Use of Genus Cistus in Phytotechnologies: Application in a Closed Mercury Mine
Abstract
:1. Introduction
2. Materials and Methods
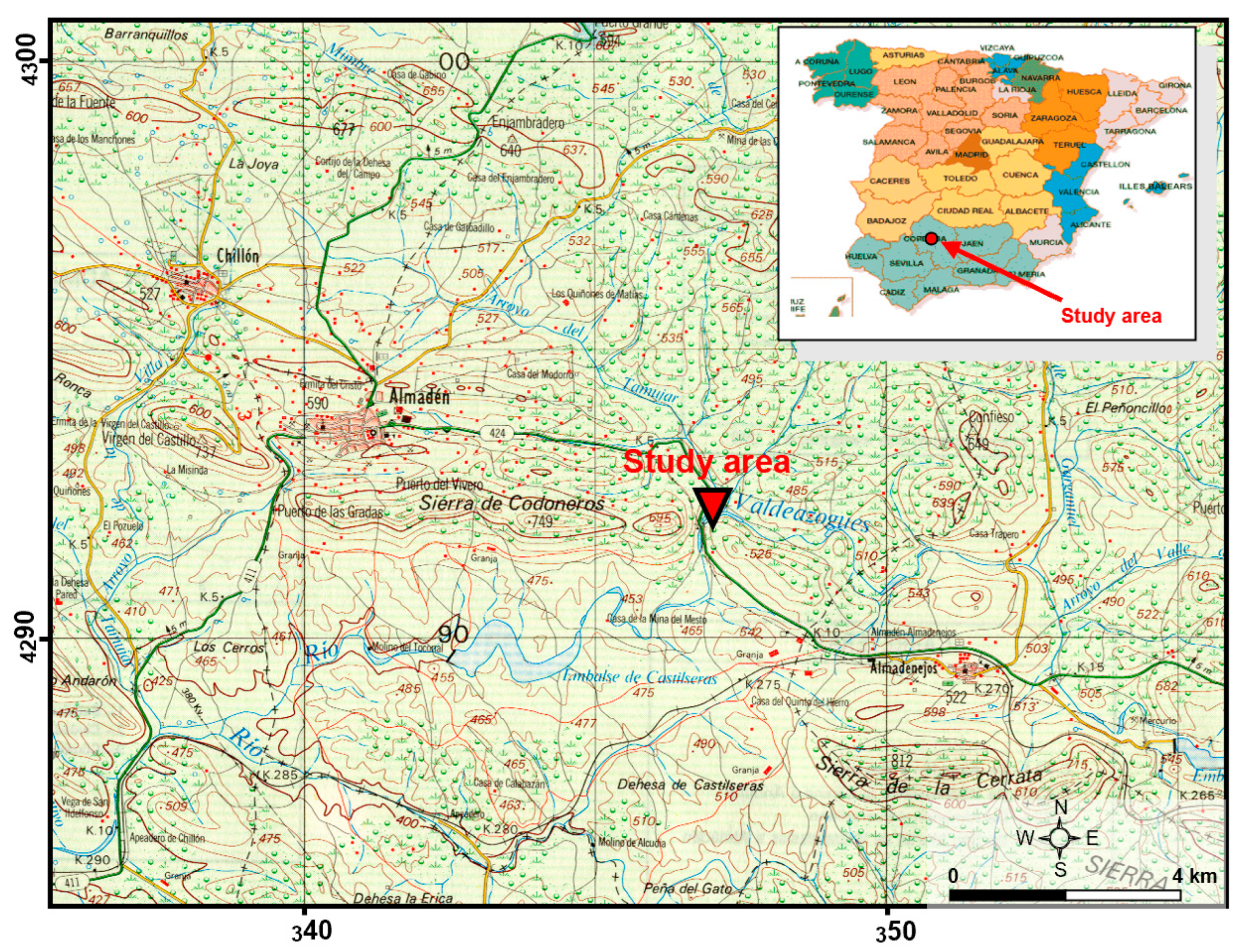
2.1. Plot Description
2.2. Measurement of Total Mercury Concentration in Soil and Plant
2.3. Measurement of Soluble and Exchangeable Mercury Concentrations
2.4. Statistical Analysis
3. Results
3.1. Assessment of Plot Homogeneity Assumptions
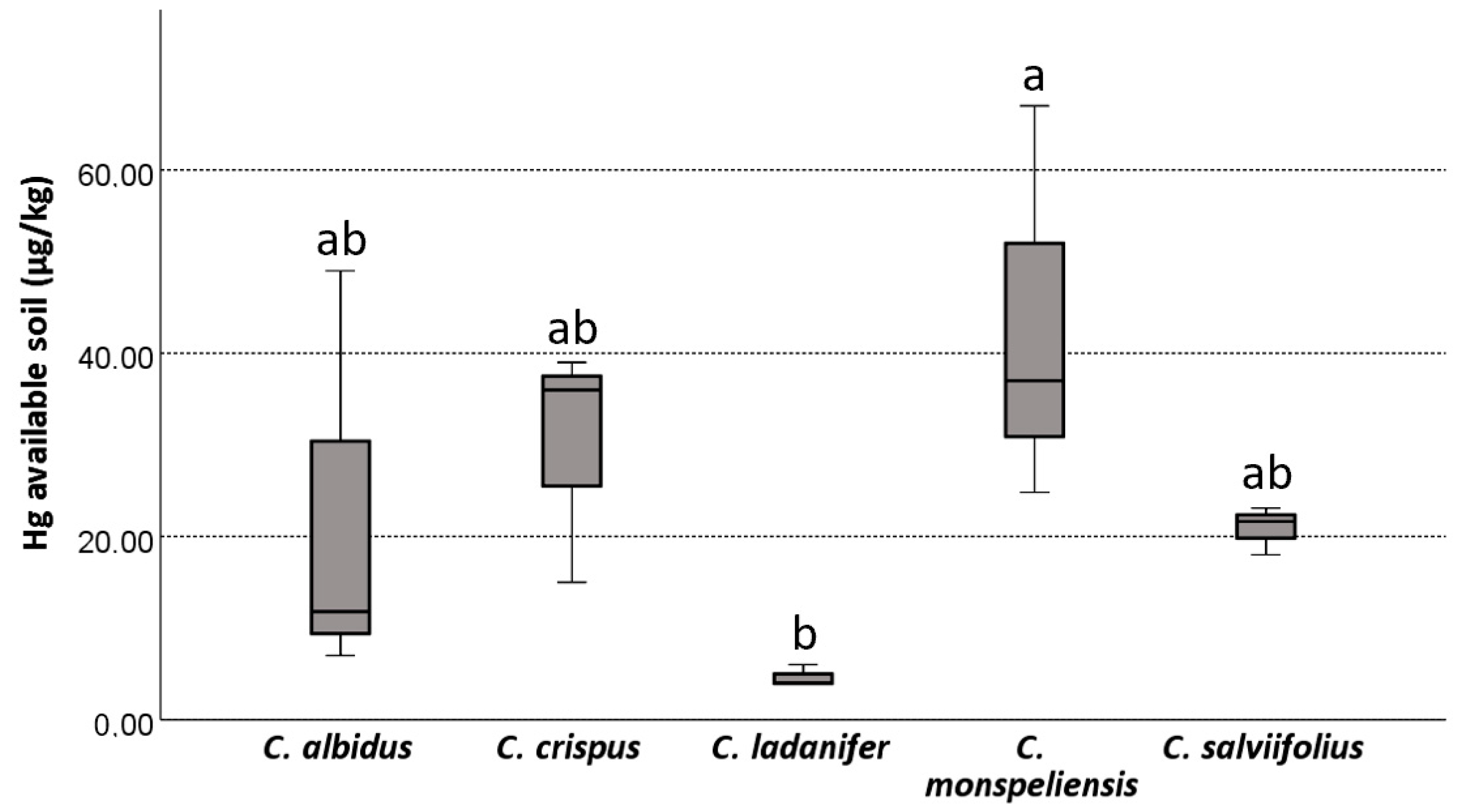
3.2. Assessment of Mercury Concentration in Soil
3.3. Assessment of Mercury Concentration in Cistus Plants
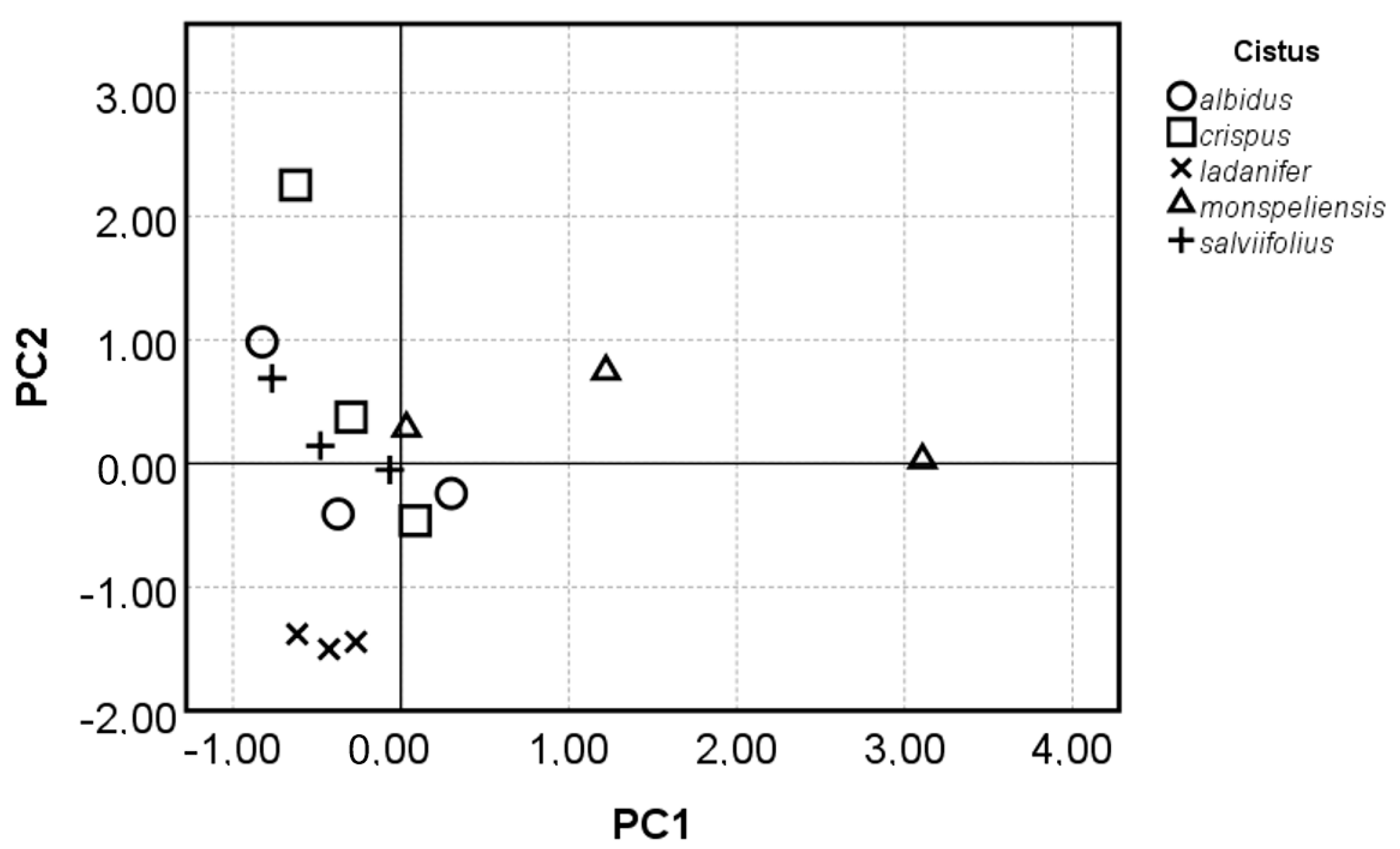
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández, A.; Jébrak, M.; Higueras, P.; Oyarzun, R.; Morata, D.; Munhá, J. The Almadén mercury mining district, Spain. Miner. Depos. 1999, 34, 539–548. [Google Scholar] [CrossRef]
- Barquero, J.I.; Lorenzo, S.; Esbrí, J.M.; Rivera, S.; González-Valoys, A.C.; García-Ordiales, E.; Higueras, P. Geochemical Assessment of Mineral Resource Potential in a Hg-Sb-Pb-Zn Mining Area: The Almadén and Guadalmez Synclines (South-Central Spain). Appl. Sci. 2022, 12, 11351. [Google Scholar] [CrossRef]
- Elmayel, I.; Esbrí, J.M.; Efrén, G.-O.; García-Noguero, E.-M.; Elouear, Z.; Jalel, B.; Farieri, A.; Roqueñí, N.; Cienfuegos, P.; Higueras, P. Evolution of the Speciation and Mobility of Pb, Zn and Cd in Relation to Transport Processes in a Mining Environment. Int. J. Environ. Res. Public Health 2020, 17, 4912. [Google Scholar] [CrossRef]
- Gray, J.E.; Pribil, M.J.; Higueras, P.L. Mercury isotope fractionation during ore retorting in the Almadén mining district, Spain. Chem. Geol. 2013, 357, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Bisquert, D.S.; Castejón, J.M.P.; Fernández, G.G. The impact of atmospheric dust deposition and trace elements levels on the villages surrounding the former mining areas in a semi-arid environment (SE Spain). Atmos. Environ. 2017, 152, 256–269. [Google Scholar] [CrossRef]
- Higueras, P.; Esbrí, J.M.; Oyarzun, R.; Llanos, W.; Martínez-Coronado, A.; Lillo, J.; López-Berdonces, M.A.; García-Noguero, E.M. Industrial and natural sources of gaseous elemental mercury in the Almadén district (Spain): An updated report on this issue after the ceasing of mining and metallurgical activities in 2003 and major land reclamation works. Environ. Res. 2013, 125, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, R.; Maserti, B.E.; Anderson, M.; Edner, H.; Ragnarson, P.; Svanberg, S.; Hernández, A. Atmospheric mercury concentrations and fluxes in the Almadén district (Spain). Atmos. Environ. 1998, 32, 3897–3904. [Google Scholar] [CrossRef]
- Berzas Nevado, J.J.; García Bermejo, L.F.; Rodríguez Martín-Doimeadios, R.C. Distribution of mercury in the aquatic environment at Almadén, Spain. Environ. Pollut. 2003, 122, 261–271. [Google Scholar] [CrossRef]
- Higueras, P.; Oryazum, R.; Biester, H.; Lillo, J.; Lorenzo, S. A first insight into mercury distribution and speciation in soils from the Almadén mining district, Spain. J. Geochem. Explor. 2003, 80, 95–104. [Google Scholar] [CrossRef]
- Gray, J.E.; Hines, M.E.; Higueras, P.L.; Adatto, I.; Lasorsa, B.K. Mercury speciation and microbial transformations in mine wastes, stream sediments and surface waters at the Almadén mining district, Spain. Environ. Sci. Technol. 2004, 38, 4285–4292. [Google Scholar] [CrossRef] [Green Version]
- Millán, R.; Gamarra, R.; Schmid, T.; Sierra, M.; Quejido, A.; Sánchez, D.; Cardona, A.; Fernández, M.; Vera, R. Mercury content in vegetation and soils of the Almadén mining area (Spain). Sci. Total. Environ. 2006, 368, 79–87. [Google Scholar] [CrossRef]
- Garcia-Ordiales, E.; Higueras, P.; Esbrí, J.M.; Roqueñí, N.; Loredo, J. Seasonal and spatial distribution of mercury in stream sediments from Almadén mining district. Geochem. Explor. Environ. Anal. 2018, 19, 121–128. [Google Scholar] [CrossRef]
- Lominchar, M.A.; Sierra, M.J.; Jiménez-Moreno, M.; Guirado, M.; Rodríguez Martín-Doimeadios, R.M.; Millán, R. Mercury species accumulation and distribution in Typha domingensis under real field conditions (Almadén, Spain). Environ. Sci. Pollut. Res. 2019, 26, 3138–3144. [Google Scholar] [CrossRef]
- Desauziers, V.; Castre, N.; Le Cloirec, P. Sortion of methylmercury by clays and mineral oxides. Environ. Technol. 1997, 18, 1009–1018. [Google Scholar] [CrossRef]
- Reis, A.T.; Lopes, C.B.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Extraction of available and labile fractions of mercury from contaminated soils: The role of operational parameters. Geoderma 2015, 259–260, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, L.; Alonso-Azcárate, J.; Gómez, R.; Rodríguez-Castellanos, L. Comparison of extractants used for the assessment of mercury availability in a soil from the Almadén mining district (Spain). Environ. Sci. Pollut. Res. 2017, 24, 12963–12970. [Google Scholar] [CrossRef] [Green Version]
- Skyllberg, U.; Bloom, P.R.; Qian, J.; Lin, C.-M.; Bleam, W.F. Complexation of Mercury(II) in Soil Organic Matter: EXAFS Evidence for Linear Two-Coordination with Reduced Sulfur Groups. Environ. Sci. Technol. 2006, 40, 4174–4180. [Google Scholar] [CrossRef]
- Schuster, E. The behavior of mercury in the soil with special emphasis on complexation and adsorption processes—A review of the literature. Water Air Soil Pollut. 1991, 56, 667–680. [Google Scholar] [CrossRef]
- Behra, P. Migration or retention of mercury II salts when percolating through a porous medium constituted of a natural quarz sand? Environ. Contam. 1986, 2, 318–320. [Google Scholar]
- Huang, J.-H.; Shetaya, W.H.; Osterwalder, S. Determination of (Bio)-available mercury in soils: A review. Environ. Pollut. 2020, 263, 114323. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Gamarra, R.G.; Carpena-Ruiz, R.O.; Millán, R.; Peñalosa, J.; Esteban, E. Mercury bioaccumulation and phytotoxicity in two wild plant species of Almadén area. Chemosphere 2006, 63, 1969–1973. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Jackson, D.R.; Huckabee, J.W.; Jansen, S.A.; Levin, M.J.; Lund, J.R. Atmospheric Emission and Plant Uptake of Mercury from Agricultural Soils near the Almadén Mercury Mine. J. Environ. Qual. 1979, 8, 572–578. [Google Scholar] [CrossRef]
- Millán, R.; Gamarra, R.; Vera, R.; Schmid, T. Mercury uptake for plant species from an Almadén test plot. In Proceedings of the 7th International Conference on the Biogeochemistry of Trace Elements; Gobran, G.R., Lepp, L., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2003; Volume 4, pp. 15–19. [Google Scholar]
- Millán, R.; Gamarra, R.; Schmid, T.; Vera, R.; Sierra, M.J.; Quejido, A.J. Mercury content in natural vegetation of three plots in the mining area of Almadén (Spain). Mater. Geoenviron. 2004, 51, 155–158. [Google Scholar]
- Millan, R.; Schmid, T.; Sierra, M.J.; Carrasco-Gil, S.; Villadóniga, M.; Rico, C.; Ledesma, D.M.S.; Puente, F.J.D. Spatial variation of biological and pedological properties in an area affected by a metallurgical mercury plant: Almadenejos (Spain). Appl. Geochem. 2011, 26, 174–181. [Google Scholar] [CrossRef]
- Hildebrand, S.G.; Huckabee, J.W.; Sanz Díaz, F.; Jansen, S.A.; Solomon, J.A.; Kumar, K.D. Distribution of Mercury in the Environment of Almadén, Spain (ORNL/TM-7446); Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1980; 87p. [Google Scholar]
- Higueras, P.; Molina, J.A.; Oyarzún, R.; Lillo, J.; Esbrí, J.M. Identification of the plant-communities and hyperaccumulators in mercury contaminated sectors of the Almadén district Spain. Mater. Geoenviron. 2004, 51, 103–107. [Google Scholar]
- Sierra, M.J.; Rodriguez-Alonso, J.; Millan, R. Impact of the lavender rhizosphere on the mercury uptake in field conditions. Chemosphere 2012, 89, 1457–1466. [Google Scholar] [CrossRef]
- Millán, R.; Lominchar, M.A.; López-Tejedor, I.; Rodríguez-Alonso, J.; Schmid, T.; Sierra, M.J. Behaviour of mercury in soils from the Valdeazogues riverbank and transfer to Nerium oleander L. J. Geochem. Explor. 2012, 123, 136–142. [Google Scholar] [CrossRef]
- Millán, R.; Lominchar, M.A.; Rodríguez-Alonso, J.; Schmid, T.; Sierra, M.J. Riparian vegetation role in mercury uptake (Val-deazogues River, Almadén, Spain). J. Geochem. Expl. 2014, 140, 104–110. [Google Scholar] [CrossRef]
- Lominchar, M.; Sierra, M.; Millán, R. Accumulation of mercury in Typha domingensis under field conditions. Chemosphere 2015, 119, 994–999. [Google Scholar] [CrossRef]
- Golia, E.; Angelaki, A.; Giannoulis, K.; Skoufogianni, E.; Bartzialis, D.; Cavalaris, C.; Vleioras, S. Evaluation of soil properties, irrigation and solid waste application levels on cu and zn uptake by industrial hemp. Agron. Res. 2021, 19, 92–99. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Millán, R.; Carpena, R.O.; Schmid, T.; Sierra, M.J.; Moreno, E.; Peñalosa, J.; Gamarra, R.; Esteban, E. Rehabilitación de suelos contaminados con mercurio: Estrategias aplicables en el área de Almadén. Ecosistemas 2007, 16, 56–66. [Google Scholar]
- Rivas Martínez, S.; Loidi Arregui, J. Biogeography of the Iberian Peninsula. Itinera Geobot. 1999, 13, 49–67. [Google Scholar]
- Rodríguez-Alonso, J.; Cabrales-García, C.; Millán, R. Factors influencing the cleaning of plant samples with ultrasonic technology. Int. J. Phytoremediation 2013, 25, 359–367. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. Available online: https://tienda.aenor.com/norma-iso-10390-2021-075243 (accessed on 30 July 2023).
- EPA 9081 SW-846; Test Method 9081: Cation-Exchange Capacity of Soils (Sodium Acetate). Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-9081-cation-exchange-capacity-soils-sodium-acetate (accessed on 30 July 2023).
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. Available online: https://www.iso.org/standard/19243.html (accessed on 30 July 2023).
- Sierra, M.; Millán, R.; Esteban, E. Mercury uptake and distribution in Lavandula stoechas plants grown in soil from Almadén mining district (Spain). Food Chem. Toxicol. 2009, 47, 2761–2767. [Google Scholar] [CrossRef]
- Quejido, A.J.; Sánchez, D.M.; Fernández, M.; Millán, R.; Vera, R.; Schmid, T. Determination Of solid-phase associations of mercury in contaminated soils from Almadén area. In Proceedings of the CSI XXXIII Colloquium Spectroscopicum International, Granada, Spain, 7–12 September 2003; p. 362. [Google Scholar]
- Sánchez, D.M.; Quejido, A.J.; Fernández, M.; Hernández, C.; Schmid, T.; Millán, R.; González, M.; Aldea, M.; Martín, R.; Morante, R. Mercury and trace element fractionation in Almaden soils by application of different sequential extraction procedures. Anal. Bioanal. Chem. 2005, 381, 1507–1513. [Google Scholar] [CrossRef]
- Jones, B. Laboratory Guide for Consulting Soil Test and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; ISBN 0-8493-0206-4. [Google Scholar]
- Adriano, D.C. Trace elements in terrestrial environments. Chapter 11. In Biogeochemistry, Bioavailability and Risks of Metals Mercury; Springer: Berlin/Heidelberg, Germany, 2001; pp. 411–458. [Google Scholar]
- González-Alcaraz, M.; Jiménez-Cárceles, F.; Álvarez, Y.; Álvarez-Rogel, J. Gradients of soil salinity and moisture, and plant distribution, in a Mediterranean semiarid saline watershed: A model of soil–plant relationships for contributing to the management. Catena 2014, 115, 150–158. [Google Scholar] [CrossRef]
- Porta, J.; López-Acebedo, M.; Roquero, C. Edafología Para la Agricultura y el Medio Ambiente. Capítulo 20: Salinización y Sodificación: Suelos de Regadío; Mundi-Prensa: Madrid, Spain, 1999; p. 657. [Google Scholar]
- Carvalho, L.C.; Santos, E.S.; Saraiva, J.A.; Magalhães, M.C.F.; Macías, F.; Abreu, M.M. The Potential of Cistus salviifolius L. to Phytostabilize Gossan Mine Wastes Amended with Ash and Organic Residues. Plants 2022, 11, 588. [Google Scholar] [CrossRef]
- Molina, J.A.; Oyarzun, R.; Esbrí, J.M.; Higueras, P. Mercury accumulation in soils and plants in the Almadén mining district, Spain: One of the most contaminated sites on Earth. Environ. Geochem. Health 2006, 28, 487–498. [Google Scholar] [CrossRef]
- Kovalevsky, A.L. Biogeochemical Exploration for Mineral Deposits, 2nd ed.; Brooks, R.R., Ed.; Trans. Russia; VNU Science Press: Utrecht, The Netherland, 1987; Volume 224. [Google Scholar]
- Arenas-Lago, D.; Santos, E.S.; Carvalho, L.S.; Abreu, M.M.; Andrade, M.L. Cistus monspeliensis L. as a potential species for rehabilitation of soils with multielemental contamination under Mediterranean conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 6443–6455. [Google Scholar] [CrossRef] [Green Version]
- McGrath, S.P.; Zhao, F.-J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean plant species are valuable resources: The example of Cistus ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef]
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef]
- Batista, M.J.; Gonzalez-Fernandez, O.; Abreu, M.M.; Queralt, I.; Carvalho, M.L. Pioneer Mediterranean Shrub Species Revegetating Soils Developed on Mining Soils/Spoils. Land Degrad. Dev. 2016, 28, 718–730. [Google Scholar] [CrossRef]
- Lázaro, J.D.; Kidd, P.; Martínez, C.M. A phytogeochemical study of the Trás-os-Montes region (NE Portugal): Possible species for plant-based soil remediation technologies. Sci. Total Environ. 2006, 354, 265–277. [Google Scholar] [CrossRef]
- Duarte, B.; Pires, V.; Carreiras, J.; de Carvalho, R.C.; Ferreira, R.; Pereira, M.F.; Maurício, A.M.; Martins-Dias, S.; Caçador, I. Cistus ladanifer metal uptake and physiological performance in biochar amended mine soils. S. Afr. J. Bot. 2023, 153, 246–257. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Nabais, C.; Saraiva, J.A. Trace elements and activity of antioxidative enzymes in Cistus ladanifer L. growing on an abandoned mine area. Ecotoxicology 2009, 18, 860–868. [Google Scholar] [CrossRef]
- Jiménez, M.N.; Bacchetta, G.; Navarro, F.B.; Casti, M.; Fernández-Ondoño, E. Native Plant Capacity for Gentle Remediation in Heavily Polluted Mines. Appl. Sci. 2021, 11, 1769. [Google Scholar] [CrossRef]
- Abreu, M.; Santos, E.; Ferreira, M.; Magalhães, M. Cistus salviifolius a promising species for mine wastes remediation. J. Geochem. Explor. 2011, 113, 86–93. [Google Scholar] [CrossRef]
- Parra, A.; Zornoza, R.; Conesa, E.; López, G.; Faz, A. Evaluation of the suitability of three Mediterranean shrub species for phytostabilization of pyritic mine soils. Catena 2016, 136, 59–65. [Google Scholar] [CrossRef]
- Roca-Perez, L.; Boluda, R.; Rodríguez-Martín, J.A.; Ramos-Miras, J.; Tume, P.; Roca, N.; Bech, J. Potentially harmful elements pollute soil and vegetation around the Atrevida mine (Tarragona, NE Spain). Environ. Geochem. Health 2023. [Google Scholar] [CrossRef]






| Species | Plot | Soil Sample | Plant Sample |
|---|---|---|---|
| C. albidus | 1 | S_CA1 | P_CA1 |
| 2 | S_CA2 | P_CA2 | |
| 3 | S_CA3 | P_CA3 | |
| C. crispus | 1 | S_CC1 | P_CC1 |
| 2 | S_CC2 | P_CC2 | |
| 3 | S_CC3 | P_CC3 | |
| C. ladanifer | 1 | S_CL1 | P_CL1 |
| 2 | S_CL2 | P_CL2 | |
| 3 | S_CL3 | P_CL3 | |
| C. monspeliensis | 1 | S_CM1 | P_CM1 |
| 2 | S_CM2 | P_CM2 | |
| 3 | S_CM3 | P_CM3 | |
| C. salvifolius | 1 | S_CS1 | P_CS1 |
| 2 | S_CS2 | P_CS2 | |
| 3 | S_CS3 | P_CS3 |
| Soil Subplot | pH | OM (%) | CEC (cmol+/kg) | EC (µS/cm) |
|---|---|---|---|---|
| SC_ 1 | 6.34 ± 0.98 a | 2.12 ± 0.62a | 9.73 ± 1.17 a | 158 ± 18 a |
| SC_ 2 | 6.26 ± 0.08 a | 2.66 ± 0.65 a | 10.08 ± 2.01 a | 158 ± 2 a |
| SC_ 3 | 6.60 ± 0.23 a | 1.10 ± 0.78 a | 5.94 ± 1.12 a | 102 ± 8 b |
| ANOVA | ||||
| F | 1.39 | 2.036 | 2.352 | 3.754 |
| Sig. | 0.286 | 0.173 | 0.137 | 0.054 |
| n.s. | n.s. | n.s. | * | |
| Soil Subplot | Hg Total Soil (mg/kg) | Hg Soluble (µg/kg) | Hg Exchangeable (µg/kg) | Hg Available (µg/kg) |
|---|---|---|---|---|
| SC_ 1 | 7.07 ± 3.91 a | 5.68 ± 1.71 a | 14.4 ± 6.5 a | 22.0 ± 7.2 a |
| SC_ 2 | 4.56 ± 1.13 a | 5.67 ± 2.43 a | 13.8 ± 5.7 a | 19.6 ± 5.5 a |
| SC_ 3 | 10.8 ± 6.2 a | 14.9 ± 7.8 a | 16.8 ± 8.4 a | 31.0 ± 11.5 a |
| ANOVA | ||||
| F | 0.532 | 1.196 | 0.053 | 0.497 |
| Sig. | 0.600 | 0.336 | 0.949 | 0.620 |
| n.s. | n.s. | n.s. | n.s. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Sanz, A.; Millán, R.; Sierra, M.J.; Schmid, T.; García, G. Use of Genus Cistus in Phytotechnologies: Application in a Closed Mercury Mine. Land 2023, 12, 1533. https://doi.org/10.3390/land12081533
Pérez-Sanz A, Millán R, Sierra MJ, Schmid T, García G. Use of Genus Cistus in Phytotechnologies: Application in a Closed Mercury Mine. Land. 2023; 12(8):1533. https://doi.org/10.3390/land12081533
Chicago/Turabian StylePérez-Sanz, Araceli, Rocío Millán, María José Sierra, Thomas Schmid, and Gregorio García. 2023. "Use of Genus Cistus in Phytotechnologies: Application in a Closed Mercury Mine" Land 12, no. 8: 1533. https://doi.org/10.3390/land12081533





