Diversity Levels under Different Grazing Intensities in Semi-Wet Grasslands
Abstract
1. Introduction
2. Materials and Methods
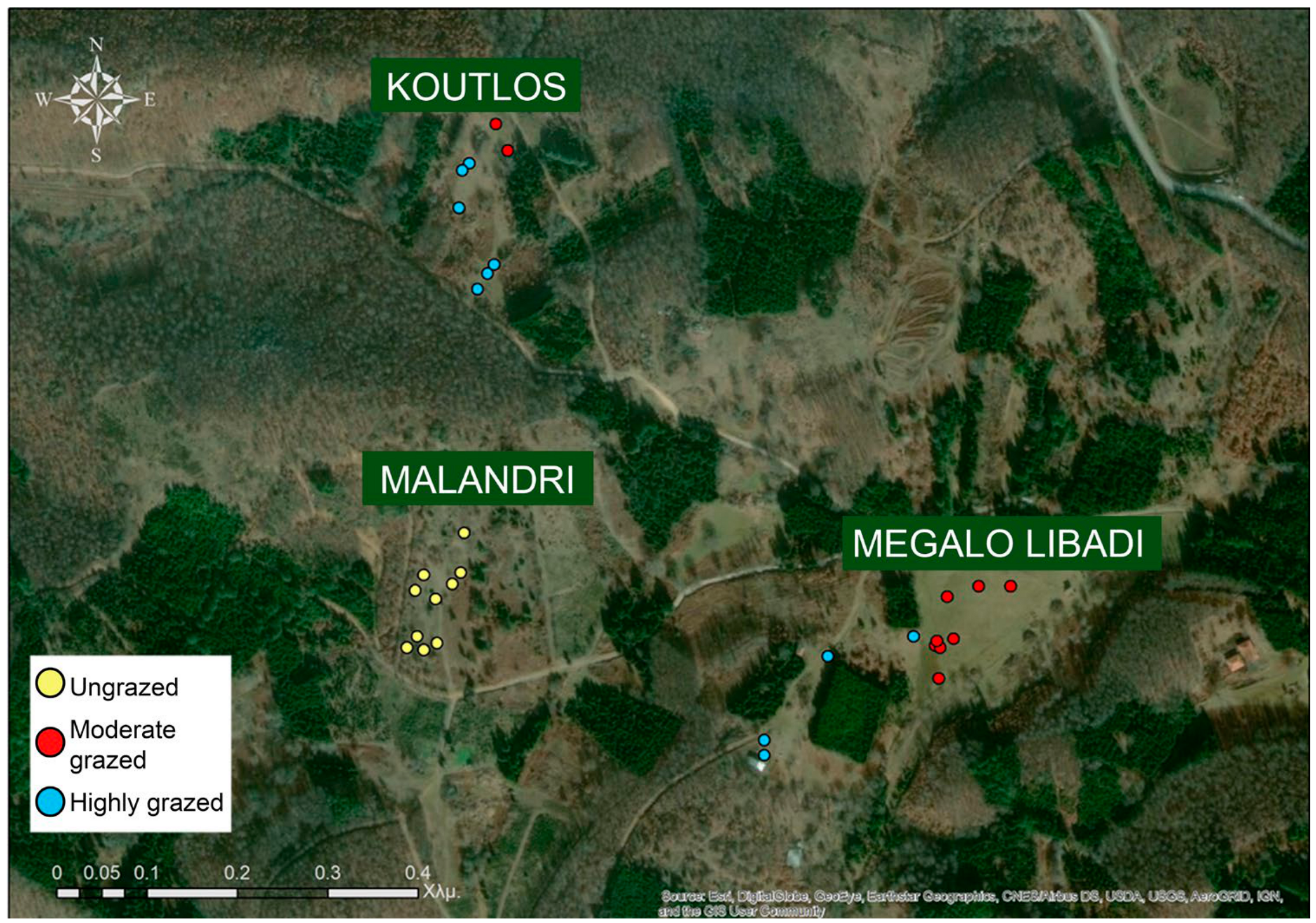
2.1. Study Area
2.2. Vegetation Cover and Composition
2.3. Floristic Diversity
2.4. Functional Traits of Plant Species
2.5. Statistical Analysis
3. Results
3.1. Plant Cover and Species Composition
3.2. Floristic Diversity
3.3. Functional Traits of Plant Species
3.3.1. Specific Leaf Area, Leaf Dry Matter Content, and Shoot Dry Matter Content
3.3.2. Vegetative and Reproductive Height
3.3.3. Leaf Nitrogen and Phosphorus Concentration
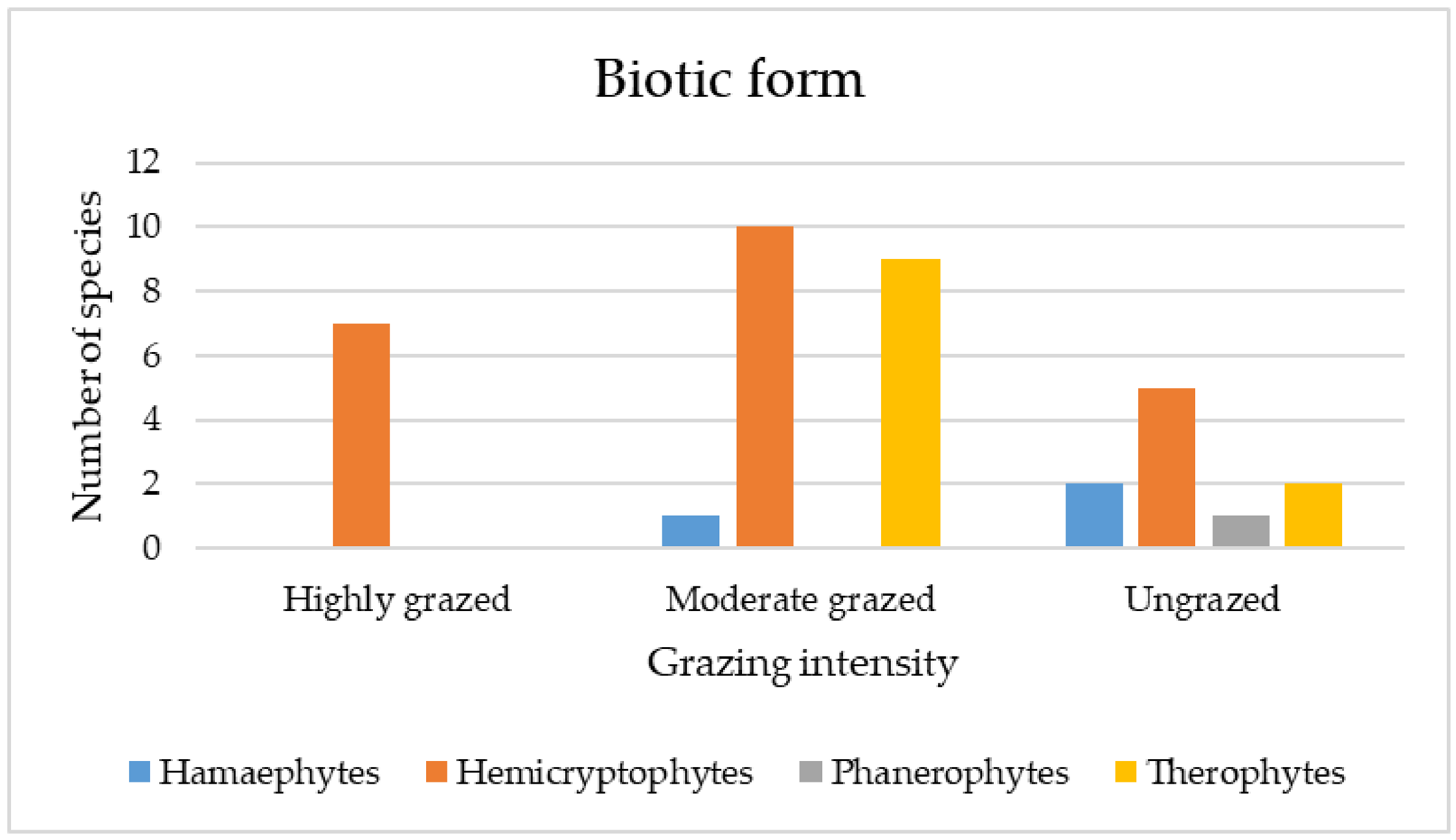
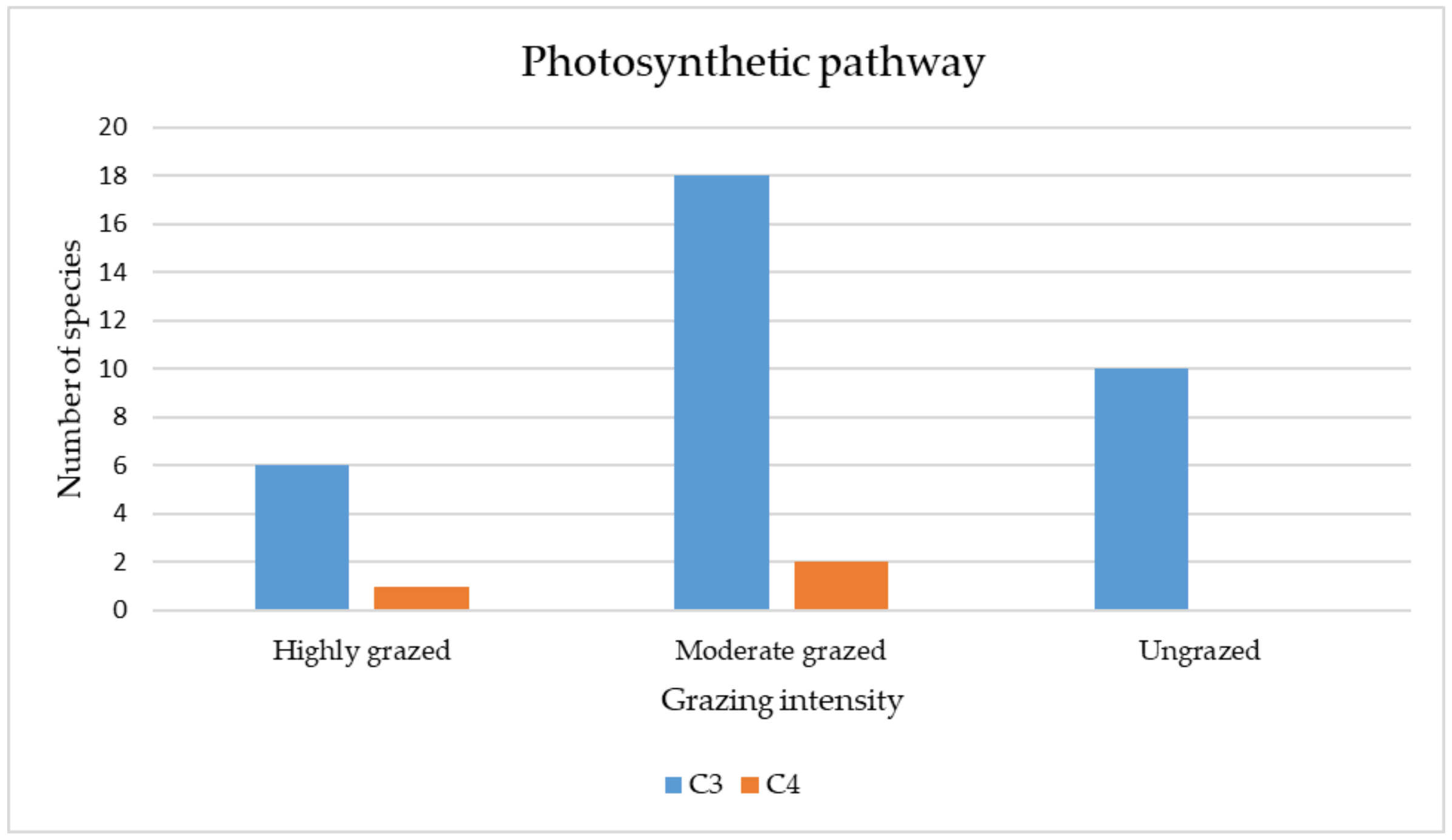
3.3.4. Qualitative (Categorical) Traits
4. Discussion
4.1. Plant Cover and Species Composition
4.2. Floristic Diversity
4.3. Functional Traits of Plant Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Ecology: Global Desertification: Building a Science for Dryland Development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Estell, R.E.; Havstad, K.M.; Cibils, A.F.; Fredrickson, E.L.; Anderson, D.M.; Schrader, T.S.; James, D.K. Increasing Shrub Use by Livestock in a World with Less Grass. Rangel. Ecol. Manag. 2012, 65, 553–562. [Google Scholar] [CrossRef]
- Papanastasis, V.P.; Noitsakis, V.I. Rangelands Ecology; Giahoudis–Giapoulis Publications: Thessaloniki, Greece, 1992; p. 244. (In Greek) [Google Scholar]
- Habel; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M.; Habel, J.C.; Dengler, J.; Janišová, M.; Török, P. European Grassland Ecosystems: Threatened Hotspots of Biodiversity. Biodivers Conserv 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Wallis de Vries, M.F.; van Swaay, C.A.M. Grasslands as Habitats for Butterflies in Europe. In Grasslands in Europe of Highnature Value; Veen, P., Jefferson, R., de Smitt, J., van der Straaten, J., Eds.; KNNV Publishing: Leiden, The Netherlands, 2009; pp. 26–34. [Google Scholar]
- Poschlod, P.; WallisDeVries, M.F. The Historical and Socioeconomic Perspective of Calcareous Grasslands—Lessons from the Distant and Recent Past. Biol. Conserv. 2002, 104, 361–376. [Google Scholar] [CrossRef]
- Butaye, J.; Adriaens, D.; Honnay, O. Conservation and Restoration of Calcareous Grasslands: A Concise Review of the Effects of Fragmentation and Management on Plant Species. Biotechnol. Agron. Soc. Environ. 2005, 9, 111–118. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.D.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.; Viedma, O.; Arianoutsou, M.; Curt, T.; Koutsias, N.; Rigolot, E.; Barbati, A.; Corona, P.; Vaz, P.; Xanthopoulos, G.; et al. Landscape—Wildfire Interactions in Southern Europe: Implications for Landscape Management. J. Environ. Manag. 2011, 92, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulos, A.P.; Arabatzis, G.; Abraham, E.M.; Parissi, Z.M. Threats to Mediterranean Rangelands: A Case Study Based on the Views of Citizens in the Viotia Prefecture, Greece. J. Environ. Manag. 2013, 129, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Huston, M. A General Hypothesis of Species Diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Kondoh, M. Unifying the Relationships of Species Richness to Productivity and Disturbance. Proc. R. Soc. B Biol. Sci. 2001, 268, 269–271. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Sala, O.E.; Lauenroth, W.K. A Generalized Model of the Effects of Grazing by Large Herbivores on Grassland Community Structure. Am. Nat. 1988, 132, 87–106. [Google Scholar] [CrossRef]
- Mackey, R.L.; Currie, D.J. The Diversity-Disturbance Relationship: Is It Generally Strong and Peaked? Ecology 2001, 82, 3479. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Noy-Meir, I.; Díaz, S. Grazing Effects on Rangeland Diversity: A Synthesis of Contemporary Models. Ecol. Appl. 2005, 15, 757–773. [Google Scholar] [CrossRef]
- Pulungan, M.A.; Suzuki, S.; Gavina, M.K.A. Author Correction: Grazing Enhances Species Diversity in Grassland Communities. Sci. Rep. 2020, 10, 12519. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mahecha, M.D.; Migliavacca, M.; van der Plas, F.; Benavides, R.; Ratcliffe, S.; Kattge, J.; Richter, R.; Musavi, T.; Baeten, L.; et al. Inferring Plant Functional Diversity from Space: The Potential of Sentinel-2. Remote Sens. Environ. 2019, 233, 111368. [Google Scholar] [CrossRef]
- Aguiar, M.R.; Parvelo, J.M.; Sala, O.E.; Lauenroth, W.K. Ecosystem Responses to Changes in Plant Functional Type Composition: An Example from the Patagonian Steppe. J. Veg. Sci. 1996, 7, 381–390. [Google Scholar] [CrossRef]
- Gitay, H.; Noble, I.R. Plant Functional Types; Smith, T.M., Shugart, H.H., Woodward, F.I., Eds.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Weiher, E.; van der Werf, A.; Thompson, K.; Roderick, M.; Garnier, E.; Eriksson, O. Challenging Theophrastus: A Common Core List of Plant Traits for Functional Ecology. J. Veg. Sci. 1999, 10, 609–620. [Google Scholar] [CrossRef]
- De Bello, F.; Leps, J.; Sebastia, M.T. Grazing Effects on the Species-Area Relationship Variation along a Climatic Gradient in NE Spain. J. Veg. Sci. 2007, 18, 25–34. [Google Scholar]
- Díaz, S.; Lavorel, S.; McIntyre, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant Trait Responses to Grazing—A Global Synthesis. Glob. Chang. Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Gubsch, M.; Buchmann, N.; Schmid, B.; Schulze, E.D.; Lipowsky, A.; Roscher, C. Differential Effects of Plant Diversity on Functional Trait Variation of Grass Species. Ann. Bot. 2011, 107, 157–169. [Google Scholar] [CrossRef]
- Salem, H.B.; Papachristou, T.G. Methodology for Studying Vegetation of Grazing Lands and Determination of Grazing Animal Responses. Options Méditerranéennes Sér. Sémin. Méditerranéens 2005, 305, 291–305. [Google Scholar]
- Heady, H.F. Rangeland Management; McGraw-Hill: New York, NY, USA, 1975. [Google Scholar]
- Cook, C.W.; Stubbendieck, J. Range Research: Basic Problems and Techniques; Society for Range Management: Littleton, CO, USA, 1986. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burgens, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 1st ed.; Cambridge University Press: Cambridge, UK, 1968; Volume 2. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burgens, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Thomas, Cambridge University Press: Cambridge, UK, 1972; Volume 3. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burgens, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Thomas, Cambridge University Press: Cambridge, UK, 1976; Volume 4. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burgens, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 1st ed.; Cambridge University Press: Cambridge, UK, 1980; Volume 5. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burgens, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1. [Google Scholar]
- Strid, K.; Tan, K. Flora Hellenica; Koeltz Scientific Books: Koenigstein, Germany, 1997; Volume 1. [Google Scholar]
- Strid, K.; Tan, K. Flora Hellenica; Koeltz Scientific Books: Koenigstein, Germany, 2002; Volume 2. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece; Turland, N.J., Ed.; Botanic Garden and Botanical Museum: Berlin, Germany, 2013; ISBN 978-3-921800-88-1. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Lezama, F.; Baeza, S.; Altesor, A.; Cesa, A.; Chaneton, E.J.; Paruelo, J.M. Variation of Grazing-Induced Vegetation Changes across a Large-Scale Productivity Gradient. J. Veg. Sci. 2014, 25, 8–21. [Google Scholar] [CrossRef]
- Werner, W.; Paulissen, D. Program VEGBASE database of indicator values of vascular plants after ELLENBERG and their evaluation to the personal computer. In Indicator Values of Plants in Central Europe. Scripta Geobotanica XVIII; Ellenberg, H., Weber, H.E., Duell, R., Wirth, V., Werner, W., Paulissen, D., Eds.; Goltze Publisher: Göttingen, Germany, 1992; pp. 238–248. [Google Scholar]
- Gusmeroli, F.; Della Marianna, G.; Puccio, C.; Corti, M.; Maggioni, L. Foraging Indices of Woody and Herbaceous Alpine Species for Goat Livestock. Quad. SoZooAlp 2007, 4, 73–82. [Google Scholar]
- Whitakker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.T.; Morgan, H.D.; Heijden, M.G.A.V.D.; et al. A Handbook of Protocols for Standardised and Easy Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 21.0; IBM Corporation: Armonk, NY, USA, 2012. [Google Scholar]
- Fisher, R.A. The Design of Experiments, 9th ed.; McMillan Publishers: New York, NY, USA, 1935. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.2-0; R Core Team: Vienna, Austria, 2011. [Google Scholar]
- Souther, S.; Loeser, M.; Crews, T.E.; Sisk, T. Complex Response of Vegetation to Grazing Suggests Need for Coordinated, Landscape-Level Approaches to Grazing Management. Glob. Ecol. Conserv. 2019, 20, e00770. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Poore, A.G.B.; Ruiz-Colmenero, M.; Letnic, M.; Soliveres, S. Ecosystem Structure, Function, and Composition in Rangelands Are Negatively Affected by Livestock Grazing. Ecol. Appl. 2016, 26, 1273–1283. [Google Scholar] [CrossRef]
- Biondini, M.E.; Patton, B.D.; Nyren, P.E. Grazing Intensity and Ecosystem Processes in a Northern Mixed-Grass Prairie, USA. Ecol. Appl. 1998, 8, 469–479. [Google Scholar] [CrossRef]
- Arévalo, J.R.; de Nascimento, L.; Fernández-Lugo, S.; Mata, J.; Bermejo, L. Grazing Effects on Species Composition in Different Vegetation Types (La Palma, Canary Islands). Acta Oecologica 2011, 37, 230–238. [Google Scholar] [CrossRef]
- Aharon, H.; Henkin, Z.; Ungar, E.D.; Kababya, D.; Baram, H.; Perevolotsky, A. Foraging Behaviour of the Newly Introduced Boer Goat Breed in a Mediterranean Woodland: A Research Observation. Small Rumin. Res. 2007, 69, 144–153. [Google Scholar] [CrossRef]
- Aich, A.E.; Assouli, N.E.; Fathi, A.; Morand-Fehr, P.; Bourbouze, A. Ingestive Behavior of Goats Grazing in the Southwestern Argan (Argania Spinosa) Forest of Morocco. Small Rumin. Res. 2007, 70, 248–256. [Google Scholar] [CrossRef]
- Osoro, K.; Ferreira, L.M.M.; García, U.; Jáuregui, B.M.; Martínez, A.; García, R.R.; Celaya, R. Diet Selection and Performance of Sheep and Goats Grazing on Different Heathland Vegetation Types. Small Rumin. Res. 2013, 109, 119–127. [Google Scholar] [CrossRef]
- Manousidis, T.; Kyriazopoulos, A.P.; Papageorgiou, A.C.; Parissi, Z.M.; Abraham, E.M.; Koutroubas, S.D.; Abas, Z. Diet Selection of Grazing Goats in an Oak Silvopastoral System in Northern Greece Using a Markov Chain Monte Carlo Simulation. Options Méditerranéennes Ser. Mediterr. Semin. 2016, 82, 79–82. [Google Scholar]
- Perevolotsky, A.; Seligman, N.G. Role of Grazing in Mediterranean Rangeland Ecosystems Inversion of a Paradigm. BioScience 1998, 48, 1007–1117. [Google Scholar] [CrossRef]
- Anderson, P.M.L.; Hoffman, M.T. The Impacts of Sustained Heavy Grazing on Plant Diversity and Composition in Lowland and Upland Habitats across the Kamiesberg Mountain Range in the Succulent Karoo, South Africa. J. Arid Environ. 2007, 70, 686–700. [Google Scholar] [CrossRef]
- Allred, B.W.; Fuhlendorf, S.D.; Hamilton, R.G. The Role of Herbivores in Great Plains Conservation: Comparative Ecology of Bison and Cattle. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Altesor, A.; Piñeiro, G.; Lezama, F.; Jackson, R.B.; Sarasola, M.; Paruelo, J.M. Ecosystem Changes Associated with Grazing in Subhumid South American Grasslands. J. Veg. Sci. 2006, 17, 323–332. [Google Scholar] [CrossRef]
- Scott-Shaw, R.; Morris, C.D. Grazing Depletes Forb Species Diversity in the Mesic Grasslands Of. Afr. J. Range Forage Sci. 2015, 32, 21–31. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, X.; Zhou, X.; Lv, P.; Lian, J.; Yue, X. Long-Term Grazing Effects on Vegetation Characteristics and Soil Properties in a Semiarid Grassland, Northern China. Environ. Monit. Assess. 2017, 189, 216. [Google Scholar] [CrossRef]
- Sternberg, M.; Golodets, C.; Gutman, M.; Perevolotsky, A.; Ungar, E.D.; Kigel, J.; Henkin, Z. Testing the Limits of Resistance: A 19-Year Study of Mediterranean Grassland Response to Grazing Regimes. Glob. Chang. Biol. 2015, 21, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Koerner, S.E.; Collins, S.L. Interactive Effects of Grazing, Drought, and Fire on Grassland Plant Communities in North America and South Africa. Ecology 2014, 95, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.S.; Wesche, K.; Chen, D.D.; Zhang, S.H.; Wu, G.L.; Du, G.Z.; Comerford, N.B. Grazing Depresses Soil Carbon Storage through Changing Plant Biomass and Composition in a Tibetan Alpine Meadow. Plant Soil Environ. 2011, 57, 271–278. [Google Scholar] [CrossRef]
- Abraham, E.M.; Aftzalanidou, A.; Ganopoulos, I.; Osathanunkul, M.; Xanthopoulou, A.; Avramidou, E.; Sarrou, E.; Aravanopoulos, F.; Madesis, P. Genetic Diversity of Thymus Sibthorpii Bentham in Mountainous Natural Grasslands of Northern Greece as Related to Local Factors and Plant Community Structure. Ind. Crops Prod. 2018, 111, 651–659. [Google Scholar] [CrossRef]
- Yates, C.J.; Norton, D.A.; Hobbs, R.J. Grazing Effects on Plant Cover, Soil and Microclimate in Fragmented Woodlands in South-Western Australia: Implications for Restoration. Austral Ecol. 2000, 25, 36–47. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Contrasting Responses of Plant and Insect Diversity to Variation in Grazing Intensity. Biol. Conserv. 2002, 106, 293–302. [Google Scholar] [CrossRef]
- Benot, M.L.; Morvan-Bertrand, A.; Mony, C.; Huet, J.; Sulmon, C.; Decau, M.L.; Prud’homme, M.P.; Bonis, A. Grazing Intensity Modulates Carbohydrate Storage Pattern in Five Grass Species from Temperate Grasslands. Acta Oecol. 2019, 95, 108–115. [Google Scholar] [CrossRef]
- Hendrickson, B.J.; Olson, B. Chapter 4: Understanding Plant Response to Grazing Targeted Grazing: Section I. In Targeted Grazing: A Natural Approach to Vegetation Management and Landscape Enhancement; American Sheep Industry Association: Washington, DC, USA, 2006; pp. 32–39. [Google Scholar]
- Pavlů, V.; Hejcman, M.; Pavlů, L.; Gaisler, J. Effect of Rotational and Continuous Grazing on Vegetation of an Upland Grassland in the Jizerské Hory Mts., Czech Republic. Folia Geobot. 2003, 38, 21–34. [Google Scholar] [CrossRef]
- Hejcman, M.; Žáková, I.; Bílek, M.; Bendová, P.; Hejcmanová, P.; Pavlů, V.; Stránská, M. Sward Structure and Diet Selection after Sheep Introduction on Abandoned Grassland in the Giant Mts, Czech Republic. Biologia 2008, 63, 506–514. [Google Scholar] [CrossRef]
- Török, P.; Penksza, K.; Tóth, E.; Kelemen, A.; Sonkoly, J.; Tóthmérész, B. Vegetation Type and Grazing Intensity Jointly Shape Grazing Effects on Grassland Biodiversity. Ecol. Evol. 2018, 8, 10326–10335. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.S.; Stenton-Dozey, J.; Plew, D.R.; Fang, J.; Gall, M. An Ecosystem Model for Optimising Production in Integrated Multitrophic Aquaculture Systems. Ecol. Model. 2012, 246, 34–46. [Google Scholar] [CrossRef]
- Škornik, S.; Vidrih, M.; Kaligarič, M. The Effect of Grazing Pressure on Species Richness, Composition and Productivity in North Adriatic Karst Pastures. Plant Biosyst. 2010, 144, 355–364. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Han, G.; Schellenberg, M.P.; Wu, Q.; Gu, C. Grazing Induced Changes in Plant Diversity Is a Critical Factor Controlling Grassland Productivity in the Desert Steppe, Northern China. Agric. Ecosyst. Environ. 2018, 265, 73–83. [Google Scholar] [CrossRef]
- Haarmeyer, D.H.; Schmiedel, U.; Dengler, J.; Bösing, B.M. How Does Grazing Intensity Affect Different Vegetation Types in Arid Succulent Karoo, South Africa? Implications for Conservation Management. Biol. Conserv. 2010, 143, 588–596. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Y. A Global Meta-Analyses of the Response of Multi-Taxa Diversity to Grazing Intensity in Grasslands. Environ. Res. Lett. 2019, 14, 114003. [Google Scholar] [CrossRef]
- Song, S.; Zhu, J.; Zheng, T.; Tang, Z.; Zang, Z.; Ji, C.; Sheng, Z.; Zhu, J. Long-Term Grazing Exclusion Reduces Species Diversity but Increases Community Heterogeneity in an Alpine Grassland. Front. Ecol. Evol. 2020, 8, 66. [Google Scholar] [CrossRef]
- HegedüŠová, K.; Senko, D. Successional Changes of Dry Grasslands in Southwestern Slovakia after 46 Years of Abandonment. Plant Biosyst. 2011, 145, 666–687. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Li, C.; Seastedt, T.R.; Liang, C.; Wang, L.; Sun, W.; Liang, M.; Li, Y. Feces Nitrogen Release Induced by Different Large Herbivores in a Dry Grassland. Ecol. Appl. 2018, 28, 201–211. [Google Scholar] [CrossRef]
- Xiong, D.; Shi, P.; Zhang, X.; Zou, C.B. Effects of Grazing Exclusion on Carbon Sequestration and Plant Diversity in Grasslands of China—A Meta-Analysis. Ecol. Eng. 2016, 94, 647–655. [Google Scholar] [CrossRef]
- Chaneton, E.J.; Perelman, S.B.; Omacini, M.; León, R.J.C. Grazing, Environmental Heterogeneity, and Alien Plant Invasions in Temperate Pampa Grasslands. Biol. Invasions 2002, 4, 7–24. [Google Scholar] [CrossRef]
- Altesor, A.; Oesterheld, M.M.; Leoni, E.; Lezama, F.; Rodríguez, C. Effect of Grazing on Community Structure and Productivity of a Uruguayan Grassland. Plant Ecol. 2005, 179, 83–91. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; John Wiley & Sons: New York, NY, USA, 1979. [Google Scholar]
- Horn, H. The Ecology of Secondary Succession. Annu. Rev. Ecol. Systematics 1974, 5, 25–37. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Gao, J.; Carmel, Y. Can the Intermediate Disturbance Hypothesis Explain Grazing–Diversity Relations at a Global Scale? Oikos 2020, 129, 493–502. [Google Scholar] [CrossRef]
- Moradi, E.; Heshmati, G.A.; Ghilishli, F.; Mirdeilami, S.Z.; Pessarakli, M. Grazing Intensity and Environmental Factors Effects on Species Composition and Diversity in Rangelands of Iran. J. Plant Nutr. 2016, 39, 2002–2014. [Google Scholar] [CrossRef]
- Yan, R.; Xin, X.; Yan, Y.; Wang, X.; Zhang, B.; Yang, G.; Liu, S.; Deng, Y.; Li, L. Impacts of Differing Grazing Rates on Canopy Structure and Species Composition in Hulunber Meadow Steppe. Rangel. Ecol. Manag. 2012, 68, 54–64. [Google Scholar] [CrossRef]
- Kang, X.; Qi, W.; Knops, J.M.H.; Luo, S.; Jia, P.; Du, G.; Zhang, A.; Li, W.; Chen, H. Climate Factors Impact Different Facets of Grassland Biodiversity Both Directly and Indirectly through Soil Conditions. Landsc. Ecol. 2020, 38, 327–340. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Ogaya, R.; Estiarte, M.; Peñuelas, J. Effects of Decadal Experimental Drought and Climate Extremes on Vegetation Growth in Mediterranean Forests and Shrublands. J. Veg. Sci. 2020, 31, 768–779. [Google Scholar] [CrossRef]
- Li, F.; Yan, Y.; Zhang, J.; Zhang, Q.; Niu, J. Taxonomic, Functional, and Phylogenetic Beta Diversity in the Inner Mongolia Grassland. Glob. Ecol. Conserv. 2021, 28, e01634. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, X. Are N, P, and N:P Stoichiometry Limiting Grazing Exclusion Effects on Vegetation Biomass and Biodiversity in Alpine Grassland? Glob. Ecol. Conserv. 2020, 24, e01315. [Google Scholar] [CrossRef]
- Pueyo, Y.; Alados, C.L.; Ferrer-Benimeli, C. Is the Analysis of Plant Community Structure Better than Common Species-Diversity Indices for Assessing the Effects of Livestock Grazing on a Mediterranean Arid Ecosystem? J. Arid Environ. 2006, 64, 698–712. [Google Scholar] [CrossRef]
- Papadimitriou, M. Characteristics and Functional Groups of Plants in Relation to the Evolution of Vegetation in Grassland Landscapes. PhD Thesis, AUTH, Thessaloniki, Greece, 2011. [Google Scholar]
- Kahmen, S.; Poschlod, P. Plant Functional Trait Responses to Grassland Succession over 25 Years. J. Veg. Sci. 2004, 15, 21–32. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Lu, X.; Wang, Y.; Bai, Y. Intraspecific Trait Variation Governs Grazing-Induced Shifts in Plant Community above- and below-Ground Functional Trait Composition. Agric. Ecosyst. Environ. 2023, 346, 108357. [Google Scholar] [CrossRef]
- Louault, F.; Pillar, V.D.; Aufrère, J.; Garnier, E.; Soussana, J.-F. Plant Traits and Functional Types in Response to Reduced Disturbance in a Semi-natural Grassland. J. Veg. Sci. 2005, 16, 151–160. [Google Scholar] [CrossRef]
- Cruz, P.; Quadros, F.L.F.D.; Theau, J.P.; Frizzo, A.; Jouany, C.; Duru, M.; Carvalho, P.C.F. Leaf Traits as Functional Descriptors of the Intensity of Continuous Grazing in Native Grasslands in the South of Brazil. Rangel. Ecol. Manag. 2010, 63, 350–358. [Google Scholar] [CrossRef]
- Jäschke, Y.; Heberling, G.; Wesche, K. Environmental Controls Override Grazing Effects on Plant Functional Traits in Tibetan Rangelands. Funct. Ecol. 2020, 34, 747–760. [Google Scholar] [CrossRef]
- Peco, B.; Pablos, I.D.; Traba, J.; Levassor, C. The Effect of Grazing Abandonment on Species Composition and Functional Traits: The Case of Dehesa Grasslands. Basic Appl. Ecol. 2005, 6, 175–183. [Google Scholar] [CrossRef]
- Moog, D.; Kahmen, S.; Poschlod, P. Application of CSR- and LHS-Strategies for the Distinction of Differently Managed Grasslands. Basic Appl. Ecol. 2005, 6, 133–143. [Google Scholar] [CrossRef]
- Poorter, H.; Pepin, S.; Rijkers, T.; Jong, Y.D.; Evans, J.R.; Körner, C. Construction Costs, Chemical Composition and Payback Time of High- and Low-Irradiance Leaves. J. Exp. Bot. 2006, 57, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Streit, H.; Menezes, L.S.; Pillar, V.D.; Overbeck, G.E. Intraspecific Trait Variation of Grassland Species in Response to Grazing Depends on Resource Acquisition Strategy. J. Veg. Sci. 2022, 33, 13129. [Google Scholar] [CrossRef]
- Golodets, C.; Sternberg, M.; Kigel, J. A Community-level Test of the Leaf-height-seed Ecology Strategy Scheme in Relation to Grazing Conditions. J. Veg. Sci. 2009, 20, 392–402. [Google Scholar] [CrossRef]
- Castro, H.; Lehsten, V.; Lavorel, S.; Freitas, H. Functional Response Traits in Relation to Land Use Change in the Montado. Agric. Ecosyst. Environ. 2010, 137, 183–191. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Li, Z.; Liu, H.; Wang, L.; Wang, W.; Wang, Y.; Liang, C. Single Grazing Is More Detrimental to Grasslands Than Mixed Grazing: Evidence From the Response of Functional Traits of Dominant Plants to Grazing Systems. Front. Ecol. Evol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Lang, B.; Ahlborn, J.; Oyunbileg, M.; Geiger, A.; Wehrden, H.V.; Wesche, K.; Oyuntsetseg, B.; Römermann, C. Grazing Effects on Intraspecific Trait Variability Vary with Changing Precipitation Patterns in Mongolian Rangelands. Ecol. Evol. 2019, 10, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, S.; Hejda, M.; Ejtehadi, H.; Farzam, M.; Memariani, F.; Pyšek, P. Effects of Livestock Grazing on Soil, Plant Functional Diversity, and Ecological Traits Vary between Regions with Different Climates in Northeastern Iran. Ecol. Evol. 2019, 9, 8225–8237. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley and Sons: Chichester, UK, 2001. [Google Scholar]
- Parissi, Z.M.; Papaioannou, A.; Abraham, E.M.; Kyriazopoulos, A.P.; Sklavou, P.; Tsiouvaras, C.N. Influence of Combined Grazing by Wild Boar and Small Ruminant on Soil and Plant Nutrient Contents in a Coppice Oak Forest. J. Plant Nutr. Soil Sci. 2014, 177, 783–791. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Bodegom, P.M.V.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A Global Study of Relationships between Leaf Traits, Climate and Soil Measures of Nutrient Fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Klimes, L. Basic and Applied Ecology Life-Forms and Clonality of Vascular Plants along an Altitudinal Gradient in E Ladakh (NW Himalayas)*. Basic Appl. Ecol. 2003, 328, 317–328. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer Nature: Cham, Switzerland, 2021; ISBN 978-3-030-59538-8. [Google Scholar]
- Bonet, A.; Pausas, J.G. Mediterranean Basin: Patterns and Processes in Semiarid Southeast Spain. In Old Fields: Dynamics and Restoration of Abandoned Farmland; Chapter 13; Cramer, V.A., Hobs, R.J., Eds.; Island Press: Washington, DC, USA, 2006; pp. 247–264. [Google Scholar]
- Lavorel, S.; Díaz, S.; Cornelissen, J.H.C.; Garnier, E.; Harrison, S.P.; McIntyre, S.; Pausas, J.G.; Pérez-Harguindeguy, N.; Roumet, C.; Urcelay, C. The Plant Traits That Drive Ecosystems: Evidence from Three Continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar]
- Karakosta, C. Patterns of Grassland Vegetation Succession in Abandoned Fields. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2012. [Google Scholar]
- Papachristou, T.G. Foraging Behaviour of Goats and Sheep on Mediterranean Kermes Oak Shrublands. Small Rumin. Res. 1997, 24, 85–93. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using Plant Functional Traits to Understand Ecological Processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Saatkamp, A.; Römermann, C.; Dutoit, T. Plant Functional Traits Show Non-Linear Response to Grazing. Folia Geobot. 2010, 45, 239–252. [Google Scholar] [CrossRef]
- Díaz, S.; Noy-Meir, I.; Cabido, M. Can Grazing Response of Herbaceous Plants Be Predicted from Simple Vegetative Traits? J. Appl. Ecol. 2001, 38, 497–508. [Google Scholar] [CrossRef]





| Grazing Intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | |||||||
| Category | Highly grazed | Moderate grazed | Ungrazed | Highly grazed | Moderate grazed | Ungrazed | Highly grazed | Moderate grazed | Ungrazed |
| Plant cover (%) | 90.9 b | 95.9 a 1 | 95.3 ab | 87.6 a | 91.1 a | 91.3 a 1 | 94.9 b | 98.4 a | 99 a 1 |
| Litter (%) | 5.8 a | 3.6 a | 4,2 a | 7.2 a | 6.4 a | 8.5 a | 4.1 a | 1.10 b | 1.0 b |
| Soil (%) | 3.3 a | 0.5 b | 0.5 b | 5.2 a | 2.50 ab | 0.20 b | 1.0 a | 0.5 ab | 0.0 b |
| Grazing Intensity | |||||||||
| 2013 | 2014 | 2015 | |||||||
| Functional groups | Highly grazed | Moderate grazed | Ungrazed | Highly grazed | Moderate grazed | Ungrazed | Highly grazed | Moderate grazed | Ungrazed |
| Grasses (%) | 63.53 a 1 | 64.00 a | 28.90 b | 59.15 a 1 | 54.76 a | 39.86 a | 69.88 a | 74.64 a 1 | 50.44 b |
| Legumes (%) | 16.05 a | 11.23 a | 7.50 a | 12.24 a | 8.44 b | 0.65 c | 17.65 a | 4.16 b | 1.72 b |
| Forbs (%) | 20.41 b | 23.17 b | 40.77 a | 24.61 a | 34.58 a | 34.19 a | 12.36 b | 19.36 ab | 28.46 a 1 |
| Woody (%) | 0.0 b | 1.60 b | 22.83 a | 0.0 b | 2.21 b | 25.29 a | 0.11 b | 1.84 b | 19.38 a 1 |
| ΔGEdc(M) 1 | ΔGEdc(G) | |
|---|---|---|
| Chrysopogon gryllus (L.) Trin. | 0.97 | −0.54 |
| Agrostis canina L. subsp. canina | −0.33 | −0.82 |
| Plantago lanceolate L. | 0.72 | 0.82 |
| Festuca ovina aggr. | 0.85 | 0.74 |
| Bromus hordeaceous L. | 0.94 | 0.63 |
| Trifolium repens L. | 0.98 | 0.83 |
| Cynodon dactylon (L.) Pers. | 1.00 | 1.00 |
| Lotus corniculatus L. | 0.15 | 0.96 |
| Lolium perenne L. | 1.00 | 1.00 |
| Anthoxanthum odoratum L. | −0.23 | −0.61 |
| Cynosurus cristatus L. | 1.00 | 1.00 |
| Year of Measurements | ||||
|---|---|---|---|---|
| Grazing intensity | 2013 | 2014 | 2015 | Means |
| Ungrazed | 3.1 | 2.8 | 3.6 | 3.2 |
| Moderate grazed | 4.7 | 5.0 | 4.9 | 4.9 |
| Highly grazed | 5.0 | 4.2 | 5.4 | 4.9 |
| Year of Measurements | ||||
|---|---|---|---|---|
| Grazing intensity | 2013 | 2014 | 2015 | Means |
| Ungrazed | 1.40 a | 1.46 a 1 | 1.62 a 1 | 1.49 a 1 |
| Moderate grazed | 1.78 b | 1.51 a | 1.87 b | 1.72 b |
| Highly grazed | 1.45 a | 1.50 a | 1.85 b | 1.60 b |
| Means | 1.56 b | 1.49 b | 1.78 a 1 | |
| Year of Measurements | ||||
|---|---|---|---|---|
| Grazing intensity | 2013 | 2014 | 2015 | Average |
| Ungrazed | 0.37 a | 0.33 a 1 | 0.27 a | 0.32 a 1 |
| Moderate grazed | 0.23 c | 0.26 b | 0.22 b | 0.24 b |
| Highly grazed | 0.28 b | 0.28 a | 0.21 b | 0.26 b |
| Year of Measurements | ||||
|---|---|---|---|---|
| Grazing intensity | 2013 | 2014 | 2015 | Average |
| Ungrazed | 0.72 a | 0.73 a 1 | 0.79 a 1 | 0.75 a 1 |
| Moderate grazed | 0.75 a | 0.72 a | 0.79 a | 0.75 a |
| Highly grazed | 0.80 b | 0.80 b | 0.82 b | 0.81 b |
| Average | 0.76 ab | 0.75 a 1 | 0.80 b | |
| Year of Measurement | ||||
|---|---|---|---|---|
| Grazing intensity | 2013 | 2014 | 2015 | Mean |
| Ungrazed | 7.48 (62) b 64.06 b | 7.95 (46) ab 51.45 b | 8.55 (44) b 80.75 b | 7.99 b 65.42 b |
| Moderate grazed | 11.40 (56) a 88.08 a | 8.83 (74) a 71.83 a | 11.28 (48) a 101.93 a | 10.50 a 87.28 a |
| Highly grazed | 6.45 (29) b 51.13 c | 6.85 (41) b 59.70 b | 9.70 (39) b 93.12 ab | 9.84 a 91.93 a |
| Mean | 8.44 b 67.76 b | 7.87 b 60.99 b | 9.84 a 91.93 a | |
| 2013 | Ungrazed | Moderate Grazed | Highly Grazed |
|---|---|---|---|
| Ungrazed | - | 0.396 | 0.22 |
| Moderate grazed | 0.396 | - | 0.392 |
| Highly grazed | 0.22 | 0.392 | - |
| 2014 | Ungrazed | Moderate Grazed | Highly Grazed |
| Ungrazed | - | 0.367 | 0.278 |
| Moderate grazed | 0.367 | - | 0.588 |
| Highly grazed | 0.278 | 0.588 | - |
| 2015 | Ungrazed | Moderate Grazed | Highly Grazed |
| Ungrazed | - | 0.397 | 0.338 |
| Moderate grazed | 0.397 | - | 0.631 |
| Highly grazed | 0.338 | 0.631 | - |
| Year of Measurements | |||
|---|---|---|---|
| Grazing intensity | 2013 | 2014 | 2015 |
| Ungrazed | 5.36 a | 5.69 a | 5.99 a |
| Moderate grazed | 4.94 a | 5.74 a | 5.18 a |
| Highly grazed | 3.53 b | 4.71 b | 4.09 b |
| Grazing Intensity | SLA | LDMC | StDMC |
|---|---|---|---|
| Ungrazed | 345.40 a | 65.66 b | 327.30 a |
| Moderate grazed | 220.74 b | 339.42 a | 352.24 a |
| Highly grazed | 225.55 b | 322.70 a | 344.67 a |
| Grazing Intensity | VH | RH |
|---|---|---|
| Ungrazed | 26.66 a | 41.70 a |
| Moderate grazed | 14.90 b | 38.84 a |
| Highly grazed | 2.19 b | 8.71 b |
| Grazing Intensity | N | LNC | LP |
|---|---|---|---|
| Ungrazed | 10 | 1.24 a | 0.58 a |
| Moderate grazed | 10 | 1.30 a | 0.51 a |
| Highly grazed | 10 | 1.70 a | 0.76 a |
| Mean | 10 | 1.35 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramidou, E.; Karamichali, I.; Tsiripidis, I.; Abraham, E.M. Diversity Levels under Different Grazing Intensities in Semi-Wet Grasslands. Land 2024, 13, 488. https://doi.org/10.3390/land13040488
Avramidou E, Karamichali I, Tsiripidis I, Abraham EM. Diversity Levels under Different Grazing Intensities in Semi-Wet Grasslands. Land. 2024; 13(4):488. https://doi.org/10.3390/land13040488
Chicago/Turabian StyleAvramidou, Eleni, Ioanna Karamichali, Ioannis Tsiripidis, and Eleni M. Abraham. 2024. "Diversity Levels under Different Grazing Intensities in Semi-Wet Grasslands" Land 13, no. 4: 488. https://doi.org/10.3390/land13040488
APA StyleAvramidou, E., Karamichali, I., Tsiripidis, I., & Abraham, E. M. (2024). Diversity Levels under Different Grazing Intensities in Semi-Wet Grasslands. Land, 13(4), 488. https://doi.org/10.3390/land13040488







