Shorebird Monitoring Using Spatially Explicit Occupancy and Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Data Collection
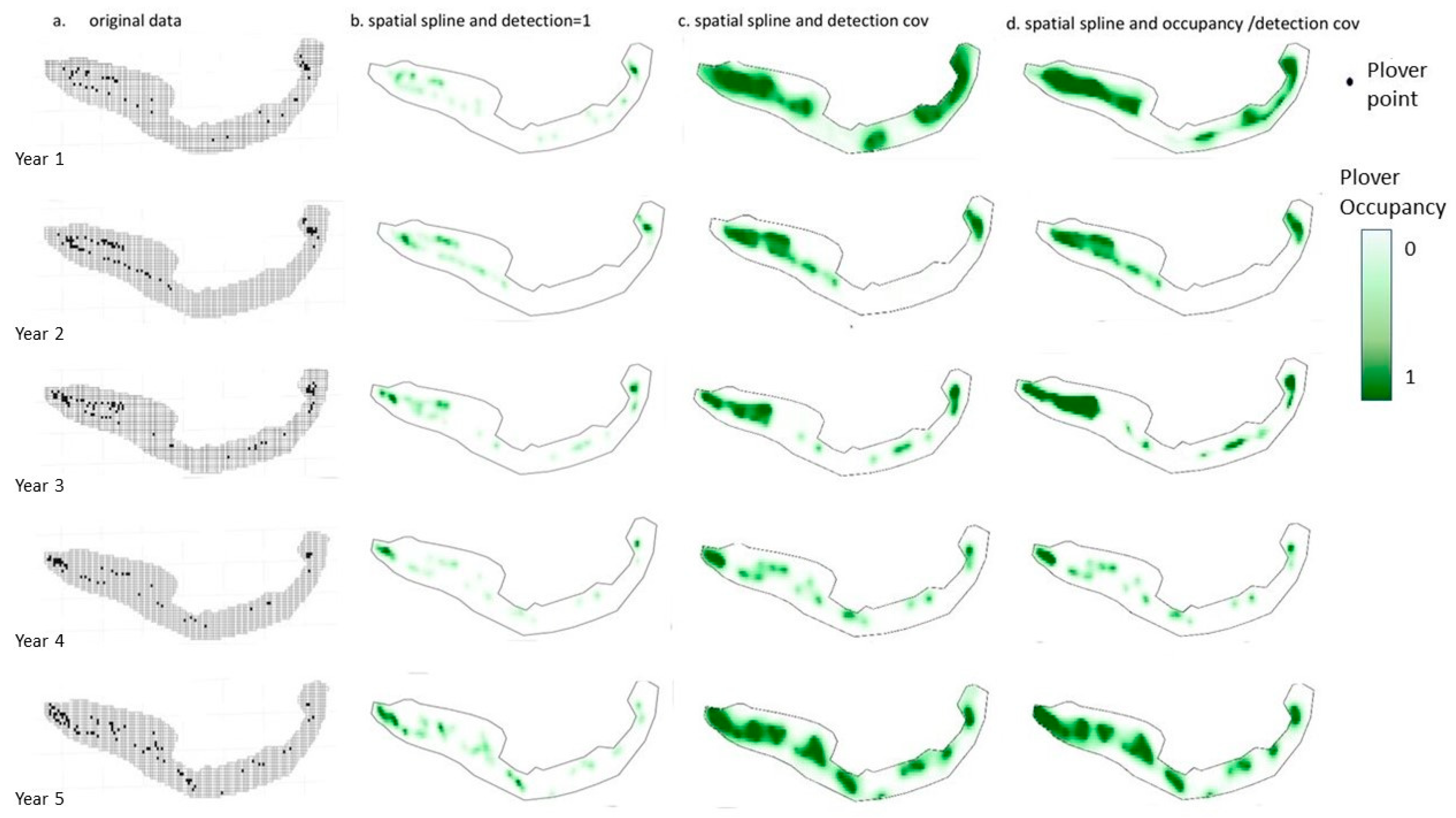
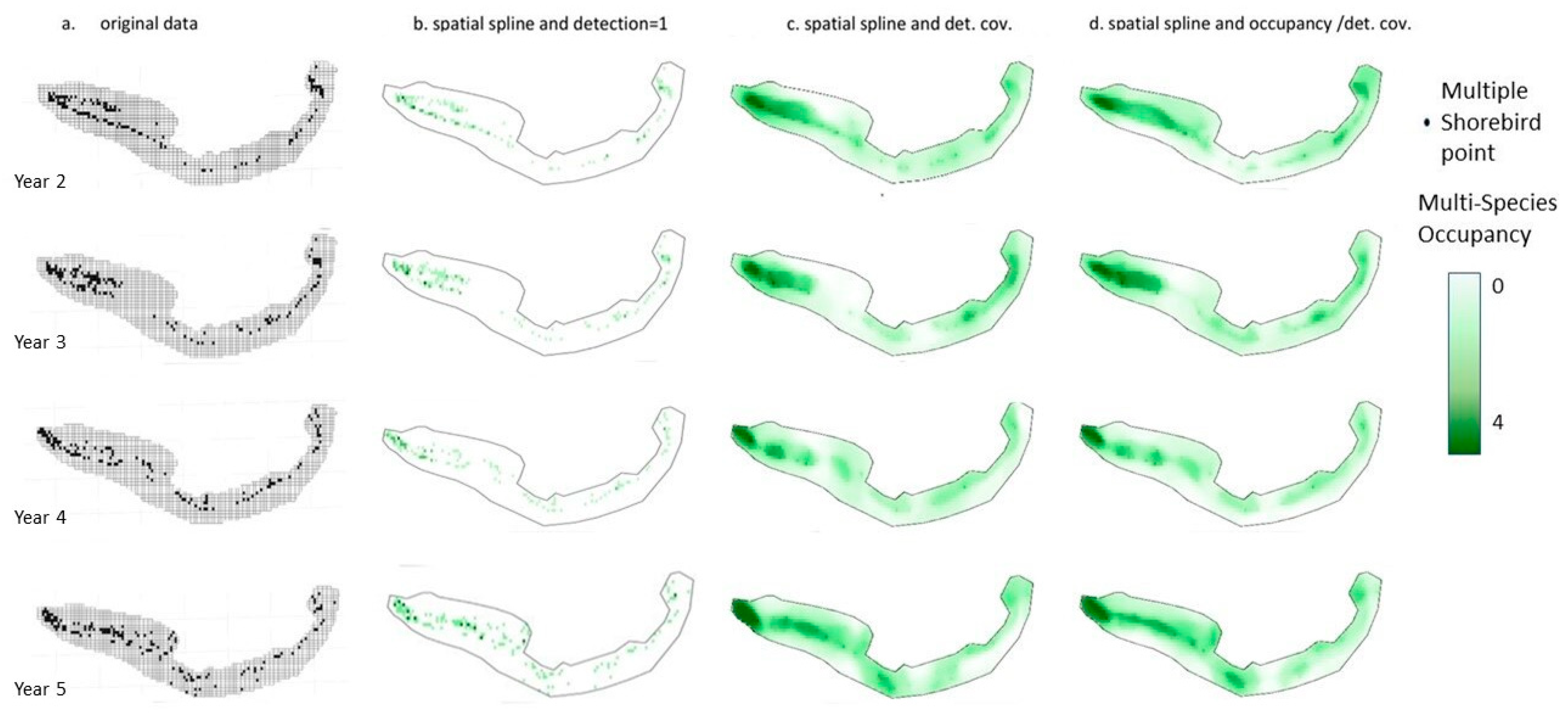
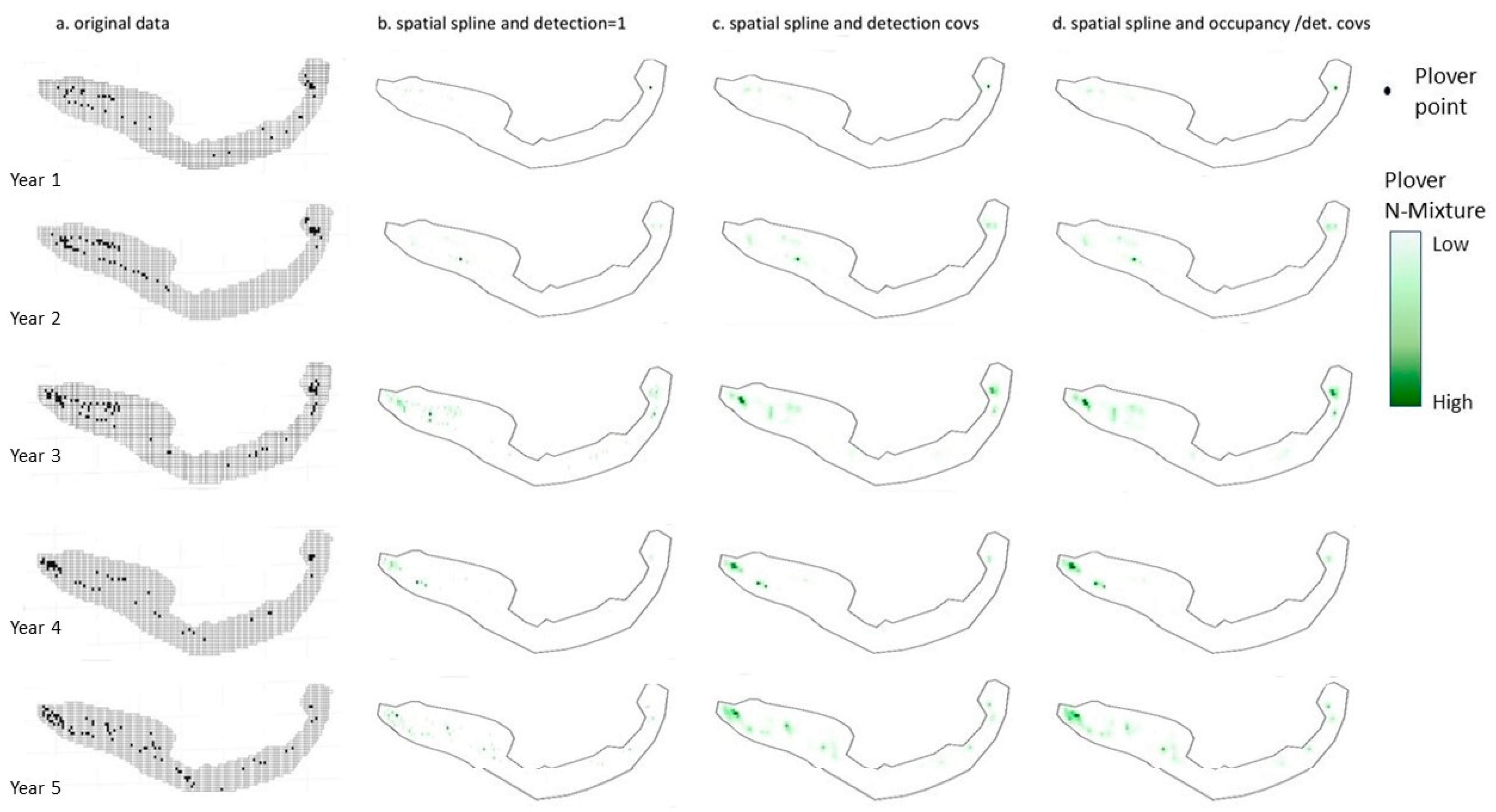
2.4. Data Analysis
2.5. Model Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, N.J.; Marra, P.P.; Fuller, R.A.; Clemens, R.S.; Dhanjal-Adams, K.; Gosbell, K.B.; Hassell, C.J.; Iwamura, T.; Melville, D.; Minton, C.D.T.; et al. The Large-Scale Drivers of Population Declines in a Long-Distance Migratory Shorebird. Ecography 2018, 41, 867–876. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, W.J.; Alves, J.A.; Amano, T.; Chang, C.H.; Davidson, N.C.; Max Finlayson, C.; Gill, J.A.; Gill, R.E.; González, P.M.; Gunnarsson, T.G.; et al. A Horizon Scanning Assessment of Current and Potential Future Threats to Migratory Shorebirds. Ibis 2012, 154, 663–679. [Google Scholar] [CrossRef]
- Zurell, D.; Graham, C.H.; Gallien, L.; Thuiller, W.; Zimmermann, N.E. Long-Distance Migratory Birds Threatened by Multiple Independent Risks from Global Change. Nat. Clim. Chang. 2018, 8, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Soanes, L.M.; Bright, J.A.; Angel, L.P.; Arnould, J.P.Y.; Bolton, M.; Berlincourt, M.; Lascelles, B.; Owen, E.; Simon-Bouhet, B.; Green, J.A. Defining Marine Important Bird Areas: Testing the Foraging Radius Approach. Biol. Conserv. 2016, 196, 69–79. [Google Scholar] [CrossRef]
- Maslo, B.; Leu, K.; Faillace, C.; Weston, M.A.; Pover, T.; Schlacher, T.A. Selecting Umbrella Species for Conservation: A Test of Habitat Models and Niche Overlap for Beach-Nesting Birds. Biol. Conserv. 2016, 203, 233–242. [Google Scholar] [CrossRef]
- Maslo, B.; Zeigler, S.L.; Drake, E.C.; Pover, T.; Plant, N.G. A Pragmatic Approach for Comparing Species Distribution Models to Increasing Confidence in Managing Piping Plover Habitat. Conserv. Sci. Pract. 2020, 2, e150. [Google Scholar] [CrossRef] [Green Version]
- Chelgren, N.D.; Adams, M.J.; Bailey, L.L.; Bury, R.B. Using Multilevel Spatial Models to Understand Salamander Site Occupancy Patterns after Wildfire. Ecology 2011, 92, 408–421. [Google Scholar] [CrossRef]
- Guélat, J.; Kéry, M. Effects of Spatial Autocorrelation and Imperfect Detection on Species Distribution Models. Methods Ecol. Evol. 2018, 9, 1614–1625. [Google Scholar] [CrossRef]
- Poley, L.G.; Pond, B.A.; Schaefer, J.A.; Brown, G.S.; Ray, J.C.; Johnson, D.S. Occupancy Patterns of Large Mammals in the Far North of Ontario under Imperfect Detection and Spatial Autocorrelation. J. Biogeogr. 2014, 41, 122–132. [Google Scholar] [CrossRef]
- Legendre, P. Spatial Autocorrelation: Trouble or New Paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Legendre, P.; Fortin, M.J. Spatial Pattern and Ecological Analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to Account for Spatial Autocorrelation in the Analysis of Species Distributional Data: A Review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef] [Green Version]
- Besag, J. Spatial Interaction and the Statistical Analysis of Lattice Systems. J. R. Stat. Soc. Ser. B (Methodol.) 1974, 36, 192–225. [Google Scholar] [CrossRef]
- Besag, J.E. Nearest-Neighbour Systems and the Auto-Logistic Model for Binary Data. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 75–83. [Google Scholar] [CrossRef]
- Hefley, T.J.; Broms, K.M.; Brost, B.M.; Buderman, F.E.; Kay, S.L.; Scharf, H.R.; Tipton, J.R.; Williams, P.J.; Hooten, M.B. The Basis Function Approach for Modeling Autocorrelation in Ecological Data. Ecology 2017, 98, 632–646. [Google Scholar] [CrossRef] [Green Version]
- Hoeting, J.A.; Leecaster, M.; Bowden, D. An Improved Model for Spatially Correlated Binary Responses. J. Agric. Biol. Environ. Stat. 2000, 5, 102. [Google Scholar] [CrossRef]
- Wintle, B.A.; Bardos, D.C. Modeling Species-Habitat Relationships with Spatially Autocorrelated Observation Data. Ecol. Appl. 2006, 16, 1945–1958. [Google Scholar] [CrossRef]
- Bardos, D.C.; Guillera-Arroita, G.; Wintle, B.A. Valid Auto-models for Spatially Autocorrelated Occupancy and Abundance Data. Methods Ecol. Evol. 2015, 6, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Webb, M.H.; Wotherspoon, S.; Stojanovic, D.; Heinsohn, R.; Cunningham, R.; Bell, P.; Terauds, A. Location Matters: Using Spatially Explicit Occupancy Models to Predict the Distribution of the Highly Mobile, Endangered Swift Parrot. Biol. Conserv. 2014, 176, 99–108. [Google Scholar] [CrossRef]
- Burton, A.C.; Sam, M.K.; Balangtaa, C.; Brashares, J.S. Hierarchical Multi-Species Modeling of Carnivore Responses to Hunting, Habitat and Prey in a West African Protected Area. PLoS ONE 2012, 7, e38007. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, B.J.; Zipkin, E.F.; Gardner, B.; Blank, P.J.; Sauer, J.R.; Royle, J.A. Explaining Local-Scale Species Distributions: Relative Contributions of Spatial Autocorrelation and Landscape Heterogeneity for an Avian Assemblage. PLoS ONE 2013, 8, e55097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, C.; Johnson, D.S.; Dunk, J.R.; Zielinski, W.J. Hierarchical Bayesian Spatial Models for Multispecies Conservation Planning and Monitoring: Hierarchical Spatial Habitat Models. Conserv. Biol. 2010, 24, 1538–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. (Eds.) An Introduction to Statistical Learning: With Applications in R; Springer Texts in Statistics; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7137-0. [Google Scholar]
- Kery, M.; Royle, J.A. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS: Volume 2: Dynamic and Advanced Models; Elsevier Science: Amsterdam, The Netherlands, 2020; Volume 2. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 2017; ISBN 978-1-315-37027-9. [Google Scholar]
- Crainiceanu, C.M.; Goldsmith, A.J. Bayesian Functional Data Analysis Using WinBUGS. J. Stat. Soft. 2010, 32, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, O.; Crainiceanu, C.; Barbraud, C.; Jenouvrier, S.; Morgan, B.J.T. Semiparametric Regression in Capture-Recapture Modeling. Biometrics 2006, 62, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Collier, B.A.; Groce, J.E.; Morrison, M.L.; Newnam, J.C.; Campomizzi, A.J.; Farrell, S.L.; Mathewson, H.A.; Snelgrove, R.T.; Carroll, R.J.; Wilkins, R.N. Predicting Patch Occupancy in Fragmented Landscapes at the Rangewide Scale for an Endangered Species: An Example of an American Warbler: Occupancy Distribution of an American Warbler. Divers. Distrib. 2012, 18, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Kindinger, J.; Buster, N.; Flocks, J.; Bernier, J.; Kulp, M. Louisiana Barrier Island Comprehensive Monitoring (BICM) Program Summary Report: Data and Analyses 2006 through 2010; U.S. Department of the Interior U.S. Geological Survey: Tallahassee, FL, USA, 2013.
- Enwright, N.; SooHoo, W.; Dugas, J.; Conzelmann, C.; Laurenzano, C.; Lee, D.; Mouton, K.; Stelly, S. Louisiana Barrier Island Comprehensive Monitoring Program: Mapping Habitats in Beach, Dune, and Intertidal Environments Along the Louisiana Gulf of Mexico Shoreline, 2008 and 2015–16; U.S. Department of the Interior U.S. Geological Survey: Tallahassee, FL, USA, 2020.
- Coastal Engineering Consultants. Louisiana Barrier Islands Restoration; Coastal Engineering Consultants: Denham Springs, LA, USA, 2017. [Google Scholar]
- Berg, R. Tropical Storm Bill; National Hurricane Center: Miami, FL, USA, 2015.
- Berg, R. Tropical Storm Cindy; National Hurricane Center: Miami, FL, USA, 2018.
- Zenzal, T.J.; Anderson, A.N.; Geary, B.; Schulz, J.L.; Dobbs, R.C.; Barrow, W.C.; Waddle, J.H. Early Season Tropical Storms Impact Birds Breeding on a Barrier Island; U.S. Geological Survey: Tallahassee, FL, USA, 2023. Unpublished (In Press)
- Zenzal, T.J.; Anderson, A.N.; Geary, B.J.; Schulz, J.; Dobbs, R.; Barrow, W.C.; Waddle, H. Immediate Impacts of Tropical Storms on the Avian Breeding Community at Whiskey Island, Louisiana from 2015–2020; U.S. Geological Survey: Tallahassee, FL, USA, 2022.
- Haig, S.M.; Ferland, C.L. A Complete Species Census and Evidence for Regional Declines in Piping Plovers. J. Wildl. Manag. 2005, 69, 160–173. [Google Scholar] [CrossRef]
- USFWS Endangered and Threatened Wildlife and Plants; Final Determinations of Critical Habitat for Wintering Piping Plovers; Final Rule. Federal Register 1985, 50, 50726–50734. Available online: https://archives.federalregister.gov/issue_slice/1985/12/11/50706-50724.pdf#page=15 (accessed on 25 May 2018).
- USFWS Endangered and Threatened Wildlife and Plants; Final Determination of Critical Habitat for Wintering Piping Plovers 2001. Available online: https://www.federalregister.gov/documents/2001/07/10/01-16905/endangered-and-threatened-wildlife-and-plants-final-determination-of-critical-habitat-for-wintering (accessed on 21 May 2019).
- Roche, E.A.; Cohen, J.B.; Catlin, D.H.; Amirault-Langlais, D.L.; Cuthbert, F.J.; Gratto-Trevor, C.L.; Felio, J.; Fraser, J.D. Range-Wide Piping Plover Survival: Correlated Patterns and Temporal Declines. J. Wildl. Manag. 2010, 74, 1784–1791. [Google Scholar] [CrossRef]
- Schulz, J.L.; Leberg, P.L. Factors Affecting Prey Availability and Habitat Use of Nonbreeding Piping Plovers (Charadrius melodus) in Coastal Louisiana. J. Coast. Res. 2019, 35, 861. [Google Scholar] [CrossRef]
- Gratto-Trevor, C.; Amirault-Langlais, D.; Catlin, D.; Cuthbert, F.; Fraser, J.; Maddock, S.; Roche, E.; Shaffer, F. Connectivity in Piping Plovers: Do Breeding Populations Have Distinct Winter Distributions? J. Wildl. Manag. 2012, 76, 348–355. [Google Scholar] [CrossRef]
- Nicholls, J.; Baldassarre, G. Winter Distribution of Piping Plovers along the Atlantic and Gulf Coasts of the United States. Wilson Bull. 1990, 102, 400–412. [Google Scholar]
- Ellis, K.S.; Anteau, M.J.; Cuthbert, F.J.; Gratto-Trevor, C.L.; Jorgensen, J.G.; Newstead, D.J.; Powell, L.A.; Ring, M.M.; Sherfy, M.H.; Swift, R.J.; et al. Impacts of Extreme Environmental Disturbances on Piping Plover Survival Are Partially Moderated by Migratory Connectivity. Biol. Conserv. 2021, 264, 109371. [Google Scholar] [CrossRef]
- Zeigler, S.; Sturdivant, E.; Gutierrez, B. Evaluating Barrier Island Characteristics and Piping Plover (Charadrius Melodus) Habitat Availability Along the U.S. Atlantic Coast—Geospatial Approaches and Methodology; Open-File Report; USGS: Tallahassee, FL, USA, 2019. Available online: https://pubs.usgs.gov/of/2019/1071/ofr20191071.pdf (accessed on 20 May 2019).
- MacKenzie, D.I.; Nichols, J.D.; Hines, J.E.; Knutson, M.G.; Franklin, A.B. Estimating Site Occupancy, Colonization, and Local Extinction When a Species Is Detected Imperfectly. Ecology 2003, 84, 2200–2207. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Andrew Royle, J.; Langtimm, C.A. Estimating Site Occupancy Rates When Detection Probabilities Are Less than One. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- An, L.; Bohnett, E.; Battle, C.; Dai, J.; Lewison, R.; Jankowski, P.; Carter, N.; Ghimire, D.; Dhakal, M.; Karki, J.; et al. Sex-Specific Habitat Suitability Modeling for Panthera Tigris in Chitwan National Park, Nepal: Broader Conservation Implications. Sustainability 2021, 13, 13885. [Google Scholar] [CrossRef]
- Dorazio, R.M.; Royle, J.A. Estimating Size and Composition of Biological Communities by Modeling the Occurrence of Species. J. Am. Stat. Assoc. 2005, 100, 389–398. [Google Scholar] [CrossRef]
- Bohnett, E.; Fandjinou, K.; Ahmad, B.; Mammo, S.; Hulse, D.; Hoctor, T. Determining Community-Level Carnivore Response to Landscape Factors in Bukit Barisan Selatan National Park, Sumatra, Indonesia. J. Wildl. Biodivers. 2021, 5, 68–88. [Google Scholar] [CrossRef]
- Bohnett, E.; Goossens, B.; Bakar, M.S.A.; Abidin, T.R.; Lim, H.-Y.; Hulse, D.; Ahmad, B.; Hoctor, T.; Gardner, P. Examining Diversity of Terrestrial Mammal Communities across Forest Reserves in Sabah, Borneo. Biodivers. Conserv. 2022, 31, 1709–1734. [Google Scholar] [CrossRef]
- Barker, N.K.S.; Slattery, S.M.; Darveau, M.; Cumming, S.G. Modeling Distribution and Abundance of Multiple Species: Different Pooling Strategies Produce Similar Results. Ecosphere 2014, 5, art158. [Google Scholar] [CrossRef] [Green Version]
- Ferrier, S.; Guisan, A. Spatial Modelling of Biodiversity at the Community Level. J. Appl. Ecol. 2006, 43, 393–404. [Google Scholar] [CrossRef]
- Zipkin, E.F.; DeWan, A.; Andrew Royle, J. Impacts of Forest Fragmentation on Species Richness: A Hierarchical Approach to Community Modelling. J. Appl. Ecol. 2009, 46, 815–822. [Google Scholar] [CrossRef]
- Royle, J.A. N-Mixture Models for Estimating Population Size from Spatially Replicated Counts. Biometrics 2004, 60, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.N.; Elkin, C.; Martin, T.G.; Possingham, H.P. Modeling Abundance Using N-Mixture Models: The Importance of Considering Ecological Mechanisms. Ecol. Appl. 2009, 19, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royle, J.A.; Kery, M. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS: Volume 1:Prelude and Static Models; Elsevier Science & Technology: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kéry, M. Estimating Abundance from Bird Counts: Binomial Mixture Models Uncover Complex Covariate Relationships. The Auk 2008, 125, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Kéry, M.; Royle, J.A.; Schmid, H. Modeling Avian Abundance from Replicated Counts Using Binomial Mixture Models. Ecol. Appl. 2005, 15, 1450–1461. [Google Scholar] [CrossRef]
- Martin, J.; Royle, J.A.; Mackenzie, D.I.; Edwards, H.H.; Kéry, M.; Gardner, B. Accounting for Non-Independent Detection When Estimating Abundance of Organisms with a Bayesian Approach: Correlated Behaviour and Abundance. Methods Ecol. Evol. 2011, 2, 595–601. [Google Scholar] [CrossRef]
- Mordecai, R.S.; Mattsson, B.J.; Tzilkowski, C.J.; Cooper, R.J. Addressing Challenges When Studying Mobile or Episodic Species: Hierarchical Bayes Estimation of Occupancy and Use: Hierarchical Bayes Multi-Scale Occupancy. J. Appl. Ecol. 2011, 48, 56–66. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 2016; ISBN 978-1-315-38272-2. [Google Scholar]
- Beale, C.M.; Lennon, J.J.; Yearsley, J.M.; Brewer, M.J.; Elston, D.A. Regression Analysis of Spatial Data. Ecol. Lett. 2010, 13, 246–264. [Google Scholar] [CrossRef]
- Levy, O.; Ball, B.A.; Bond-Lamberty, B.; Cheruvelil, K.S.; Finley, A.O.; Lottig, N.R.; Punyasena, S.W.; Xiao, J.; Zhou, J.; Buckley, L.B.; et al. Approaches to Advance Scientific Understanding of Macrosystems Ecology. Front. Ecol. Environ. 2014, 12, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Crainiceanu, C.; Ruppert, D.; Wand, M.P. Bayesian Analysis for Penalized Spline Regression Using WinBUGS. J. Stat. Softw. 2005, 14, 1–24. [Google Scholar] [CrossRef]
- Kellner, K. Package “JagsUI” 2019.
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Kéry, M. Introduction to WinBUGS for Ecologists: A Bayesian Approach to Regression, ANOVA, Mixed Models and Related Analyses, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2010; ISBN 978-0-12-378605-0. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. CRAN Repository 2022. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 1 May 2022).
- Martin, J.; Sabatier, Q.; Gowan, T.A.; Giraud, C.; Gurarie, E.; Calleson, C.S.; Ortega-Ortiz, J.G.; Deutsch, C.J.; Rycyk, A.; Koslovsky, S.M. A Quantitative Framework for Investigating Risk of Deadly Collisions between Marine Wildlife and Boats. Methods Ecol. Evol. 2016, 7, 42–50. [Google Scholar] [CrossRef]

| Species | Total Detections | Year1 | Year2 | Year3 | Year4 | Year5 |
|---|---|---|---|---|---|---|
| American oystercatcher | 40 | - | 16 | 7 | 10 | 7 |
| Piping plover | 526 | 57 | 117 | 112 | 123 | 117 |
| Red knot | 40 | - | 4 | 7 | 4 | 25 |
| Snowy plover | 210 | - | 26 | 53 | 67 | 62 |
| Wilson’s plover | 408 | - | 116 | 111 | 91 | 81 |
| Species | Total Detections | Year1 | Year2 | Year3 | Year4 | Year5 |
|---|---|---|---|---|---|---|
| American oystercatcher | 16 | - | 2 | 4 | 7 | 3 |
| Piping plover | 325 | 41 | 70 | 87 | 53 | 74 |
| Red knot | 22 | - | 2 | 5 | 3 | 12 |
| Snowy plover | 121 | - | 17 | 37 | 27 | 38 |
| Wilson’s plover | 72 | - | 13 | 25 | 18 | 12 |
| Null Model | Spline with Detection = 1 | Spline with Detection Covariates | Spline with Occupancy and Detection Covariates | |||||
|---|---|---|---|---|---|---|---|---|
| Year | Mean | (2.5%, 97.5%) | Mean | (2.5%, 97.5%) | Mean | (2.5%, 97.5%) | Mean | (2.5%, 97.5%) |
| 1 | 0.071 | (0.039, 0.130) | 0.026 | (0.002, 0.109) | 0.424 | (0.075, 0.915) | 0.323 | (0.096, 0.736) |
| 2 | 0.066 | (0.050, 0.091) | 0.039 | (0.006, 0.121) | 0.160 | (0.024, 0.377) | 0.148 | (0.019, 0.329) |
| 3 | 0.081 | (0.059, 0.115) | 0.042 | (0.007, 0.130) | 0.155 | (0.032, 0.376) | 0.167 | (0.076, 0.346) |
| 4 | 0.053 | (0.034, 0.083) | 0.028 | (0.004, 0.104) | 0.122 | (0.013, 0.442) | 0.077 | (0.011, 0.272) |
| 5 | 0.142 | (0.086, 0.243) | 0.044 | (0.005, 0.151) | 0.341 | (0.071, 0.795) | 0.300 | (0.062, 0.672) |
| Number of occupied grid cells (out of 1690 grid cells across the island) for single species plover occupancy models for each of the five years of the survey, reporting the mean and 97.5% confidence interval (CI). | ||||||||
| 1 | 121 | (72, 213) | 41 | (41, 41) | 717 | (489, 853) | 546 | (397, 760) |
| 2 | 112 | (87, 146) | 63 | (63, 63) | 268 | (143, 360) | 250 | (119, 339) |
| 3 | 140 | (106, 184) | 70 | (70, 70) | 261 | (161, 341) | 282 | (232, 329) |
| 4 | 89 | (65, 131) | 46 | (46, 46) | 205 | (90, 373) | 129 | (79, 218) |
| 5 | 240 | (155, 410) | 72 | (72, 72) | 574 | (434, 746) | 507 | (377, 645) |
| Null Model | Spline with Detection = 1 | Spline with Detection Covariates | Spline with Occupancy and Detection Covariates | |||||
|---|---|---|---|---|---|---|---|---|
| Year | Mean | (2.5%, 97.5%) | Mean | (2.5%, 97.5%) | Mean | (2.5%, 97.5%) | Mean | (2.5%, 97.5%) |
| 1 | 294 | (183, 469) | 80 | (80, 80) | 19,531 | (7938, 31,930) | 10,820 | (4924, 24,171) |
| 2 | 376 | (260, 528) | 128 | (128, 128) | 27,857 | (6949, 55,364) | 16,619 | (4821, 23,816) |
| 3 | 262 | (191, 349) | 102 | (102, 102) | 13,413 | (4314, 29,283) | 10,817 | (4733, 30,194) |
| 4 | 428 | (318, 575) | 148 | (148, 148) | 46,662 | (16,216, 80,486) | 26,036 | (11,788, 52,175) |
| 5 | 508 | (347, 784) | 120 | (120, 120) | 8651 | (4278, 14,533) | 13,806 | (4013, 22,503) |
| Estimates for N across grid cells for single species plover N-mixture models for each of the five years of the survey, reporting the mean and 97.5% confidence interval (CI). | ||||||||
| 1 | 0.17 | (0.05, 1.05) | 0.05 | (0.05, 0.05) | 11.55 | (1.86, 43.00) | 6.4 | (1.01, 26.73) |
| 2 | 0.22 | (0.08, 1.1) | 0.08 | (0.08, 0.08) | 16.48 | (2.22, 57.91) | 9.83 | (1.67, 29.32) |
| 3 | 0.15 | (0.06, 1.06) | 0.06 | (0.06, 0.06) | 7.94 | (1.07, 29.82) | 6.4 | (0.91, 27.07) |
| 4 | 0.25 | (0.09, 1.14) | 0.09 | (0.09, 0.09) | 27.61 | (5.50, 85.39) | 15.41 | (3.86, 48.31) |
| 5 | 0.3 | (0.07, 1.41) | 0.07 | (0.07, 0.07) | 5.12 | (0.63, 18.95) | 8.17 | (0.92, 30.43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohnett, E.; Schulz, J.; Dobbs, R.; Hoctor, T.; Hulse, D.; Ahmad, B.; Rashid, W.; Waddle, H. Shorebird Monitoring Using Spatially Explicit Occupancy and Abundance. Land 2023, 12, 863. https://doi.org/10.3390/land12040863
Bohnett E, Schulz J, Dobbs R, Hoctor T, Hulse D, Ahmad B, Rashid W, Waddle H. Shorebird Monitoring Using Spatially Explicit Occupancy and Abundance. Land. 2023; 12(4):863. https://doi.org/10.3390/land12040863
Chicago/Turabian StyleBohnett, Eve, Jessica Schulz, Robert Dobbs, Thomas Hoctor, Dave Hulse, Bilal Ahmad, Wajid Rashid, and Hardin Waddle. 2023. "Shorebird Monitoring Using Spatially Explicit Occupancy and Abundance" Land 12, no. 4: 863. https://doi.org/10.3390/land12040863
APA StyleBohnett, E., Schulz, J., Dobbs, R., Hoctor, T., Hulse, D., Ahmad, B., Rashid, W., & Waddle, H. (2023). Shorebird Monitoring Using Spatially Explicit Occupancy and Abundance. Land, 12(4), 863. https://doi.org/10.3390/land12040863







