Ecological Connectivity of Vicuña (Vicugna vicugna) in a Remote Area of Chile and Conservation Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
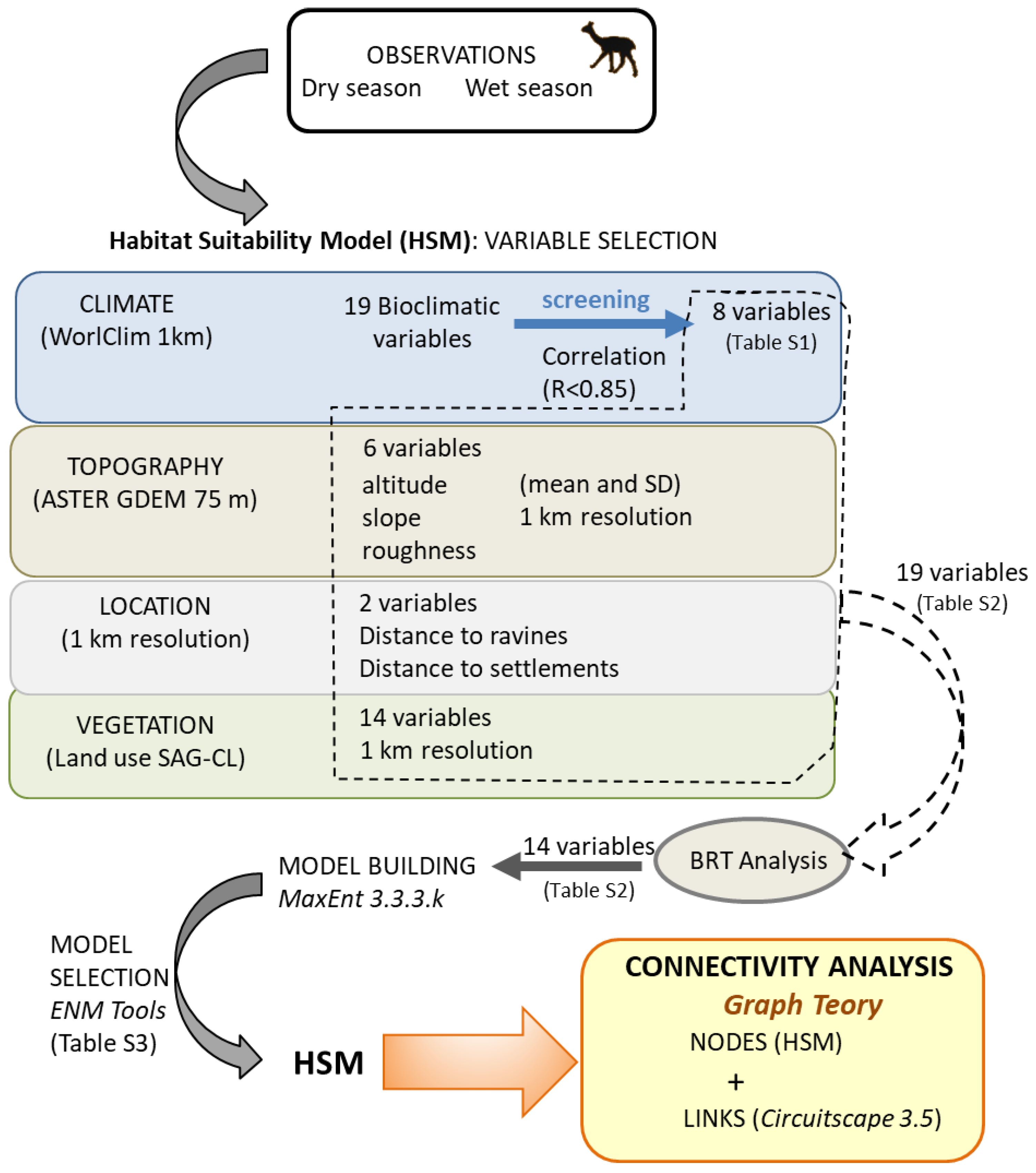
2.2. Data Collection
2.3. Connectivity Analysis
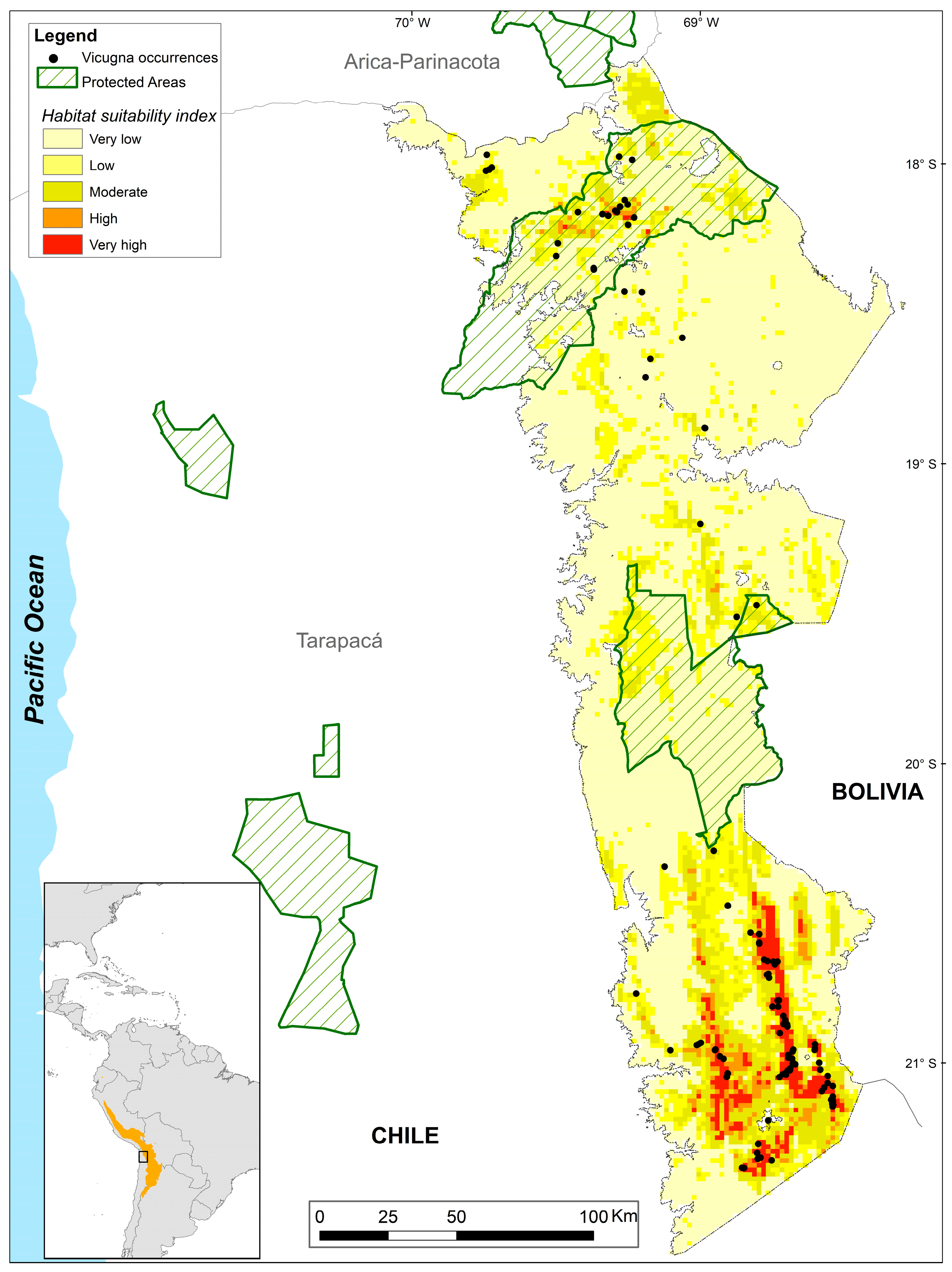
3. Results
3.1. Habitat Suitability Models
3.2. Connectivity Analysis
| Threshold | ECAnorm | dPCintra | dPCflux | dPCconnector |
|---|---|---|---|---|
| MaxSS | 7.70 | 55.99 | 43.44 | 0.57 |
| AvPP | 8.98 | 66.5 | 33.32 | 0.18 |

| Threshold | Node | dPC | dPCintra | dPCflux | dPCconnector | Area (Ha) |
|---|---|---|---|---|---|---|
| MaxSS | 1 | 93.833 | 71.140 | 22.511 | 0.182 | 1146 |
| 2 | 4.019 | 0.046 | 3.943 | 0.031 | 29 | |
| 3 | 3.113 | 0.135 | 2.698 | 0.280 | 50 | |
| 4 | 2.331 | 0.020 | 2.312 | 0.000 | 19 | |
| 5 | 2.049 | 0.016 | 2.007 | 0.026 | 17 | |
| 6 | 1.627 | 0.176 | 1.431 | 0.019 | 57 | |
| 7 | 1.410 | 0.125 | 1.279 | 0.006 | 48 | |
| 8 | 1.405 | 0.236 | 1.169 | 0.000 | 66 | |
| 9 | 1.351 | 0.005 | 1.346 | 0.000 | 10 | |
| 10 | 1.034 | 0.014 | 0.845 | 0.174 | 16 | |
| AvPP | 1 | 98.589 | 79.569 | 18.811 | 0.208 | 519 |
| 2 | 6.165 | 0.130 | 6.026 | 0.009 | 21 | |
| 3 | 3.627 | 0.043 | 3.585 | 0.000 | 12 | |
| 4 | 2.464 | 0.019 | 2.445 | 0.000 | 8 | |
| 5 | 2.239 | 0.019 | 2.220 | 0.000 | 8 | |
| 6 | 0.878 | 0.003 | 0.876 | 0.000 | 3 | |
| 7 | 0.874 | 0.003 | 0.871 | 0.000 | 3 | |
| 8 | 0.698 | 0.003 | 0.696 | 0.000 | 3 | |
| 9 | 0.650 | 0.003 | 0.647 | 0.000 | 3 | |
| 10 | 0.608 | 0.001 | 0.607 | 0.000 | 2 |
4. Discussion
Conservation Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CMS (Convention on the Conservation of Migratory Species). Improving Ways of Addressing Connectivity in the Conservation of Migratory Species, Resolution 12.26 (REV.COP13); Convention on Migratory Species: Gandhinagar, India, 2020; Available online: https://www.cms.int/sites/default/files/document/cms_cop13_doc.26.4.4_addressing-connectivity-in-conservation-of-migratory-species_e.pdf (accessed on 29 November 2023).
- Taylor, P.D.; Fahrig, L.; With, K.A. Landscape connectivity: A return to the basics. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 29–43. [Google Scholar]
- Crooks, K.R.; Sanjayan, M. Connectivity Conservation; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Baguette, M.; Blanchet, S.; Legrand, D.; Stevens, V.M.; Turlure, C. Individual dispersal, landscape connectivity and ecological networks. Biol. Rev. 2013, 88, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Morelli, T.L.; Maher, S.P.; Lim, M.C.W.; Kastely, C.; Eastman, L.M.; Flint, L.E.; Flint, A.L.; Beissinger, S.R.; Moritz, C. Climate change refugia and habitat connectivity promote species persistence. Clim. Chang. Responses 2017, 4, 8. [Google Scholar] [CrossRef]
- Costanza, J.K.; Terando, A.J. Landscape Connectivity Planning for Adaptation to Future Climate and Land-Use Change. Curr. Landsc. Ecol. Rep. 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Carroll, C.; Rohlf, D.J.; Li, Y.W.; Hartl, B.; Phillips, M.K.; Noss, R.F. Connectivity Conservation and Endangered Species Recovery: A Study in the Challenges of Defining Conservation-Reliant Species. Conserv. Lett. 2015, 8, 132–138. [Google Scholar] [CrossRef]
- Kauffman, M.J.; Cagnacci, F.; Chamaillé-Jammes, S.; Hebblewhite, M.; Hopcraft, J.G.C.; Merkle, J.A.; Mueller, T.; Mysterud, A.; Peters, W.; Roettger, C.; et al. Mapping out a future for ungulate migrations. Science 2021, 372, 566–569. [Google Scholar] [CrossRef]
- Rabinowitz, A.; Zeller, K. A rangewide model of landscape connectivity and conservation for the jaguar, Panthera onca. Biol. Conserv. 2010, 143, 939–945. [Google Scholar] [CrossRef]
- Ziółkowska, E.; Ostapowicz, K.; Kuemmerle, T.; Perzanowski, K.; Radeloff, V.C.; Kozak, J. Potential habitat connectivity of European bison (Bison bonasus) in the Carpathians. Biol. Conserv. 2012, 146, 188–196. [Google Scholar] [CrossRef]
- Kabir, M.; Hameed, S.; Ali, H.; Bosso, L.; Din, J.U.; Bischof, R.; Redpath, S.; Nawaz, M.A. Habitat suitability and movement corridors of grey wolf (Canis lupus) in Northern Pakistan. PLoS ONE 2017, 12, e0187027. [Google Scholar] [CrossRef]
- Batter, T.J.; Bush, J.P.; Sacks, B.N. Assessing genetic diversity and connectivity in a tule elk (Cervus canadensis nannodes) metapopulation in Northern California. Conserv. Genet. 2021, 22, 889–901. [Google Scholar] [CrossRef]
- González, B.A.; Donoso, D.S. Capítulo 4. Distribución geográfica actual de la vicuña austral. In La Vicuña Austral; González, B.A., Ed.; Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Corporación Nacional Forestal y Grupo Especialista en Camélidos Sudamericanos Silvestres: Santiago, Chile, 2020; ISBN 978-956-7669-74-5. [Google Scholar]
- Yacobaccio, H. The historical relationship between people and the vicuña. In The Vicuña; Gordon, I.J., Ed.; Springer: Boston, MA, USA, 2009; pp. 7–20. [Google Scholar] [CrossRef]
- Wheeler, J.C.; Laker, J. The vicuña in the Andean altiplano. In The Vicuña: The Theory and Practice of Community-Based Wildlife Management; Gordon, I., Ed.; Springer: Boston, MA, USA, 2009; pp. 21–33. [Google Scholar] [CrossRef]
- Jungius, H. Bolivia and the vicuna. Oryx 1972, 11, 335–346. [Google Scholar] [CrossRef]
- Boswall, J. Vicuna in Argentina. Oryx 1972, 11, 449–456. [Google Scholar] [CrossRef]
- Grimwood, I. Notes on the Distribution and Status of Some Peruvian Mammals; Zoological Society: New York, NY, USA, 1969; Volume 21. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R. Mammal Population Losses and the Extinction Crisis. Science 2002, 296, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Acebes, P.; Wheeler, J.; Baldo, J.; Tuppia, P.; Lichtenstein, G.; Hoces, D.; Franklin, W.L. Vicugna vicugna: The IUCN Red List of Threatened Species; e.T22956A18540534; International Union for Conservation of Nature: Gland, Switzerland, 2018. [Google Scholar]
- Arzamendia, Y.; Carbajo, A.E.; Vilá, B. Social group dynamics and composition of managed wild vicuñas (Vicugna vicugna vicugna) in Jujuy, Argentina. J. Ethol. 2018, 36, 125–134. [Google Scholar] [CrossRef]
- González, B.A.; Donoso, D.S.; Villalobos, R.; Lagos, N.; Iriarte, A. Box 6.1. Ámbito de hogar y preferencia de hábitat de individuos de vicuña austral en el altiplano de Chile. In La Vicuña Austral; González, B.A., Ed.; Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Corporación Nacional Forestal y Grupo Especialista en Camélidos Sudamericanos: Santiago, Chile, 2020; ISBN 978-956-7669-74-5. [Google Scholar]
- Karandikar, H.; Donadio, E.; Smith, J.A.; Bidder, O.R.; Middleton, A.D. Spatial ecology of the Vicuña (Lama vicugna) in a high Andean protected area. J. Mammal. 2023, 104, 509–518. [Google Scholar] [CrossRef]
- Malo, J.E.; González, B.A.; Mata, C.; Vielma, A.; Donoso, D.S.; Fuentes, N.; Estades, C.F. Low habitat overlap at landscape scale between wild camelids and feral donkeys in the Chilean desert. Acta Oecol. 2016, 70, 1–9. [Google Scholar] [CrossRef]
- González, B.A.; Marín, J.C.; Toledo, V.; Espinoza, E. Wildlife forensic science in the investigation of poaching of vicuña. Oryx 2016, 50, 14–15. [Google Scholar] [CrossRef]
- Mata, C.; Malo, J.E.; Galaz, J.L.; Cadorzo, C.; Lagunas, H. A three-step approach to minimise the impact of a mining site on vicuña (Vicugna vicugna) and to restore landscape connectivity. Environ. Sci. Pollut. Res. 2016, 23, 13626–13636. [Google Scholar] [CrossRef]
- Franklin, W.L. Contrasting socioecologies of South American’s wild camelids: The vicuña and guanaco. In Advances in the Study of Mammalian Behavior; Eisenberg, J.F., Kleiman, D.G., Eds.; American Society of Mammalogists Special Publication: Topeka, KS, USA, 1983; Volume 7, pp. 573–629. [Google Scholar]
- Tirado, C.; Cortés, A.; Miranda-Urbina, E.; Carretero, M.A. Trophic preferences in an assemblage of mammal herbivores from Andean Puna (Northern Chile). J. Arid Environ. 2012, 79, 8–12. [Google Scholar] [CrossRef]
- Arzamendia, Y.; Cassini, M.H.; Vilá, B. Habitat use by vicuña Vicugna vicugna in Laguna Pozuelos Reserve, Jujuy, Argentina. Oryx 2006, 40, 198–203. [Google Scholar] [CrossRef]
- Moraga, C.; Donoso, D.S.; González, B.A. Capítulo 5. Metodologías de monitoreo en vicuñas y estimaciones de abundancia y densidad de vicuña austral en Chile. In La Vicuña Austral; González, B.A., Ed.; Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Corporación Nacional Forestal y Grupo Especialista en Camélidos Sudamericanos: Santiago, Chile, 2020. [Google Scholar]
- Miller, S.D.; Rottmann, J.; Raedeke, K.J.; Taber, R.D. Endangered mammals of Chile: Status and conservation. Biol. Conserv. 1983, 25, 335–352. [Google Scholar] [CrossRef]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago de Chile, Chile, 2018. [Google Scholar]
- IUCN (International Union for Conservation of Nature). Vicugna vicugna: The IUCN Red List of Threatened Species; Version 2022-2; International Union for Conservation of Nature: Gland, Switzerland, 2022. [Google Scholar]
- Hughes, A.C.; Orr, M.C.; Ma, K.; Costello, M.J.; Waller, J.; Provoost, P.; Yang, Q.; Zhu, C.; Huijie Qiao, H. Sampling biases shape our view of the natural world. Ecography 2021, 44, 1259–1269. [Google Scholar] [CrossRef]
- Bunn, A.G.; Urban, D.L.; Keitt, T.H. Landscape connectivity: A conservation application of graph theory. J. Environ. Manag. 2000, 59, 265–278. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Comparison and development of new graph-based landscape connectivity indices: Towards the priorization of habitat patches and corridors for conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either- or presence-absence. Acta Oecol. 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G.; Pearson, R. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Saura, S.; Estreguil, C.; Mouton, C.; Rodríguez-Freire, M. Network analysis to assess landscape connectivity trends: Application to European forests (1990–2000). Ecol. Indic. 2011, 11, 407–416. [Google Scholar] [CrossRef]
- Sutherland, G.D.; Harestad, A.S.; Price, K.; Lertzman, K.P. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000, 4, 16. [Google Scholar] [CrossRef]
- Bowman, J.; Jaeger, J.A.; Fahrig, L. Dispersal distance of mammals is proportional to home range size. Ecology 2002, 83, 2049–2055. [Google Scholar] [CrossRef]
- Bonacic, C. Sustainable Use of the Vicuña in Chile. Master’s Thesis, University of Reading, Reading, UK, 1996. [Google Scholar]
- Saura, S.; Rubio, L. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Saura, S.; Torné, J. Conefor Sensinode 2.2: A software package for quantify the importance of habitat patches for landscape connectivity. Environ. Model. Softw. 2009, 24, 135–139. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Beger, M.; Metaxas, A.; Balbar, A.C.; McGowan, J.A.; Daigle, R.; Kuempel, C.D.; Treml, E.A.; Possingham, H.P. Demystifying ecological connectivity for actionable spatial conservation planning. Trends Ecol. Evol. 2022, 37, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- González, B.A.; Vásquez, J.P.; Gómez-Uchida, D.; Cortés, J.; Rivera, R.; Aravena, N.; Chero, A.M.; Agapito, A.M.; Varas, V.; Wheleer, J.C.; et al. Phylogeography and Population Genetics of Vicugna vicugna: Evolution in the Arid Andean High Plateau. Front. Genet. 2019, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Unnithan Kumar, S.; Turnbull, J.; Hartman Davies, O.; Hodgetts, T.; Cushman, S.A. Moving beyond landscape resistance: Considerations for the future of connectivity modelling and conservation science. Lands Ecol. 2022, 37, 2465–2480. [Google Scholar] [CrossRef]
- d’Arc, N.R.; Cassini, M.H.; Vilá, B.L. Habitat use by vicuñas Vicugna vicugna in the Laguna Blanca Reserve (Catamarca, Argentina). J. Arid Environ. 2000, 46, 107–115. [Google Scholar]
- Fuentes-Allende, N.; Quilodrán, C.S.; Jofré, A.; González, B.A. Behavioral responses of vicuñas to human activities at priority feeding sites associated with roads in the highland desert of northern Chile. Int. J. Agric. Nat. Resour. 2023, 50, 75–83. [Google Scholar] [CrossRef]
- Mosca-Torres, M.E.; Puig, S. Seasonal diet of vicuñas in the Los Andes protected area (Salta, Argentina): Are they optimal foragers? J. Arid Environ. 2010, 74, 450–457. [Google Scholar] [CrossRef]
- MMA (Ministerio del Medio Ambiente). Plan Nacional de Protección de Humedales 2018–2022; Ministerio del Medio Ambiente, Gobierno de Chile: Santiago, Chile, 2018.
- IUCN. Red List Categories and Criteria, Version 3.1, 2nd ed.; IUCN Library System: Gland, Switzerland; Cambridge, UK, 2012; 32p, ISBN 978-2-8317-1435-6. [Google Scholar]
- RCE (Reunión Comité Clasificación de Especies Silvestres). Ministerio del Medio Ambiente, Gobierno de Chile. 2019. Available online: https://clasificacionespecies.mma.gob.cl/wp-content/uploads/2019/12/Acta_RCE_4_Decimosexto_Proc_9_octubre_2019.pdf (accessed on 26 October 2023).
- Cushman, S.A.; Elliot, N.B.; Macdonald, D.W.; Loveridge, A.J. A multi-scale assessment of population connectivity in African lions (Panthera leo) in response to landscape change. Landsc. Ecol. 2016, 31, 1337–1353. [Google Scholar] [CrossRef]
- Quilodrán, C.S.; Montoya-Burgos, J.I.; Currat, M. Harmonizing hybridization dissonance in conservation. Commun. Biol. 2020, 3, 391. [Google Scholar] [CrossRef]
- SNASPE (Sistema Nacional de Áreas Silvestres del Estado). Biblioteca del Congreso Nacional de Chile. Información Territorial. Available online: https://www.bcn.cl/siit/mapas_vectoriales/index_html (accessed on 22 December 2022).
- Sonter, L.J.; Ali, S.H.; Watson, J.E.M. Mining and biodiversity: Key issues and research needs in conservation science. Proc. R. Soc. B 2018, 285, 20181926. [Google Scholar] [CrossRef] [PubMed]
- Krosby, M.; Tewksbury, J.; Haddad, N.M.; Hoekstra, J. Ecological connectivity for a changing climate. Conserv. Biol. 2010, 24, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Oxford Analytica. Climate change will hit the Andean region unevenly. Expert Briefs 2022. [Google Scholar] [CrossRef]
- Sarricolea Espinoza, P.; Romero Aravena, H. Variabilidad y cambios climáticos observados y esperados en el Altiplano del norte de Chile. Rev. Geogr. Norte Gd. 2015, 62, 169–183. (In Spanish) [Google Scholar] [CrossRef]
- Chavez, R.O.; Meseguer-Ruiz, O.; Olea, M.; Calderon-Seguel, M.; Yager, K.; Meneses, R.I.; Lastra, J.A.; Nuñez-Hidalgo, I.; Sarricolea, P.; Serrano-Notivoli, R.; et al. Andean peatlands at risk? Spatiotemporal patterns of extreme NDVI anomalies, water extraction and drought severity in a large-scale mining area of Atacama, northern Chile. Int. J. Appl. Earth Obs. Geoinf. 2023, 116, 103138. [Google Scholar] [CrossRef]
- SERNAGEOMIN (Servicio Nacional de Geología y Minería). Anuario de la Minería de Chile 2022; Servicio Nacional de Geología y Minería: Santiago, Chile, 2023; 235p.
- Hamann, R. Mining companies’ role in sustainable development: The ‘why’ and ‘how’ of corporate social responsibility from a business perspective. Dev. South. Afr. 2003, 20, 237–254. [Google Scholar] [CrossRef]
- Hartman, B.D.; Bookhagen, B.; Chadwick, O.A. The effects of check dams and other erosion control structures on the restoration of Andean bofedal. Restor. Ecol. 2016, 24, 761–772. [Google Scholar] [CrossRef]
| Variables | Contribution (%) | Jackknife Test of Training Gain | ||
|---|---|---|---|---|
| Only the Variable | Without the Variable | |||
| Topographic | Mean altitude | 0.57 | 0.014 | 1.048 |
| SD altitude | 10.34 | 0.168 | 0.938 | |
| Mean gradient | 0.62 | 0.010 | 1.030 | |
| Location | Distance to ravines | 11.14 | 0.222 | 0.891 |
| Distance to settlements | 4.90 | 0.025 | 1.020 | |
| Climatic | BIO 1 | 5.10 | 0.013 | 0.974 |
| BIO2 | 0.20 | 0.062 | 1.051 | |
| BIO 3 | 1.02 | 0.097 | 1.038 | |
| BIO 4 | 5.10 | 0.227 | 1.047 | |
| BIO 7 | 3.02 | 0.064 | 1.052 | |
| BIO 12 | 40.34 | 0.463 | 0.926 | |
| Vegetation | Bofedal | 1.16 | 0.002 | 1.039 |
| Steppe | 9.76 | 0.026 | 0.891 | |
| Very open shrub | 6.72 | 0.129 | 1.010 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, C.; González, B.A.; Donoso, D.S.; Fuentes-Allende, N.; Estades, C.F.; Malo, J.E. Ecological Connectivity of Vicuña (Vicugna vicugna) in a Remote Area of Chile and Conservation Implications. Land 2024, 13, 472. https://doi.org/10.3390/land13040472
Mata C, González BA, Donoso DS, Fuentes-Allende N, Estades CF, Malo JE. Ecological Connectivity of Vicuña (Vicugna vicugna) in a Remote Area of Chile and Conservation Implications. Land. 2024; 13(4):472. https://doi.org/10.3390/land13040472
Chicago/Turabian StyleMata, Cristina, Benito A. González, Denise S. Donoso, Nicolás Fuentes-Allende, Cristián F. Estades, and Juan E. Malo. 2024. "Ecological Connectivity of Vicuña (Vicugna vicugna) in a Remote Area of Chile and Conservation Implications" Land 13, no. 4: 472. https://doi.org/10.3390/land13040472
APA StyleMata, C., González, B. A., Donoso, D. S., Fuentes-Allende, N., Estades, C. F., & Malo, J. E. (2024). Ecological Connectivity of Vicuña (Vicugna vicugna) in a Remote Area of Chile and Conservation Implications. Land, 13(4), 472. https://doi.org/10.3390/land13040472






