4%), Mg (1.5–3.7%) and Al (2.6–9.7%). Finally, the micro-fraction comprised elements such as the following: Ga (below the detection limit), Nb (≤12 ppm), Ni (≤36 ppm), Y (≤47 ppm), Co (≤51 ppm), Br (≤52 ppm), Sn (≤54 ppm), Rb (≤64 ppm), Cd (≤65 ppm), Ag (≤70 ppm), Cr (≤80 ppm), V (≤110 ppm), Ba (≤120 ppm), Zr (≤154 ppm), Sb (≤220 ppm), Cu (≤268 ppm) and Sr (≤284 ppm). In the case of the control soil, the elements that were in the different fractions and arranged in increasing concentrations were Si and Ca in the major fraction; Fe, Ti, Al, Na, K, Mg and P in the minor fraction, and Zn, Pb, As, Cd, Cu, Mn, Ag, Ni, Cr, Sn, Sr, Br, Co, Zr, Rb, V, Ga, Nb, Y, Sb, Ba, S and Cl were found within the micro-fraction. The presence of elements such as As, Mn, Zn, Pb and Al, as well as other elements such as Ni, Co, Br, Sn, Cd, Ag, Cr, Zr, Sb, Cu and Sr in the micro-fraction of the waste, is noteworthy. Mining residues are often easily erodible and weatherable materials, which means that they are highly susceptible to contributing potentially toxic elements to the environment. These findings are consistent with the geochemistry reported for this area by other authors [7,8,15,16,17,18,19,24].
Using dry combustion, C, H and N were quantitatively analysed. A 100 mg dust sample was incinerated at 950 °C in excess oxygen. Using the soil standard Leco 502–062, calibration curves were generated. The resulting data are compiled in Table 4.

Table 4.
CHN analysis by dry combustion of the soils and metal mining wastes considered in this study. SP1–SP8: different sampling points. n.d.: not detected.
It should be noted that sphalerite (ZnS) is the main geological source of Zn and Cd both globally [52] and in the study area [36]. In addition, since the study area is a heavily weathered former mining area, Zn and Cd were also present in the form of oxides and other mineral phases enriched in these elements, as reflected in the mineralogical and geochemical studies presented here (Table 1 and Table 3). This behaviour should be extended to the rest of the elements studied, which will be equally affected by weathering and oxidation processes due to the mining–metallurgical processes.
In terms of carbon, only post-flotation tailings (sea-based) and agricultural soil were within the range of validity of the calibration, both rich in carbonates. In the case of agricultural soils, this carbon came from both the organic fraction and the carbonates present in the soils, whereas in the post-flotation tailings of marine origin, this carbon was mostly inorganic due to carbonates in the form of calcite, dolomite and siderite present in the waste products (Table 1).
Regarding hydrogen, the highest concentrations of this element were detected in the post-flotation tailings (land-based), in the gravity concentration tailings and in the samples from the gossan/alteration zone. Finally, nitrogen was only detected in very low amounts in the post-flotation tailings (land-based) as well as in the control soil, indicating the occurrence of microbial activity.
Taking into account the local background levels of the surface soils in the area [53], the enrichment factors (EF) of the considered mining residues were evaluated (Table 5). Low-enriched samples (EF < 10) corresponded to Ni, Cr and Co. On the other hand, moderately enriched residues (10 < EF < 100) were only found for Cu, and finally samples with a strong enrichment (EF > 100) corresponded in some cases to Cd, in most of the mining waste types to As and Sb, but especially to Zn and Pb. This proves that the mining waste still contained significant amounts of metal(loid)s in its structure.

Table 5.
Metal(loid) enrichment factors of mining waste in relation to local background levels in local soils. SP1–SP8: different sampling points. n.d.: this data is not available because this element was not detected.
Based on the overall enrichment factor of each waste type, it can be concluded that post-flotation tailings (land-based), gravity concentration tailings, the gossan/alteration zone and the molten slag zone showed the highest contamination levels, followed by post-flotation tailings (sea-based) by some distance. On the other hand, pre-concentration tailings and, finally, open-pit tailings showed the lowest level of pollution of all the mine waste considered.
3.3. Leaching Test Results
Table 6 shows the results of the leaching tests conducted on the tailings and the control soil. This table also comprises the leaching limit values (LLV) in accordance with the European Council Decision 2003/33/EC [54], which establishes standards and practices for the acceptance of residues in landfills (L/S = 10 L/kg; leachate produced according to DIN 38414-S4 [45]), as well as the regulatory levels for pH and EC [46,54].

Table 6.
Leaching test results for soils and waste samples. SP1–SP8: different sampling points. n.d.: not detected. EC = electrical conductivity (expressed in mS/cm). TDS = total dissolved solids (expressed in g/kg). SO42− and Cl− (expressed in g/kg). Zn, Pb, Cd, Ni, Cu, As and Sb expressed in mg/kg. Lim. val.: indicate the limit values of the WHO guidelines for drinking water quality. Factor: ratio of the average parameter obtained in the leaching tests to that of the WHO guidelines for drinking water. The three sets of leaching limit values (LLVs) for waste reception at landfills are provided. Values outside the WHO limit values for drinking water quality are highlighted in bold, while values outside the LLV set 1, set 2 and set 3 are indicated by *, ** and ***, respectively.
Although Pb is one of the most common elements found in the study area, its leaching values are low (Table 6). This can be explained by the low solubility of this metal [30]. However, its solubility could increase due to soil characteristics, such as high acidity and the development of Pb-organic compounds [55]. Together with the presence of anglesite in some mine residues (Table 1), a mineral that may also contribute to increased lead solubility [56], these factors could partly explain the relatively high Pb leachates from SP7 (Table 6).
The European Council Decision 2003/33/EC specifies three sets for the disposal of waste in landfills [54,57]. Group 1 establishes the threshold limits for acceptance of hazardous waste at dumps for the disposal of hazardous waste. In turn, group 2 establishes the threshold values for the acceptance of waste at landfills for non-hazardous waste disposal. Finally, group 3 specified the acceptance limits for the disposal of waste in inert waste dumps. In this regard, it was found that only post-flotation tailings (sea-based) met the waste acceptance criteria for landfill, in addition to open-pit tailings and pre-concentration tailings. Gravity concentration tailings were the most harmful wastes followed by post-flotation tailings (land-based) and the molten slag zone and to a lesser extent the gossan/alteration zone. All of them showed limitations for landfilling. Regarding the elements and anions that most affected the storage of these residues in landfills, the vast majority of the restrictions were due to the presence of Cd, Zn and Pb, and anions such as SO42− and Cl− that are associated with set 3 of LLVs for the acceptance of waste in landfills. In addition, pH and TDS are the most limiting physicochemical parameters for the disposal of mining waste in landfill.
In summary, it could be concluded that depending on the degree of compliance with the criteria for the acceptance of waste in landfills, the approximate classification of the different types of mining waste would obey the following order, from highest to lowest degree of compliance: post-flotation tailings (sea-based) ≥ open-pit tailings ≥ pre-concentration tailings >> gossan/alteration zone >> molten slag zone > post-flotation tailings (land-based) >> gravity concentration tailings.
On another level, in relation to the WHO drinking water guidelines [58], the “Factor” row of Table 6 shows the enrichment factors for each element in the leachate of the studied mining waste in comparison to the WHO recommendations. According to these values, for each litre of leachate generated by these tailings, up to 107 litres of drinking water could be contaminated. In this respect, gravity concentration tailings and post-flotation tailings (land-based), but also the gossan/alteration zone, the molten slag zone and open-pit tailings on a second level, and on a lower level, pre-concentration tailings and post-flotation tailings (sea-based) are wastes that can cause significant contamination of freshwater bodies. When analysing the relationship between the average value obtained for the different parameters in the leaching tests carried out and the limit values assigned by the WHO guidelines for drinking water [58], the values of Pb stand out with a level 107 times above the values of the WHO guidelines for drinking water, and of Cd with a value up to 78 times above this limit established to consider the water as drinkable (Table 6). To a lesser extent, this factor is also notable for parameters such as Zn, TDS and SO42-, with values between seven and three times higher than those established in the corresponding regulations.
Summarising all the above, as regards the impact of these residues on the quality of freshwater bodies, the level of impact is classified in the following order, from highest to lowest impact: gravity concentration tailings ≥ post-flotation tailings (land-based) >> gossan/alteration zone > molten slag zone > open-pit tailings >> pre-concentration tailings > post-flotation tailings (sea-based). Furthermore, the relationship between the parameters considered in the waste leachates studied and their corresponding WHO limit values for drinking water, in decreasing order, is as follows: Pb >> Cd >>> Zn > TDS > SO42− > EC = Cl− > Ni > As > Sb > Cu.
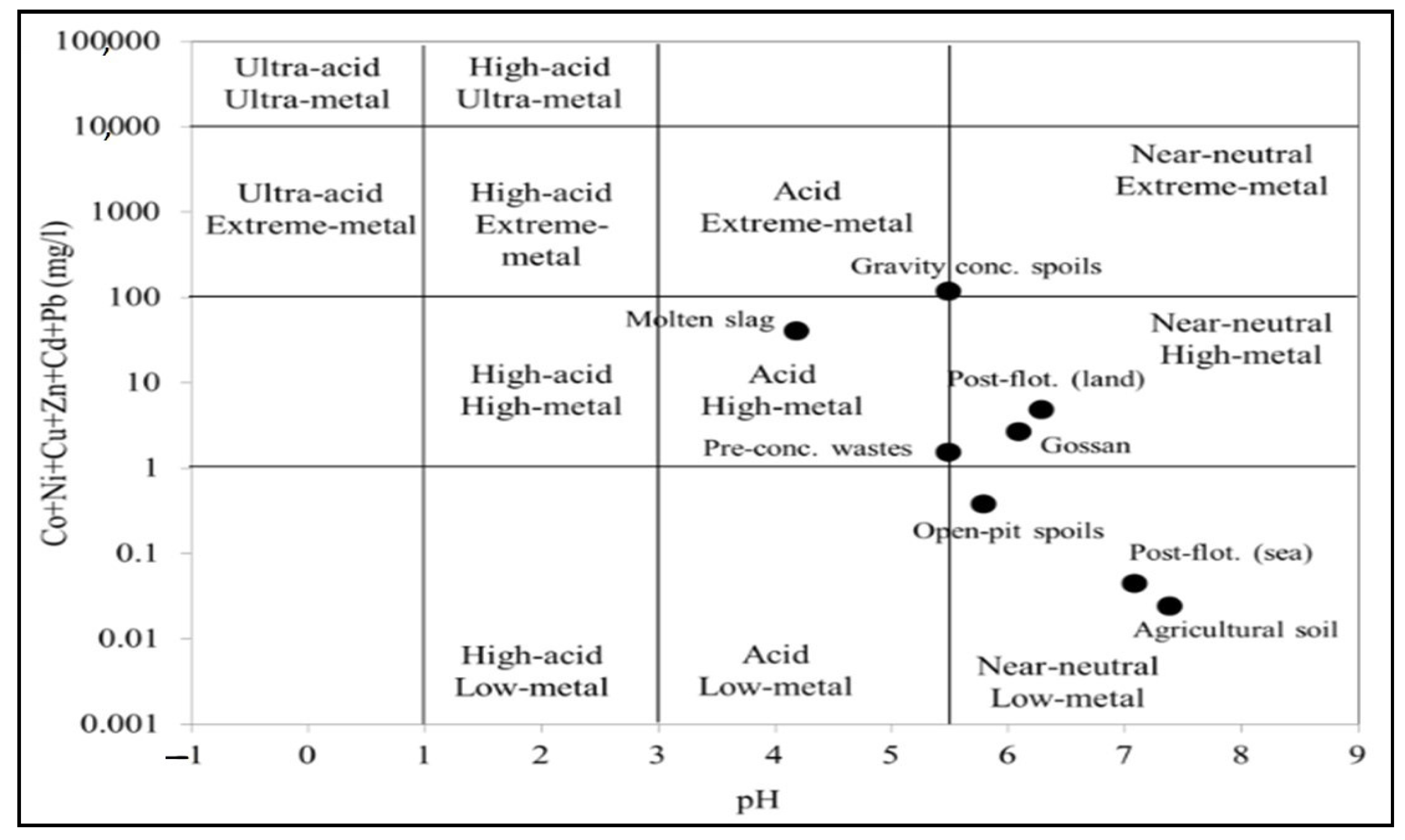
In a complementary way to the above, a classification was made using a Ficklin diagram [46] (Figure 3). This analysis was performed with the values obtained from the sum of metal(loid)s and the pH of the leachates of the studied mining waste according to the leaching test DIN 38414-S4 [45]. Summarising all this, it could be concluded that most of these leachates could be considered as high or extreme-metal content solutions, while only post-flotation tailings (sea-based) and tailings from open-pit mining could be categorised as low-metal content leachates. According to this diagram (Figure 3), the leachates from the different tailings were grouped into different categories in this diagram. In this respect, only gravity tailings could be considered extreme-metal-acid solutions, while only the pre-concentration tailings and the molten slag residues could be considered high-metal-acid solutions. On the other hand, gossan/alteration and post-flotation tailings (land-based) could be considered high-metal-quasi-neutral solutions. Finally, only post-flotation (sea-based) tailings and open-pit mining tailings could be considered low-metal-near-neutral solutions.
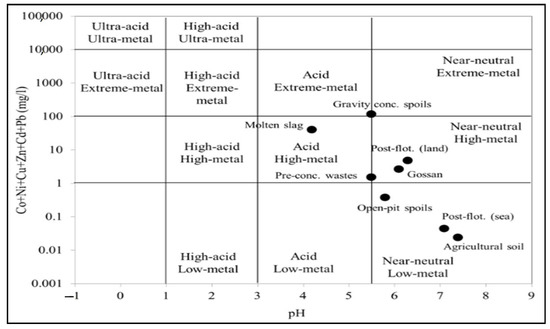
Figure 3.
Ficklin diagram showing the categories of leachates from the different soils and mining waste considered in this study based on their metal content according to the WHO guidelines for drinking water.
Based on these findings, it can be considered that these leaching solutions can cause significant impacts on the environmental quality of water bodies and soils in the vicinity of these mine tailings, which corroborates the risks calculated according to the WHO guide limits [58].
3.4. Statistical Relationships between the Different Parameters Considered
A relevant issue in assessing the chemical and geochemical behaviour of different types of mining waste is to reveal both the relationships between the different parameters and between the parameters and the types of mining waste. The innovative approach proposed in this section is to evaluate by correlation analysis and agglomerative cluster analysis on the basis of mineralogical and geochemical data in order to achieve a typification of these tailings into groups with more or less similar environmental behaviour.
First of all, the Kolmogorov–Smirnov normality test shows that the data are non-normally distributed in the waste and soil samples. Due to this non-normal character of the data, statistical analyses performed included Spearman’s correlation coefficient to assess the robustness of a monotonic relationship in paired data (Table 7). Significant correlations at the 0.01 and 0.05 levels between values are shown in bold.

Table 7.
Spearman’s rank correlation coefficient between anions, metal(loid)s, groups of minerals and properties of soils and metal mining waste (M) and their leachates (D). Significant correlations at the 0.05 (*) and 0.01 (**) levels are shown in bold. M = mineral solid form; D = dissolved in leachates; ∑M = sum of the metal(loid)s considered; Sil. = silicate minerals; Ox. = oxide minerals; Car. = carbonate minerals, Sulfa. = sulphate minerals; Sulfi. = sulphide minerals.
The occurrence of a significant correlation between different elements could result from the same source [59]. When studying the correlations between PTEs, minerals and properties, some correlations have been found (Table 7). The sum of elements in solid form appears highly correlated with the presence of Pb, Zn, Cd and Cu in their mineral form. In turn, the correlations between elements in their solid form such as Cu and Pb, as well as between Zn and Cd show very high values. Equally significant are those between the solid phases for Cu and Zn, Zn and Pb, Cd and Pb, and As and Pb, although the significance level was lower. In relation to the soluble forms present in the leachates, the sum of all the elements considered seems to be highly correlated with the presence of Zn, Ni and Cd, and to a lesser extent with that of Pb, which may be due to the lower solubility of the latter metal. Likewise, the relationships between the soluble forms of Ni with those of Zn and Pb, as well as those of Zn with Cd, are highly significant, while Ni and Cd are also correlated, but to a lesser extent.
Numerous studies have shown strong relationships between minerals and their major (Zn, Pb, etc.) and trace elements (Cd, As, etc.), like those found in this study [60,61]. It has been reported for this geological setting, Cu appears associated with mineable minerals, such as galena (PbS), as metallic impurities of primary minerals [36]. Therefore, this can explain the high correlation detected for Cu and Pb. In turn, when understanding the relationship between Zn and Cd, it must be considered that they are chemically similar elements, which often allows substitution between them in minerals [62]. In fact, previous studies have already observed a significant correlation between both elements within the sulphide mining area studied [63,64]. The similar electronic configuration, as well as their common dissolution from secondary minerals, explains the similar geochemical behaviour of these elements in mining environments [65]. It should be noted that sphalerite (ZnS) is the main geological source of Zn and Cd both globally [52] and in the study area [36].
Cd is found in significant amounts in many Pb/Zn mine tailings [6,62,66]. Like others such as Mn and Cr, Ni has been described for the study area as impurities of metallic sulphides such as sphalerite [67]. For its part, As is associated with sulphide ores of Pb, Zn and Cu [68] and, consequently, it can pollute soils and sediments during mining and smelting activities [69]. For the geological setting of this study, As and Cu, at least in part, are associated with pyrites, which are very abundant in this area [67]. Acid mine drainage in metal sulphide mining areas generates significant leaching of concentrated metal ions such as Zn, Pb and Cu among others [70], so these results coincide with those found by other authors for this area [15,63]. It is worth mentioning that the most harmful heavy metals for the environment, are Cd and Pb, in addition to Hg [29].
Respecting, the mineral groups considered, sulphates were significantly correlated with the solid phases of As and Pb, and to a lesser extent with the sum of elements evaluated. A good correlation was also found between sulphides and soluble Cu. The significant negative correlations between the oxides with silicates and sulphides are also noteworthy, while the carbonates show a good correlation with pH, which is very high with organic matter. The properties such as electrical conductivity and total dissolved solutes show a total correlation between themselves and a high correlation with Cl− and SO42−.
It is known that the mineralogical composition, dissolution and oxidation processes are determining factors in the geochemical and environmental risks associated with metallic mine waste [22,28,61,71]. As for pH, this parameter is only correlated with carbonates, without correlation with the concentration of the elements considered, unlike other similar cases [72]. The good correlation between pH and organic matter must be related to the best conditions for plants and microbiota in non-acidic soils, which correspond to the presence of carbonates in this mining area [64].
Different tools can be used to determine if the origin of the studied materials is natural or anthropogenic. In this sense, a good way to detect possible relationships between the different types of mining waste is the use of statistical tools such as principal component analysis (PCA) [22,61]. Consequently, in a complementary way to the correlation analysis, a PCA analysis was carried out to detect the mineralogical processes and relationships between the parameters within the studied mining waste (Table 8, Figure 4). To achieve a high correspondence between each variable studied with a single principal component, a Varimax rotation with Kaiser normalisation was performed. PCA is a mathematical process that involves computing the covariance matrix, removing elements that only slightly modify the datasets, and automatically scaling the values to produce new parameters [1].

Table 8.
Principal component analysis with extracted components and total variance explained.
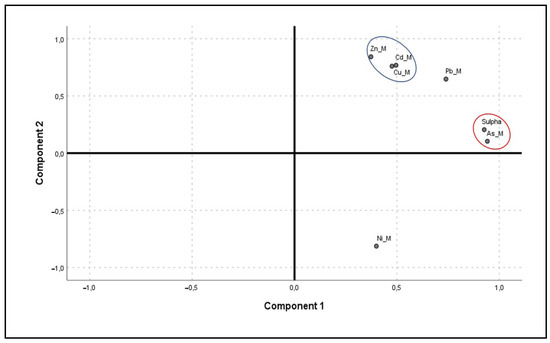
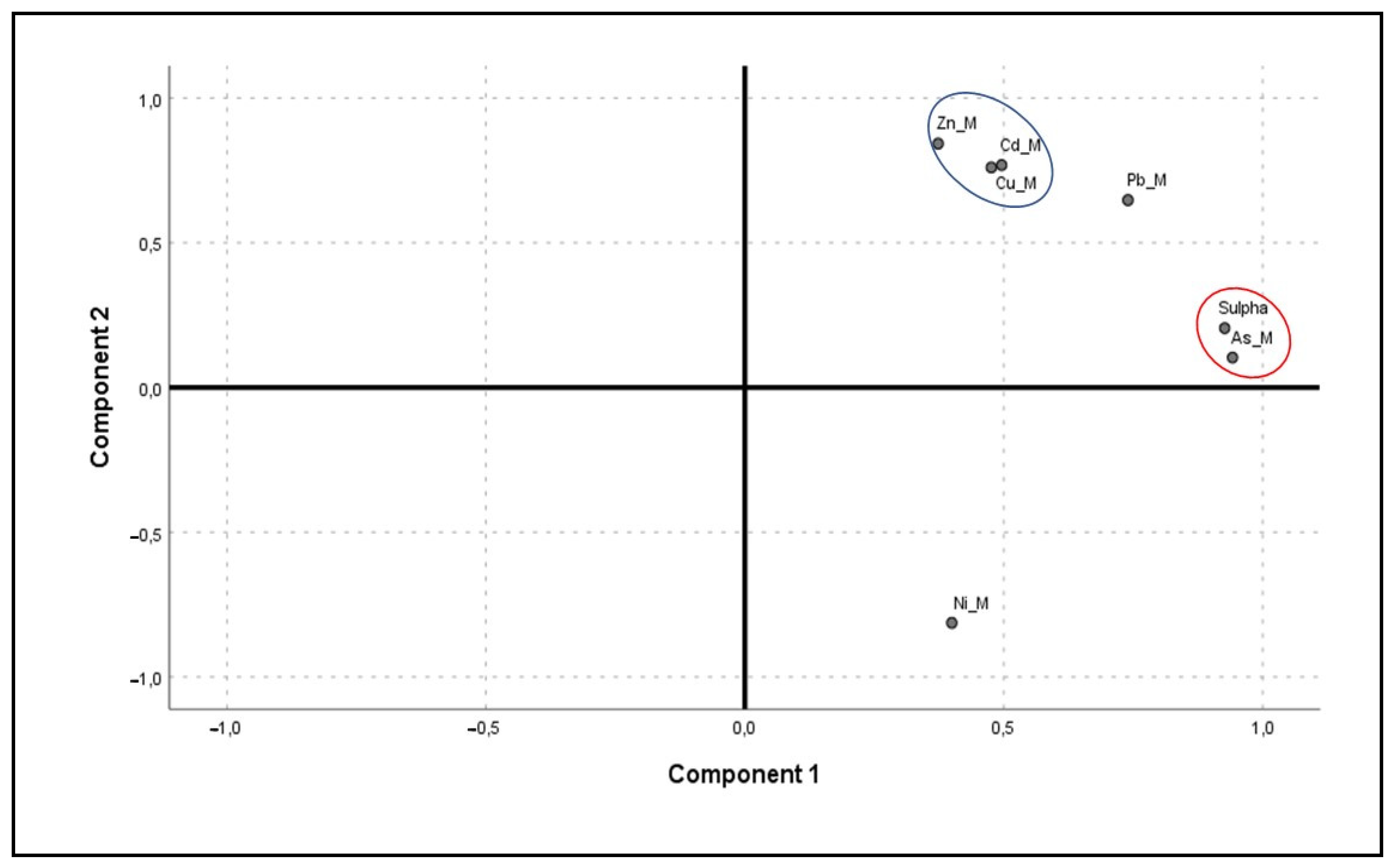
Figure 4.
Principal component analysis plot with Varimax rotation of sulphate minerals (Sulpha) and potentially toxic elements in solid form (Ni_M, Cu_M, Zn_M, As_M, Cd_M, Pb_M). The red circle corresponds to Component 1, while the blue circle corresponds to Component 2.
In light of the PCA analysis (Table 8, Figure 4), it was possible to determine up to two factors with eigenvalues greater than 1, denoted as Component 1 and Component 2, which explained 86.778% of the total variance. Component 1 accounted for 64.795% of the total variance, mainly due to As, with a loading of 0.942, and sulphates, with a loading of 0.927.
The presence of As is mainly associated with pyrite (FeS2), a very abundant mineral in this area [36]. On the other hand, sulphates are usually secondary minerals resulting from the oxidation of primary sulphides, very common in this mining area [71]. Furthermore, Component 2 explained 21.983% of the total variance and loaded on Zn (0.842), Cd (0.768) and Cu (0.760). These elements are associated with mineable minerals such as sphalerite (ZnS), for Zn and Cd, and galena (PbS) for Cu as a primary mineral impurity [36]. Together with pyrite, sphalerite and galena are the most common sulphides in this area [36], with a high presence of Zn, Cd and Cu associated with them [6,36,52,62,63,64,65].
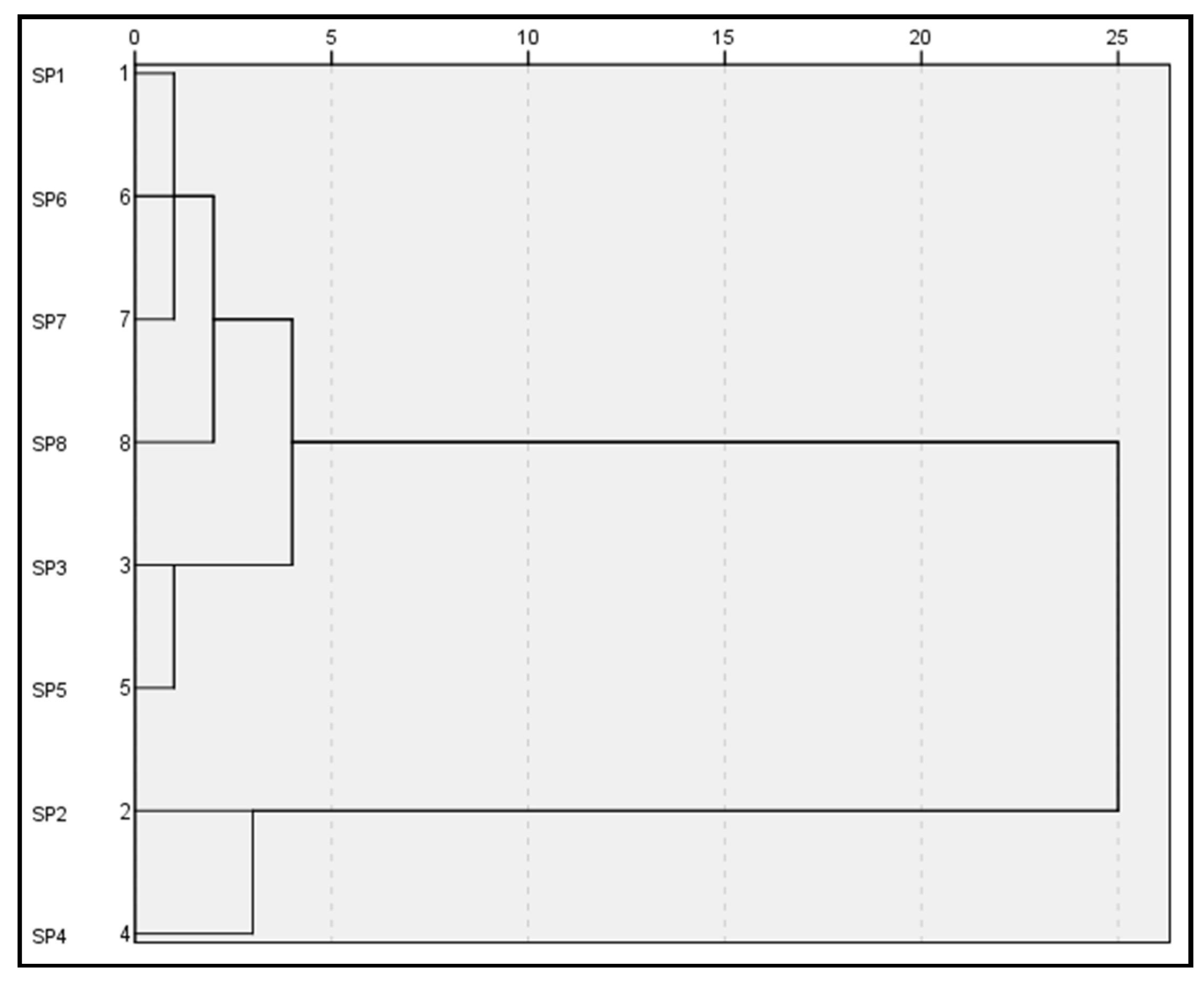
To find similarities between the geochemical behaviour of the different types of mining waste and soils, a hierarchical cluster analysis of all the results obtained was conducted. This analysis was translated into a hierarchical dendrogram to graphically visualise these possible similarities (Figure 5). To correct the distortion produced by the outliers and considering the Tukey’s transformation ladder, the correction of the positive asymmetries detected in the data was carried out by means of square root transformations. This analysis shows the existence of two main groups of residues (Figure 5). The first group includes open-pit tailings, post-flotation tailings (sea-based), the gossan/alteration zone, pre-concentration tailings and the molten slag zone. In turn, the second group includes post-flotation tailings (land-based) and gravity concentration tailings.
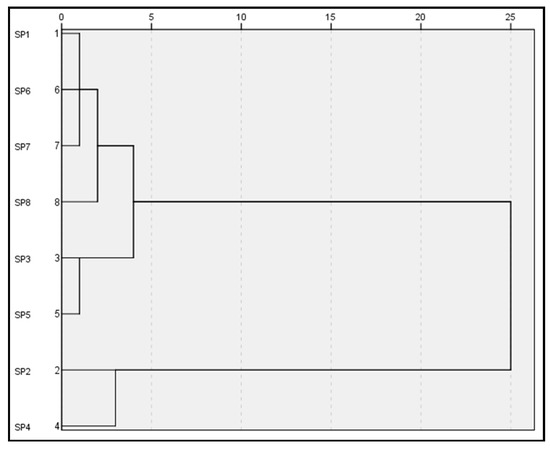
Figure 5.
Hierarchical cluster analysis dendrogram (Ward’s cluster algorithm; distances: Euclidean squared) for all soils and mining waste datasets. The ordinate axis indicates the different types of mining waste, and the abscissa axis shows the distance between the merged clusters.
Cluster analysis of soils and mining waste revealed several interesting linkages between elements. Seven types of waste and the natural soil used as a control were compared to find similarities mainly in terms of their geochemical values. The result of the cluster analysis is presented in the form of a dendrogram (Figure 5). Similar results refer to those with similar chemical and geochemical behaviour. The dendrogram was made using Ward’s method to define similarities and dissimilarities between soils and residues. The constructed dendrogram showed two large distinct clusters with four groups formed.
Open-pit tailings (SP1), the molten slag zone (SP7) and pre-concentration tailings (SP6) show good similarity and clump together in one cluster, while the post-flotation (sea-based) tailings (SP3) and the gossan/alteration zone (SP5) are grouped in another. Within this first large cluster, the control soil (SP8) is isolated in an exclusive group. The first group in relation to the control soil and these two groups in relation to each other showed a close relationship in the first of the main clusters. On the other hand, the post-flotation (land-based) tailings (SP2) and the gravity concentration tailings (SP4) were grouped in the fourth group forming the second cluster, not showing a close similarity with the rest of the groups in the dendrogram.
These results revealed proximity in the geochemical behaviour of the older (gravity) and more modern (post-flotation land-based) tailings. However, when these post-flotation tailings were deposited at sea, their behaviour was more similar to that of the residues from the mineral/gossan alteration zone than to that of the same tailings deposited on land. The other types of mining waste showed similar behaviour to each other, while the natural soil exhibited its own geochemical dynamics.
4. Conclusions
On a local scale in a Mediterranean metal mining area, the mineralogical, geochemical and environmental characterisation of different types of tailings was addressed. In addition, an innovative typification of these residues was carried out in order to determine their different behaviours and environmental impact. Although there have been numerous previous studies on mining waste in this area, none of them have carried out a typification of these tailings. This typification of the different types of metal mining waste in the studied area was carried out by means of a correlation analysis and an agglomerative cluster analysis that provided interrelationships and a hierarchy between the different types of waste according to their mineralogy, geochemistry and associated environmental risks.
The most common mineral groups found in the studied mining waste were, in decreasing abundance: silicates >> oxides >> sulphates >> sulphides >> carbonates. This corresponds to the presence of two dominant minerals, chlorite, which is a silicate, and secondarily an oxide such as quartz. When analysing mineral geochemistry, the major fraction includes Si and Fe, the minor fraction Al > Mg ≥ S > Ca > Pb > Cl ≥ Zn ≥ Na ≥ K ≥ Mn > Ti > As > P, and the micro-fraction Sr ≥ Cu ≥ Sb > Zr > Ba ≥ V > Cr ≥ Ag ≥ Ni > Nb. The dominant mineral group and the most important metal(loid) fraction differed between each type of mining waste considered.
The use of enrichment factors was a very useful tool to assess the different levels of pollution of each type of mining waste. According to this enrichment factor, the post-flotation tailings (land-based), the gravity concentration tailings, the gossan/alteration zone and the molten slag zone had the highest levels of contamination. In any case, these data reveal that mining waste still contains significant quantities of metal(loid)s to be considered toxic and hazardous waste.
Due to their high Cd, Zn and Pb content, but also SO42−, Cl−, pH and TDS, the gravity concentration tailings, post-flotation tailings (land-based), the molten slag zone and the gossan/alteration zone showed the main limitations for landfilling. On the other hand, only post-flotation tailings (sea-based), open-pit tailings and pre-concentration tailings, with some limitations, met the residues acceptance criteria for landfilling. In addition, some of these mining wastes may be hazardous to water quality. Gravity concentration tailings, post-flotation tailings (land-based), the gossan/alteration zone, the molten slag zone, open-pit tailings, pre-concentration tailings and post-flotation tailings (sea-based) are wastes that can cause significant contamination of freshwater bodies due to their Pb, Cd, Zn, TDS and SO42− content. With the exception of post-flotation (sea-based) tailings, virtually all of these leachates can be considered high- or extreme-metal content solutions. Based on all this evidence, it seems that different risks and characteristics are associated with each type of waste. This differential toxicity between the different types of waste could be considered one of the main aspects to be taken into account when addressing the management of abandoned metal mines.
For this geological setting, mineable minerals such as galena, sphalerite and pyrite are the main source of the studied PTEs. These minerals are therefore the source of contamination of soils and sediments in the area once they are mined and oxidised to sulphate and other mineral phases. The geochemical behaviour of the different types of tailing has been determined mainly by As and sulphates, but also by Zn, Cd and Cu to a lesser extent. Considering all the parameters studied, the dynamics of the different types of tailings have allowed us to separate them into several groups with a homogeneous internal response.
All these results could be positive and useful for better management of this metal mining area. Although these results on the environmental hazards associated with the different types of metal mining waste studied are mainly significant at the local level, some of the considerations and methodologies included here could be applicable to other mining areas around the world. Finally, even though these results are a snapshot of the current state, they could help to better prioritize each type of mining within a future restoration and/or management programme for this mining area. In the future, methodologies such as the one used in this research could be used and improved to obtain more accurate technical criteria for a better understanding of the functioning and problems of metal mining areas.
Author Contributions
Conceptualisation, L.A.A.-R. and R.R.-P.; methodology, L.A.A.-R., C.G.-G., V.R.-A. and A.V.C.-R.; software, L.A.A.-R., C.G.-G. and V.R.-A.; validation, L.A.A.-R. and A.V.C.-R.; formal analysis, L.A.A.-R., G.G. and R.R.-P.; investigation, L.A.A.-R., R.R.-P., R.M. and A.P.-S.; resources, R.R.-P., G.G. and C.G.-G.; data curation, L.A.A.-R. and A.V.C.-R.; writing—original draft preparation, G.G. and R.R.-P.; writing—review and editing, G.G., R.R.-P., L.A.A.-R., R.M. and A.P.-S.; visualisation, R.R.-P. and L.A.A.-R.; supervision, R.R.-P. and L.A.A.-R.; project administration, R.R.-P., L.A.A.-R. and C.G.-G.; funding acquisition, R.R.-P., G.G., R.M., A.P.-S. and L.A.A.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the infrastructure support of the Assistance Service for Technological Research at the Universidad Politécnica de Cartagena (Spain). An intensive revision of the manuscript has been carried out by a native English speaker.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010; 400p. [Google Scholar]
- Hudson-Edwards, K.A.; Jamieson, H.E.; Lottermoser, B.G. Mine wastes: Past, present, future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Nikolaidis, C.; Orfanidis, M.; Hauri, D.; Mylonas, S.; Constantinidis, T. Public health risk assessment associated with heavy metal and arsenic exposure near an abandoned mine (Kirki, Greece). Int. J. Environ. Health Res. 2013, 23, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Fields, S. The Earth’s open wounds: Abandoned and orphaned mines. Environ. Health Perspect. 2003, 111, A154–A161. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Mickus, K.; Camacho, L.M. Abandoned Pb/Zn mining wastes and their mobility as proxy to toxicity: A review. Sci. Total Environ. 2016, 565, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Gutiérrez, M.; Gouzie, D.; McAlily, L.R. State of remediation and metal toxicity in the tri-state mining district, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef]
- Robles-Arenas, V.M.; Candela, L. Hydrological conceptual model characterisation of an abandoned mine site in semiarid climate. The Sierra de Cartagena-La Unión (SE Spain). Geol. Acta 2010, 8, 235–248. [Google Scholar]
- Gomez-Ros, J.M.; Garcia, G.; Peñas, J.M. Assessment of restoration success of former metal mining areas after 30 years in a highly polluted Mediterranean mining area: Cartagena-La Unión. Ecol. Eng. 2013, 57, 393–402. [Google Scholar] [CrossRef]
- EU. Union Directive 2006/21/EC of 15 March 2006 on the management of waste from extractive industries. Off. J. Eur. Commun. 2006, L102, 15–33. [Google Scholar]
- Rybak, J.; Gorbatyuk, S.M.; Bujanovna-Syuryun, K.C.; Khairutdinov, A.M.; Tyulyaeva, Y.S.; Makarov, P.S. Utilization of mineral waste: A method for expanding the mineral resource base of a mining and smelting company. Metallurgist 2021, 64, 851–861. [Google Scholar] [CrossRef]
- Rybak, J.; Adigamov, A.; Kongar-Syuryun, C.; Khayrutdinov, M.; Tyulyaeva, Y. Renewable-Resource Technologies in Mining and Metallurgical Enterprises Providing Environmental Safety. Minerals 2021, 11, 1145. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghosh, R.; Adhikari, T.; Mukhopadhyay, S.; Chattopadhyay, A.; Pal, S.K. Development of Nanomedicine from Copper Mine Tailing Waste: A Pavement towards Circular Economy with Advanced Redox Nanotechnology. Catalysts 2023, 13, 369. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Arima, T.; Villacorte-Tabelin, M. Utilization of Palm Oil Fuel Ash (POFA) as an Admixture for the Synthesis of a Gold Mine Tailings-Based Geopolymer Composite. Minerals 2023, 13, 232. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, B.; Han, G.; Duan, W.; Wang, Z. Application and Prospect of Curtain Grouting Technology in Mine Water Safety Management in China: A Review. Water 2022, 14, 4093. [Google Scholar] [CrossRef]
- Alcolea, A.; Vázquez, M.; Caparrós, A.; Ibarra, I.; García, C.; Linares, R.; Rodríguez, R. Heavy metal removal of intermittent acid mine drainage with an open limestone channel. Miner. Eng. 2012, 26, 86–98. [Google Scholar] [CrossRef]
- Alcolea, A.; Fernández-López, C.; Vázquez, M.; Caparrós, A.; Ibarra, I.; García, C.; Zarroca, M.; Rodríguez, R. An assessment of the influence of sulfidic mine wastes on rainwater quality in a semiarid climate (SE Spain). Atmos. Environ. 2015, 107, 85–94. [Google Scholar] [CrossRef]
- González-Fernández, O.; Jurado-Roldán, A.M.; Queralt, I. Geochemical and mineralogical features of overbank and stream sediments of the Beal wadi (Cartagena-La Union mining district, SE Spain): Relation to former lead–zinc mining activities and its environmental risk. Water Air Soil Pollut. 2010, 215, 55–65. [Google Scholar] [CrossRef]
- García, C. Impacto y Riesgo Ambiental de los Residuos Minero-Metalúrgicos de la Sierra de Cartagena-La Unión (Murcia-España). Ph.D. Thesis, Universidad Politécnica de Cartagena, Murcia, Spain, 2004. [Google Scholar]
- Navarro, M.C.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Prentice Hall: Harlow, UK, 2010; 278p. [Google Scholar]
- Sanchez-Bisquert, D.; Peñas-Castejon, J.M.; García-Fernandez, G. The impact of atmospheric dust deposition and trace elements levels on the villages surrounding the former mining areas in a semi-arid environment (SE Spain). Atmos. Environ. 2017, 152, 256–269. [Google Scholar] [CrossRef]
- Carbone, C.; Dinelli, E.; Marescotti, P.; Gasparotto, G.; Lucchetti, G. The role of AMD secondary minerals in controlling environmental pollution: Indications from bulk leaching tests. J. Geochem. Explor. 2013, 132, 188–200. [Google Scholar] [CrossRef]
- Valente, T.; Grande, J.A.; De La Torre, M.L.; Santisteban, M.; Cerón, J.C. Mineralogy and environmental relevance of AMD-precipitates from the Tharsis mines, Iberian Pyrite Belt (SW, Spain). Appl. Geochem. 2013, 39, 11–25. [Google Scholar] [CrossRef]
- Pérez-Sirvent, C.; Hernández-Pérez, C.; Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Bech, J. Geochemical characterisation of surface waters, topsoils and efflorescences in a historic metal-mining area in Spain. J. Soils Sediments 2016, 16, 1238–1252. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Flohr, M.J.K.; Dillenber, R.G.; Plumlee, G.S. Characterization of secondary minerals formed as the result of weathering of the Anakeesta Formation, Great Smoky Mountains National Park, Tennessee. In U.S. Geological Survey Open-File; U.S. Geological Survey: Reston, VA, USA, 1995; pp. 95–477. [Google Scholar]
- Brunskill, G.J. Mine waste disposal in the ocean: An introduction. Oceanography 2012, 25, 166–169. [Google Scholar] [CrossRef]
- Cesar, A.; Marın, A.; Marin-Guirao, L.M.; Vita, R.; Lloret, J.; Del Valls, T.A. Integrative ecotoxicological assessment of sediment in Portmán Bay (southeast Spain). Ecotoxicol. Environ. Saf. 2009, 72, 1832–1841. [Google Scholar] [CrossRef]
- Dold, B. Submarine tailings disposal (STD)—A review. Minerals 2014, 4, 642–666. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments form Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry. Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; 884p. [Google Scholar] [CrossRef]
- Martinez-Frías, J. Mine waste pollutes Mediterranean. Nature 1997, 388, 120. [Google Scholar] [CrossRef]
- Simoneau, J. Mar Menor: Evolution Sedimentologique et Geochimique Recente du Remplissage. Ph.D. Thesis, Université Paul Sebatier de Tolouse, Toulouse, France, 1973. [Google Scholar]
- Oyarzun, R.; Manteca, J.I.; López-García, J.A.; Carmona, C. An account of the events that led to full bay infilling with sulfide tailings at Portmán (Spain), and the search for “black swans” in a potential land reclamation scenario. Sci. Total Environ. 2013, 454, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Oen, I.S.; Fernandez, J.C.; Manteca, J.I. The Lead-Zinc and associated ores of La Unión, Sierra de Cartagena, Spain. Econ. Geol. 1975, 70, 1259–1278. [Google Scholar] [CrossRef]
- Manteca Martínez, J.I.; Ovejero Zappino, G. Los yacimientos Zn, Pb, Ag-Fe del distrito minero de La Unión-Cartagena, Bética Oriental. In Recursos Minerales de España; CSIC: Madrid, Spain, 1992; pp. 1085–1102. [Google Scholar]
- Doblas, M.; Oyarzun, R. Neogene extensional collapse in the western Mediterranean (Betic-Rif Alpine orogenic belt): Implications for the genesis of the Gibraltar Arc and magmatic activity. Geology 1989, 17, 430–433. [Google Scholar] [CrossRef]
- Platt, J.P.; Vissers, R.L.M. Extensional collapse of thickened continental lithosphere: A working hypothesis for the Alboran Sea and Gibraltar arc. Geology 1989, 17, 540–543. [Google Scholar] [CrossRef]
- Oyarzun, R.; Márquez, A.; Ortega, L.; Lunar, R.; Oyarzun, J. A late Miocene metallogenic province in southeast Spain: Atypical Andean-type processes on a smaller scale. Trans. Inst. Min. Metall. Sect. B Appl. Earth Sci. 1995, 104, 197–202. [Google Scholar]
- López-García, J.A.; Oyarzun, R.; López Andrés, S.; Manteca Martínez, J.I. Scientific, educational, and environmental considerations regarding mine sites and geoheritage: A perspective from SE Spain. Geoheritage 2011, 3, 267–275. [Google Scholar] [CrossRef]
- Gil-Guirado, S.; Pérez-Morales, A. Variabilidad climática y patrones termopluviométricos en Murcia (1863–2017). Técnicas de análisis climático en un contexto de cambio global. Investig. Geográficas (Esp) 2019, 71, 27–54. [Google Scholar] [CrossRef]
- Conesa, H.M.; María-Cervantes, A.; Álvarez-Rogel, J.; González-Alcaraz, M.N. Influence of soil properties on trace element availability and plant accumulation in a Mediterranean salt marsh polluted by mining wastes: Implications for phytomanagement. Sci. Total Environ. 2011, 409, 4470–4479. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tolosa, C.A.; Tack, F.M.G.; Verloo, M.G. Characterization of selected element concentrations and enrichment ratios in background and anthropogenically impacted roadside areas. Arch. Environ. Contam. Toxicol. 2000, 38, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Blaser, P.; Zimmermann, S.; Luster, J.; Shotyk, W. Critical examination of trace element enrichments and depletions in soils: As, Cr, Cu, Ni, Pb, and Zn in Swiss forest soils. Sci. Total Environ. 2000, 249, 257–280. [Google Scholar] [CrossRef] [PubMed]
- DIN 38414-S4; German Standard Methods for the Examination of Water, Wastewater and Sludge; Sludge and Sediments (Group S); Determination of Leachability by Water (S 4). Deutsches Institut fur Normung: Berlin/Heidelberg, Germany, 1984; pp. 464–475.
- Marguí, E.; Hidalgo, M.; Queralt, I.; Rodríguez, R. Métodos de evaluación del riesgo ambiental de los residuos minero-metalúrgicos sólidos. In Los Residuos Minero-Metalúrgicos en el Medio Ambiente; Rodríguez, R., García Cortés, A., Eds.; IGME: Madrid, Spain, 2006; pp. 413–439. [Google Scholar]
- Perera, A.S.R.; Al-Tabbaa, A.; Reid, J.M.; Stegemann, J.A. State of practice report. UK Stabilisation/Solidification Treatment and Remediation. Part IV: Testing and Performance Criteria. In Proceedings of the International Conference on Stabilization/Solidification Treatment and Remediation, London, UK, 12–13 April 2005; pp. 415–435. [Google Scholar]
- Aboubakar, A.; Douaik, A.; Mewouo, Y.C.M.; Madong, R.C.B.A.; Dahchour, A.; El Hajjaji, S. Determination of background values and assessment of pollution and ecological risk of heavy metals in urban agricultural soils of Yaoundé, Cameroon. J. Soils Sediments 2021, 21, 1437–1454. [Google Scholar] [CrossRef]
- Trifi, M.; Gasmi, A.; Carbone, C.; Majzlan, J.; Nasri, N.; Dermech, M.; Charef, A.; Elfil, H. Machine learning-based prediction of toxic metals concentration in an acid mine drainage environment, northern Tunisia. Environ. Sci. Pollut. Res. 2022, 29, 87490–87508. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Ismail, M.; Ahmed, A.N. Identification of air pollution potential sources through principal component analysis (PCA). Int. J. Civ. Eng. Technol. 2018, 9, 1435–1442. [Google Scholar]
- Plumlee, G.S. The Environmental Geology of Mineral Deposit. In The Environmental Geochemistry of Mineral Deposits, Part A. Processes, Techniques, and Health Issues; Plumlee, G.S., Logsdon, M.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 71–116. [Google Scholar]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2012; Volume 22, pp. 11–50. [Google Scholar]
- Martínez-Sánchez, M.J.; Pérez-Sirvent, C. Niveles de Fondo y Niveles Genéricos de Referencia de Metales Pesados en Suelos de la Región de Murcia; Comunidad Autónoma de la Región de Murcia: Murcia, Spain, 2007. [Google Scholar]
- Council of the European Union. European Council Decision of 19 December 2002 on establish criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Off. J. Eur. Commun. 2003, L11, 27–49. Available online: http://europa.eu.int/eur-lex (accessed on 10 January 2023).
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; 561p. [Google Scholar]
- Latimer, W.M. Oxidation States of the Elements and Their Potentials in Aqueous Solution; Prentice-Hall: Hoboken, NJ, USA, 1952; 392p. [Google Scholar]
- European Commission. Critical Raw Materials for the EU; Report of the RMSG Ad-Hoc Working Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2010.
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organisation: Geneva, Switzerland, 2011.
- Li, X.; Feng, L. Multivariate and geostatistical analyses of metals in urban soil of Weinan industrial areas Northwest of China. Atmos. Environ. 2012, 47, 58–65. [Google Scholar] [CrossRef]
- Stefanov, S.H.; Ouchev, A. Gisement Plombo-Zincifère de Sidi Driss; Rapport Géol. Avec Estimation de Réserves, Rapport Interne; Office National des Mines de Tunisie: Tunis, Tunisia, 1972. [Google Scholar]
- Trifi, M.; Dermech, M.; Abdelkrim, C.; Azouzi, R.; Hjiri, B. Extraction procedures of toxic and mobile heavy metal fraction from complex mineralogical tailings affected by acid mine drainage. Arab. J. Geosci. 2018, 11, 328. [Google Scholar] [CrossRef]
- Schaider, L.A.; Senn, D.B.; Brabander, D.J.; McCarthy, K.D.; Shine, J.P. Characterization of zinc, lead and cadmium in mine waste: Implications for transport, exposure, and bioavailability. Environ. Sci. Technol. 2007, 41, 4164–4171. [Google Scholar] [CrossRef]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manage. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Fernandez-Naranjo, F.J.; Arranz-Gonzalez, J.C.; Rodriguez-Gomez, V.; Rodriguez-Pacheco, R.L.; Vadillo, L. Geochemical anomalies for the determination of surface stream sediments pollution: Case of Sierra de Cartagena-La Union mining district, Spain. Environ. Monit. Assess. 2020, 192, 247. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, S.; Vriens, B.; Sorensen, M.; Power, I.M.; Smith, L.; Hallam, S.J.; Mayer, K.U.; Beckie, R.D. Microbial and geochemical controls on waste rock weathering and drainage quality. Sci. Total Environ. 2018, 640, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Anju, M.; Banerjee, D.K. Associations of cadmium, zinc, and lead in soils from a lead and zinc mining area as studied by single and sequential extractions. Environ. Monit. Assess. 2011, 176, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.G.; Faz, A.; Martinez-Martinez, S.; Gabarrón, M.; ·Beltrá, J.C.; Martínez, J.; Acosta, J.A. Spatial distribution and pollution evaluation in dry riverbeds affected by mine tailings. Environ. Geochem. Health 2023, 45, 1–17. [Google Scholar] [CrossRef]
- Lazo, P.; Cullaj, A.; Deda, T. Arsenic in soil environment in Albania. In Arsenic in Soils and Groundwater Environment: Trace Metals and Other Contaminants in the Environment; Battacharia, P., Mukherjee, A.B., Bundschuh, J., Zevenhoven, J.R., Loeppert, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 9, pp. 237–256. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. Rev. Environ. Contam. 2009, 197, 17–60. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Andrade, M.L. Competitive sorption and desorption of heavy metals in mine soils: Influence of mine soil characteristics. J. Colloid Interface Sci. 2006, 298, 582–592. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, P.; Zhang, P. The Chemical Oxidation and Immobilization of Arsenic and Antimony in Simulated AMD in Karst Areas. Bull. Environ. Contam. Toxicol. 2022, 108, 541–548. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).