Carbon Nitrogen Isotope Coupling of Soils and Seasonal Variation Characteristics in a Small Karst Watershed in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Collection and Processing
2.3. Statistical Analysis
3. Results
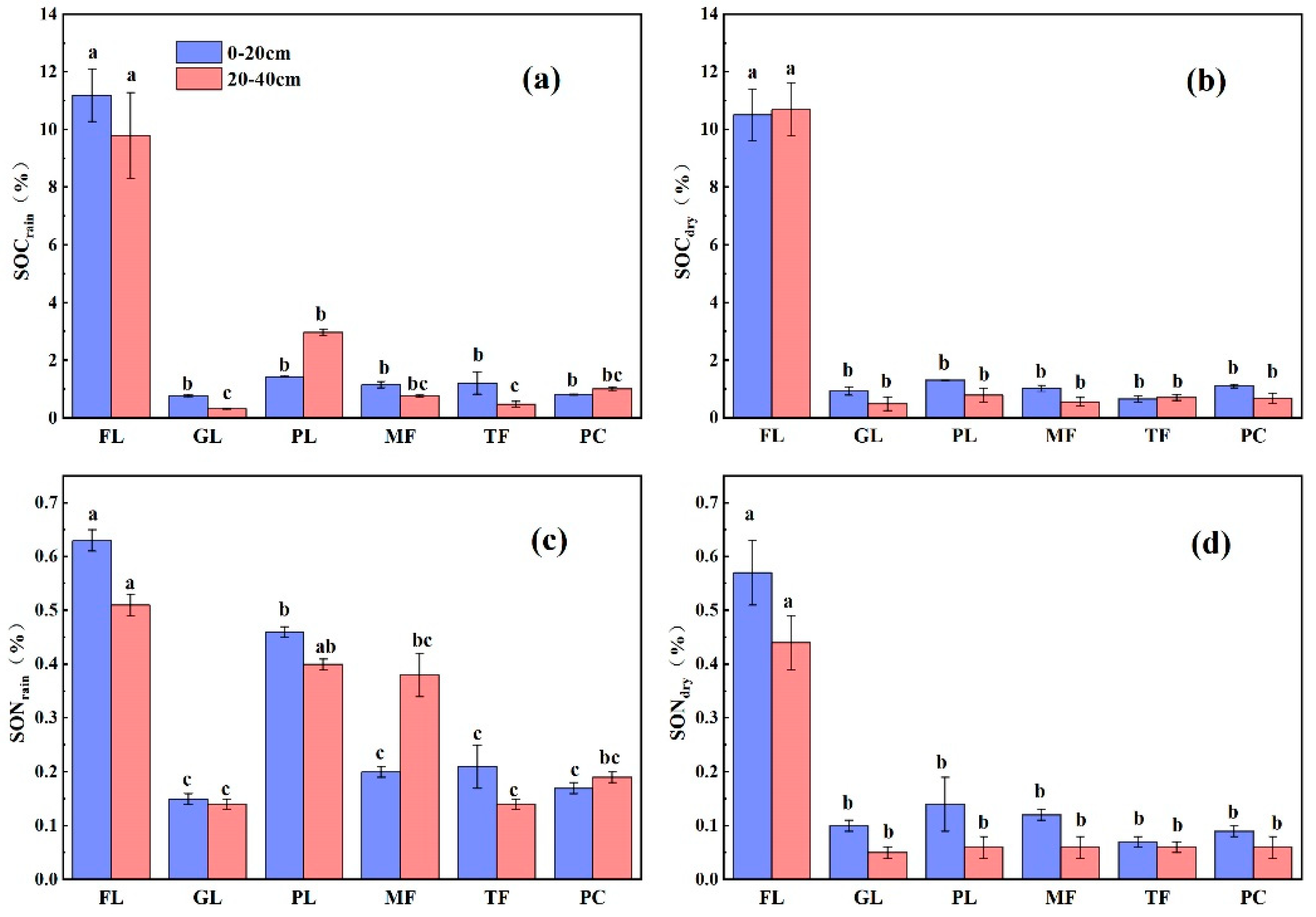
3.1. Seasonal Variation of SOC, SON, and C/N in the Soil Profiles of the Different Land Types
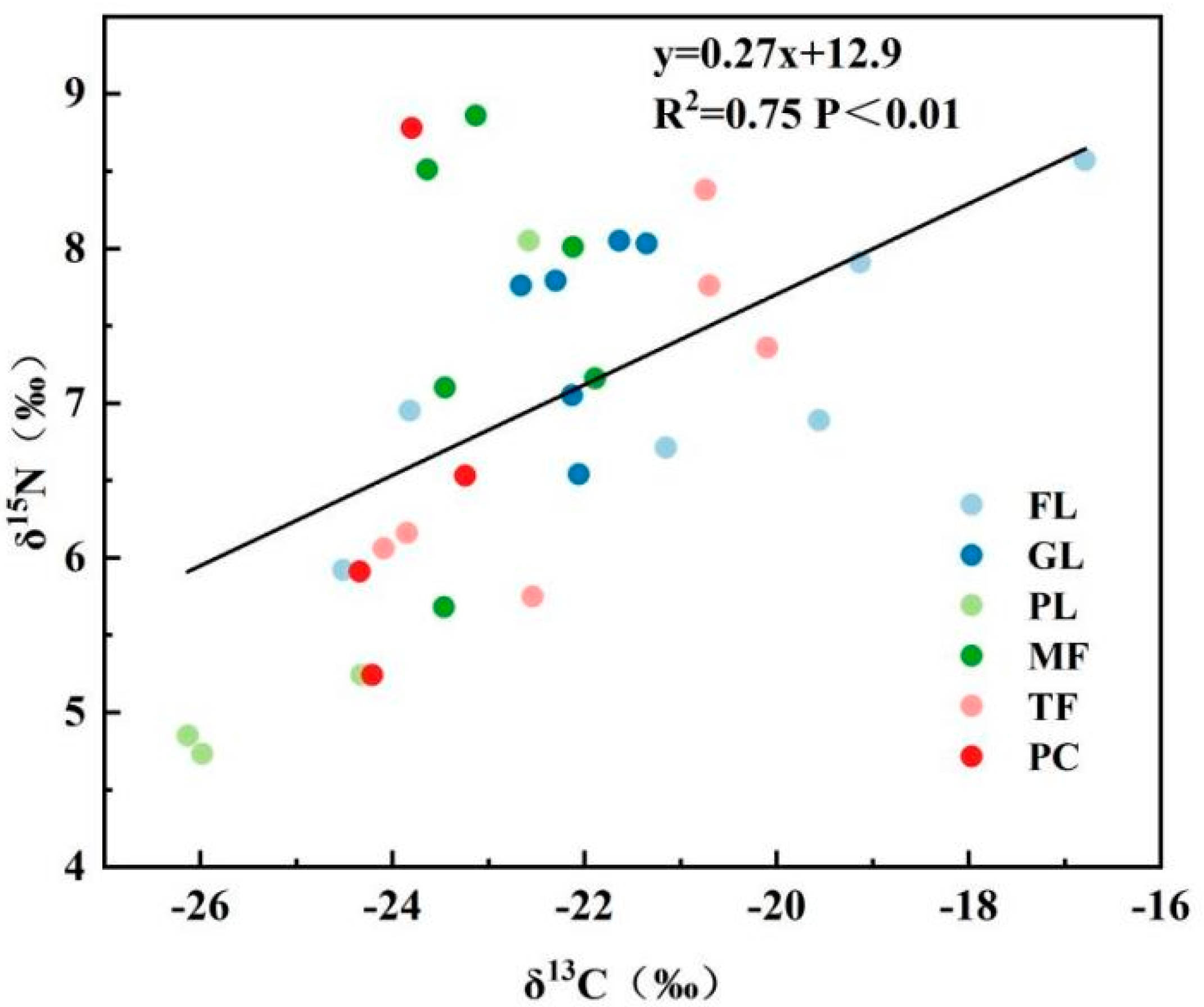
3.2. Seasonal Distribution Characteristics of Stable Isotopes δ13C and δ15N in the SOM of Different Land Types
4. Discussion
4.1. Effect of Land Use Type on SOC and SON
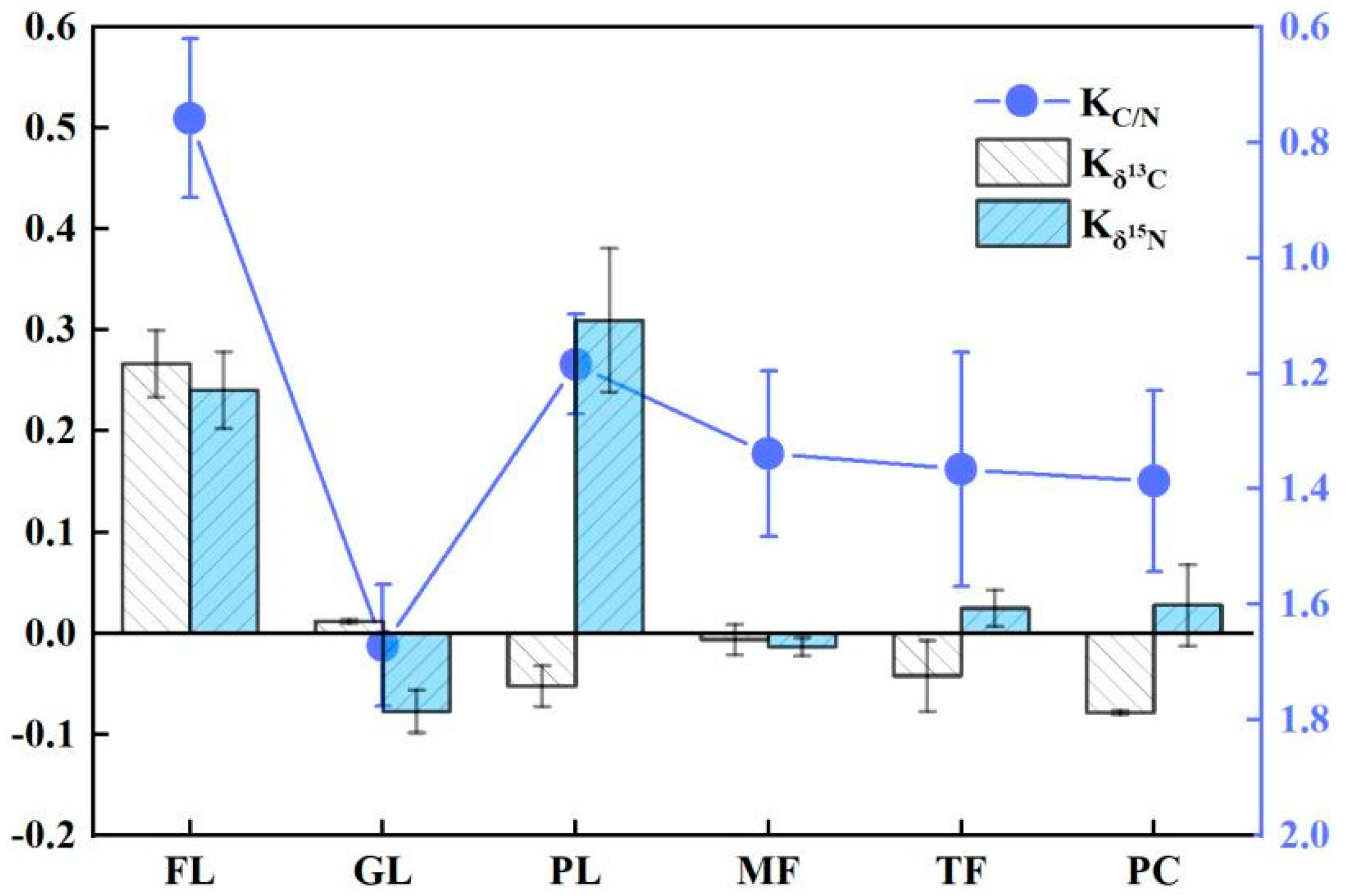
4.2. Intrinsic Correlation of Soil Ecological Stoichiometry (SOC, SON, C\N and pH) with δ13C and δ15N
4.3. Seasonal Characteristics of C and N Isotope Fractionation Coupling in Soil Organic Matter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, D.; Williams, P.D. Karst Hydrogeology and Geomorphology; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 126–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; Pugh, T.A. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sardans, J.; Zeng, C.; Zhong, C.; Li, Y.; Peñuelas, J. Responses of soil nutrient concentrations and stoichiometry to different human land uses in a subtropical tidal wetland. Geoderma 2014, 232, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Ming-Hua, S.; Shao-Qiang, W. Patterns of soil 15N and total N and their relationships with environmental factors on the Qinghai-Tibetan Plateau. Pedosphere 2014, 24, 232–242. [Google Scholar]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ni, Z.; Diao, H.; Jiang, K.; Hu, C.; Shao, L.; Huang, W. Root Endophytic Fungal Community and Carbon and Nitrogen Stable Isotope Patterns Differ among Bletilla Species (Orchidaceae). J. Fungi 2021, 7, 69. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, Y.; Shen, C.; Peng, S.; Yi, W.; Jiang, M. Organic matter turnover rates and CO2 flux from organic matter decomposition of mountain soil profiles in the subtropical area, south China. Catena 2002, 49, 217–229. [Google Scholar] [CrossRef]
- Liu, W.; Wei, J.; Cheng, J.; Li, W. Profile distribution of soil inorganic carbon along a chronosequence of grassland restoration on a 22-year scale in the Chinese Loess Plateau. Catena 2014, 121, 321–329. [Google Scholar] [CrossRef]
- Piao, S.; Huang, M.; Liu, Z.; Wang, X. Lower land-use emissions responsible for increased net land carbon sink during the slow warming period. Nat. Geosci. 2018, 11, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Sasmito, S.D.; Kuzyakov, Y.; Lubis, A.A. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Tian, H.; Shen, X.; Qiu, L. Responses of soil organic carbon and nitrogen to land-use changes in a semiarid region of northwest China. Arid Land Res. Manag. 2020, 34, 188–206. [Google Scholar] [CrossRef]
- Li, B.B.; Li, P.P.; Yang, X.M. Land-use conversion changes deep soil organic carbon stock in the Chinese Loess Plateau. Land Degrad. Dev. 2021, 32, 505–517. [Google Scholar] [CrossRef]
- Smal, H.; Ligęza, S.; Pranagal, J.; Urban, D.; Pietruczyk-Popławska, D. Changes in the stocks of soil organic carbon, total nitrogen and phosphorus following afforestation of post-arable soils: A chronosequence study. For. Ecol. Manag. 2019, 451, 117536. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Gao, Y.; He, N.; Yu, G.; Chen, W.; Wang, Q. Long-term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: A case study in China. Ecol. Eng. 2014, 67, 171–181. [Google Scholar] [CrossRef]
- Tesfaye, M.A.; Bravo, F.; Ruiz-Peinado, R.; Pando, V.; Bravo-Oviedo, A. Impact of changes in land use, species and elevation on soil organic carbon and total nitrogen in Ethiopian Central Highlands. Geoderma 2016, 261, 70–79. [Google Scholar] [CrossRef]
- Rustad, L.; Campbell, J.; Marion, G. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Buchmann, N.; Flanagan, L.B. Carbon isotope ratios in belowground carbon cycle processes. Ecol. Appl. 2000, 10, 412–422. [Google Scholar] [CrossRef]
- Campbell, J.E.; Fox, J.F.; Davis, C.M. Carbon and nitrogen isotopic measurements from southern Appalachian soils: Assessing soil carbon sequestration under climate and land-use variation. J. Environ. Eng. 2009, 135, 439–448. [Google Scholar] [CrossRef]
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef]
- Peri, P.; Ladd, B.; Pepper, D.A. Carbon (δ13C) and nitrogen (δ15N) stable isotope composition in plant and soil in S outhern P atagonia’s native forests. Glob. Chang. Biol. 2012, 18, 311–321. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H. Effect of alternate aerobic and anaerobic conditions on redox potential, organic matter decomposition and nitrogen loss in a flooded soil. Soil Biol. Biochem. 1975, 7, 87–94. [Google Scholar] [CrossRef]
- Fissore, C.; Dalzell, B.J.; Berhe, A.A.; Voegtle, M.; Evans, M.; Wu, A. Influence of topography on soil organic carbon dynamics in a Southern California grassland. Catena 2017, 149, 140–149. [Google Scholar] [CrossRef]
- Krull, E.S.; Skjemstad, J.O. δ13C and δ15N profiles in 14C-dated Oxisol and Vertisols as a function of soil chemistry and mineralogy. Geoderma 2003, 112, 1–29. [Google Scholar] [CrossRef]
- Choi, W.J.; Kwak, J.H.; Lim, S.S. Synthetic fertilizer and livestock manure differently affect δ15N in the agricultural landscape: A review. Agric. Ecosyst. Environ. 2017, 237, 1–15. [Google Scholar] [CrossRef]
- Wang, K.L.; Chen, H.S.; Yue, Y.M. Experiment and demonstration on degraded mechanism and its adaptive restoration of karst ecosystems in Northwest Guangxi. Sci. Technol. Dev. 2015, 11, 179–183. [Google Scholar]
- Xiong, K.; Li, J.; Long, M. Characteristics and key problems of soil erosion in typical karst rocky desertification control area. Acta Geogr. Sin. 2012, 67, 878–888. (In Chinese) [Google Scholar]
- Xiong, K.; Chen, Q. Discussion on karst rocky desert evolution trend based on ecologically comprehensive treatment. Caisologica Sin. 2010, 29, 50–56. (In Chinese) [Google Scholar]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Wofsy, S.C. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Ding, W.; Cai, Z. Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol. Biochem. 2005, 37, 2037–2045. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Liu, M.; Van Zwieten, L.; Yang, X.; Yu, C.; Song, Z. Carbon-nitrogen isotope coupling of soil organic matter in a karst region under land use change, Southwest China. Agric. Ecosyst. Environ. 2020, 301, 107027. [Google Scholar] [CrossRef]
- Aryal, D.R.; Morales Ruiz, D.E.; Tondopó Marroquín, C.N. Soil organic carbon depletion from forests to grasslands conversion in Mexico: A Review. Agriculture 2018, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Li, F.; Tang, Y. Variations in soil organic carbon contents and isotopic compositions under different land uses in a typical karst area in Southwest China. Geochem. J. 2015, 49, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Zhu, W.; Zhou, Y.; Yin, Y. Soil organic carbon chemical functional groups under different revegetation types are coupled with changes in the microbial community composition and the functional genes. Forests 2019, 10, 240. [Google Scholar] [CrossRef] [Green Version]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Zhang, Q. Effects of soil aggregate stability on soil organic carbon and nitrogen under land use change in an erodible region in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3809. [Google Scholar] [CrossRef] [Green Version]
- Schulp, C.J.; Nabuurs, G.J.; Verburg, P.H. Future carbon sequestration in Europe—Effects of land use change. Agric. Ecosyst. Environ. 2008, 127, 251–264. [Google Scholar] [CrossRef]
- Fang, X.; Xue, Z.; Li, B.; An, S. Soil organic carbon distribution in relation to land use and its storage in a small watershed of the Loess Plateau, China. Catena 2012, 88, 6–13. [Google Scholar] [CrossRef]
- Chaopricha, N.T.; Marín-Spiotta, E. Soil burial contributes to deep soil organic carbon storage. Soil Biol. Biochem. 2014, 69, 251–264. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, X.; Ma, J.; Ye, J.; Sun, W.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Liu, L.B.; Zhong, Q.L.; Ni, J. Ecosystem C: N: P stoichiometry and storages of a secondary plateau-surface karst forest in Guizhou Province, southwestern China. Acta Ecol. Sin. 2019, 39, 8606–8614. [Google Scholar]
- Collins, J.G.; Dijkstra, P.; Hart, S.C.; Hungate, B.A.; Flood, N.M.; Schwartz, E. Nitrogen source influences natural abundance 15N of Escherichia coli. FEMS Microbiol. Lett. 2008, 282, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Stevenson, B.A.; Parfitt, R.L.; Schipper, L.A.; Baisden, W.T.; Mudge, P. Relationship between soil δ15N, C/N and N losses across land uses in New Zealand. Agric. Ecosyst. Environ. 2010, 139, 736–741. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Song, Y.; Li, Y. Carbon and Nitrogen Stable Isotope Abundance and Soil Stoichiometry of Zanthoxylum planispinum var. dintanensis Plantations of Different Ages. Agronomy 2022, 12, 1248. [Google Scholar]
- Drollinger, S.; Kuzyakov, Y.; Glatzel, S. Effects of peat decomposition on δ13C and δ15N depth profiles of Alpine bogs. Catena 2019, 178, 1–10. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Wang, Y.; Wang, W.; Fu, X.; Singh, B.P.; Wang, H. Soil organic matter turnover depending on land use change: Coupling C/N ratios, δ13C, and lignin biomarkers. Land Degrad. Dev. 2021, 32, 1591–1605. [Google Scholar] [CrossRef]
- Wynn, J.G. Carbon isotope fractionation during decomposition of organic matter in soils and paleosols: Implications for paleoecological interpretations of paleosols. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 251, 437–448. [Google Scholar] [CrossRef]
- Lerch, T.Z.; Nunan, N.; Dignac, M.F. Variations in microbial isotopic fractionation during soil organic matter decomposition. Biogeochemistry 2011, 106, 5–21. [Google Scholar] [CrossRef]
- Wang, G.; Jia, Y.; Li, W. Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci. Rep. 2015, 5, 11043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krull, E.S.; Bestland, E.A.; Skjemstad, J.O.; Parr, J.F. Geochemistry (δ13C, δ15N, 13C NMR) and residence times (14C and OSL) of soil organic matter from red-brown earths of South Australia: Implications for soil genesis. Geoderma 2006, 132, 344–360. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, C.; Sun, Y.; Peng, S.; Yi, W.; Li, Z.A.; Jiang, M. Spatial and temporal distribution of carbon isotopes in soil organic matter at the Dinghushan Biosphere Reserve, South China. Plant Soil 2005, 273, 115–128. [Google Scholar] [CrossRef]
- Högberg, P.; Ekblad, A. Substrate-induced respiration measured in situ in a C3-plant ecosystem using additions of C4-sucrose. Soil Biol. Biochem. 1996, 28, 1131–1138. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Buchmann, N.; Kao, W.Y.; Ehleringer, J. Influence of stand structure on carbon-13 of vegetation, soils, and canopy air within deciduous and evergreen forests in Utah, United States. Oecologia 1997, 110, 109–119. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Zhang, P.; Yu, Y.; Ding, F. A synthesis of change in deep soil organic carbon stores with afforestation of agricultural soils. For. Ecol. Manag. 2013, 296, 53–63. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Fu, X.; Horton, R.; Li, Y.; Zhang, X. Distribution of soil organic C, N and P in three adjacent land use patterns in the northern Loess Plateau, China. Biogeochemistry 2009, 96, 149–162. [Google Scholar] [CrossRef]

| Sampling ID | Sampling Sites | Altitude | Soil Texture | Land-Use Type | Vegetation | Disturbance History | ||
|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | ||||||
| FL1 | 27°09′25″ N, 108°08′40″ E | 1063 | 52.92 | 33.66 | 13.42 | Forest | Pinus massoniana | More than 100 years without disturbance. |
| FL2 | 27°09′44″ N, 108°07′43″ E | 1064 | 50.75 | 33.75 | 15.50 | Forest | Cunninghamia lanceolata, Podocarpus macrophyllus | Same as above |
| FL2 | 27°08′54″ N, 108°07′33″ E | 1058 | 51.56 | 34.08 | 14.36 | Forest | Cinnamomum camphora (L.), Cyclobalanopsis glauca | Same as above |
| PC1 | 27°08′49″ N, 108°07′28″ E | 998 | 21.22 | 40.39 | 38.39 | Peach orchard | Peach tree | The planting period is 7–8 years, the land was abandoned before planting. |
| PC2 | 27°09′37″ N, 108°07′40″ E | 982 | 26.15 | 39.28 | 34.57 | Peach orchard | Peach tree | Same as above |
| PC3 | 27°09′57″ N, 108°07′32″ E | 976 | 25.72 | 35.32 | 38.96 | Peach orchard | Peach tree | The planting period is 10 years, the land was abandoned before planting. |
| PL1 | 27°08′53″ N, 108°07′45″ E | 984 | 18.37 | 38.08 | 43.55 | Paddy land | Oryza sativa L. | Rice is planted in the rainy season, drained and fallow in the dry season, the length of continuous planting is about 30 years. |
| PL2 | 27°09′10″ N, 108°07′55″ E | 981 | 19.25 | 39.93 | 40.82 | Paddy land | Oryza sativa L. | Same as above |
| PL3 | 27°06′52″ N, 108°07′47″ E | 985 | 19.30 | 38.56 | 42.14 | Paddy land | Oryza sativa L. | Same as above |
| TF1 | 27°08′55″ N, 108°07′28″ E | 987 | 26.95 | 37.96 | 35.09 | Tobacco field | Nicotiana tabacun L. | Corn and roasted tobacco are planted in rotation for more than a decade, urea and compound fertilizer are mainly applied in the cultivation process. |
| TF2 | 27°08′54″ N, 108°07′27″ E | 984 | 27.13 | 38.53 | 34.34 | Tobacco field | Nicotiana tabacun L. | Same as above |
| TF3 | 27°08′48″ N, 108°07′26″ E | 981 | 27.44 | 37.24 | 35.32 | Tobacco field | Nicotiana tabacun L. | Same as above |
| MF1 | 27°08′53″ N, 108°07′45″ E | 997 | 27.85 | 37.58 | 34.57 | Maize field | Zea mays L. | Same as above |
| MF2 | 27°09′34″ N, 108°07′43″ E | 991 | 26.92 | 38.71 | 34.37 | Maize field | Zea mays L. | Same as above |
| MF3 | 27°08′54″ N, 108°07′35″ E | 989 | 28.31 | 38.06 | 33.63 | Maize field | Zea mays L. | Same as above |
| GL1 | 27°08′53″ N, 108°07′28″ E | 1018 | 27.14 | 37.65 | 35.21 | Grass land | Weeds (Mainly C4 plant) | Natural recovery of corn land for 4–5 years after abandonment. |
| GL2 | 27°08′11″ N, 108°07′33″ E | 1010 | 27.04 | 37.12 | 35.84 | Grass land | Weeds | Same as above |
| GL3 | 27°09′51″ N, 108°07′47″ E | 1008 | 27.11 | 38.43 | 34.46 | Grass land | Weeds | Natural recovery of corn land for 5 years after abandonment. |
| Index | SOCrain | SOCdry | SONrain | SONdry | δ13Crain | δ13Cdry | δ15Nrain | δ15Ndry | C/Nrain | C/Ndry | pHrain |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOCdry | 0.719 ** | 1 | |||||||||
| SONrain | 0.769 ** | 0.640 ** | 1 | ||||||||
| SONdry | 0.739 ** | 0.903 ** | 0.623 ** | 1 | |||||||
| δ13Crain | 0.626 ** | 0.436 * | 0.306 | 0.361 * | 1 | ||||||
| δ13Cdry | 0.354 * | 0.576 ** | 0.113 | 0.558 ** | 0.349 | 1 | |||||
| δ15Nrain | −0.337 | −0.271 | −0.496 ** | −0.316 | 0.087 | 0.021 | 1 | ||||
| δ15Ndry | 0.034 | 0.083 | −0.036 | 0.084 | 0.179 | 0.597 ** | 0.165 | 1 | |||
| C/Nrain | 0.972 ** | 0.657 ** | 0.648 ** | 0.684 ** | 0.668 ** | 0.338 | −0.337 | 0.014 | 1 | ||
| C/Ndry | 0.623 ** | 0.845 ** | 0.575 ** | 0.573 ** | 0.469 ** | 0.34 | −0.242 | 0.035 | 0.578 ** | 1 | |
| pHrain | −0.361 * | −0.312 | −0.388 * | −0.328 | −0.047 | 0.129 | 0.175 | 0.274 | −0.34 | −0.244 | 1 |
| pHdry | 0.517 ** | 0.439 * | 0.421 * | 0.364 * | 0.209 | −0.021 | −0.232 | −0.106 | 0.480 ** | 0.537 ** | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Z.; Xiong, K.; Li, Y.; Lyu, X.; Cai, L. Carbon Nitrogen Isotope Coupling of Soils and Seasonal Variation Characteristics in a Small Karst Watershed in Southern China. Land 2023, 12, 501. https://doi.org/10.3390/land12020501
Liu Y, Liu Z, Xiong K, Li Y, Lyu X, Cai L. Carbon Nitrogen Isotope Coupling of Soils and Seasonal Variation Characteristics in a Small Karst Watershed in Southern China. Land. 2023; 12(2):501. https://doi.org/10.3390/land12020501
Chicago/Turabian StyleLiu, Ya, Ziqi Liu, Kangning Xiong, Yuan Li, Xiaoxi Lyu, and Lulu Cai. 2023. "Carbon Nitrogen Isotope Coupling of Soils and Seasonal Variation Characteristics in a Small Karst Watershed in Southern China" Land 12, no. 2: 501. https://doi.org/10.3390/land12020501
APA StyleLiu, Y., Liu, Z., Xiong, K., Li, Y., Lyu, X., & Cai, L. (2023). Carbon Nitrogen Isotope Coupling of Soils and Seasonal Variation Characteristics in a Small Karst Watershed in Southern China. Land, 12(2), 501. https://doi.org/10.3390/land12020501






