Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude
Abstract
1. Introduction
2. Materials and Methods
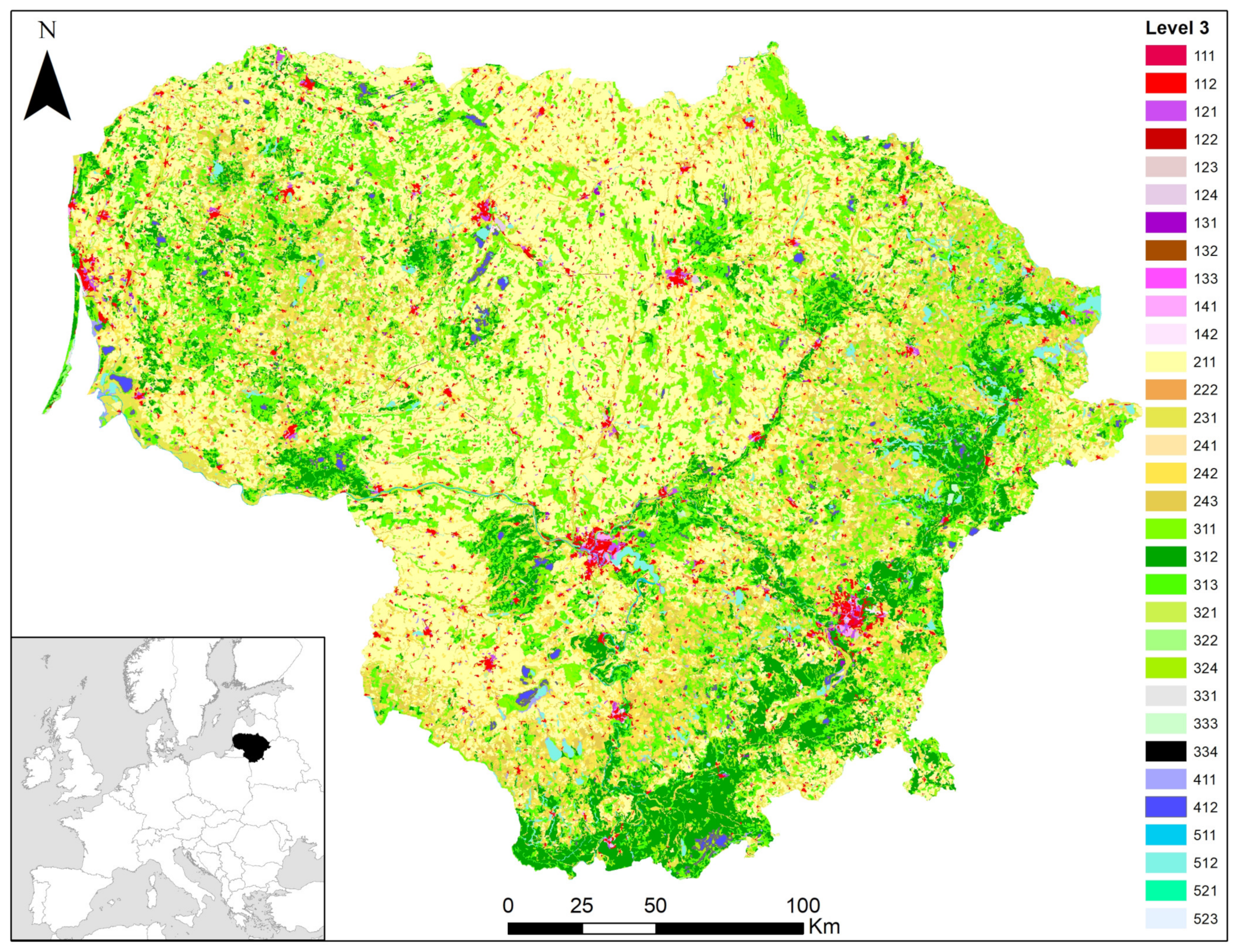
2.1. Small Mammal Habitats
- Forest (311–313 land cover classes according to the CORINE nomenclature, covering all forest ages, including clear-cuts);
- Scrub (324 land cover classes according to the CORINE nomenclature);
- Wetland (411 and 412 land cover classes according to the CORINE nomenclature);
- Grassland (231 and 321 land cover classes according to the CORINE nomenclature, including natural and sown grassland and pastures);
- Riparian habitats (no equivalent in the CORINE classification; we have included all habitats close to water, such as meadows, forests and wetlands, with the rule that they are within 50 m of the shore of a river, lake, or island);
- Mixed and fragmented habitats (no direct equivalent according to the CORINE classification; the most relevant is land use class 243, but also all cases where a catch line covered several different habitats, i.e., habitats were fragmented);
- Disturbed habitats (land use classes 131, 132, and 133 according to the CORINE nomenclature, dump sites, and breeding sites of great cormorants (Phalacrocorax carbo), i.e., sites characterized by natural or anthropogenic disturbance). We treated human-caused and biological disturbances as having similar effects on small mammals. Active landfills and recently burned areas were not used as trapping sites;
- Agricultural habitats (land cover classes 211, 222, 241 and 242 according to the CORINE nomenclature);
- Commensal habitats (111 and 112 land cover classes according to the CORINE nomenclature, but also farms, farmsteads, cattle sheds, individual houses, and similar places providing small mammals with food and shelter).
2.2. Small Mammal Trapping and Sample Size
2.3. Body Condition Index of Small Mammals
2.4. Data Analysis
3. Results
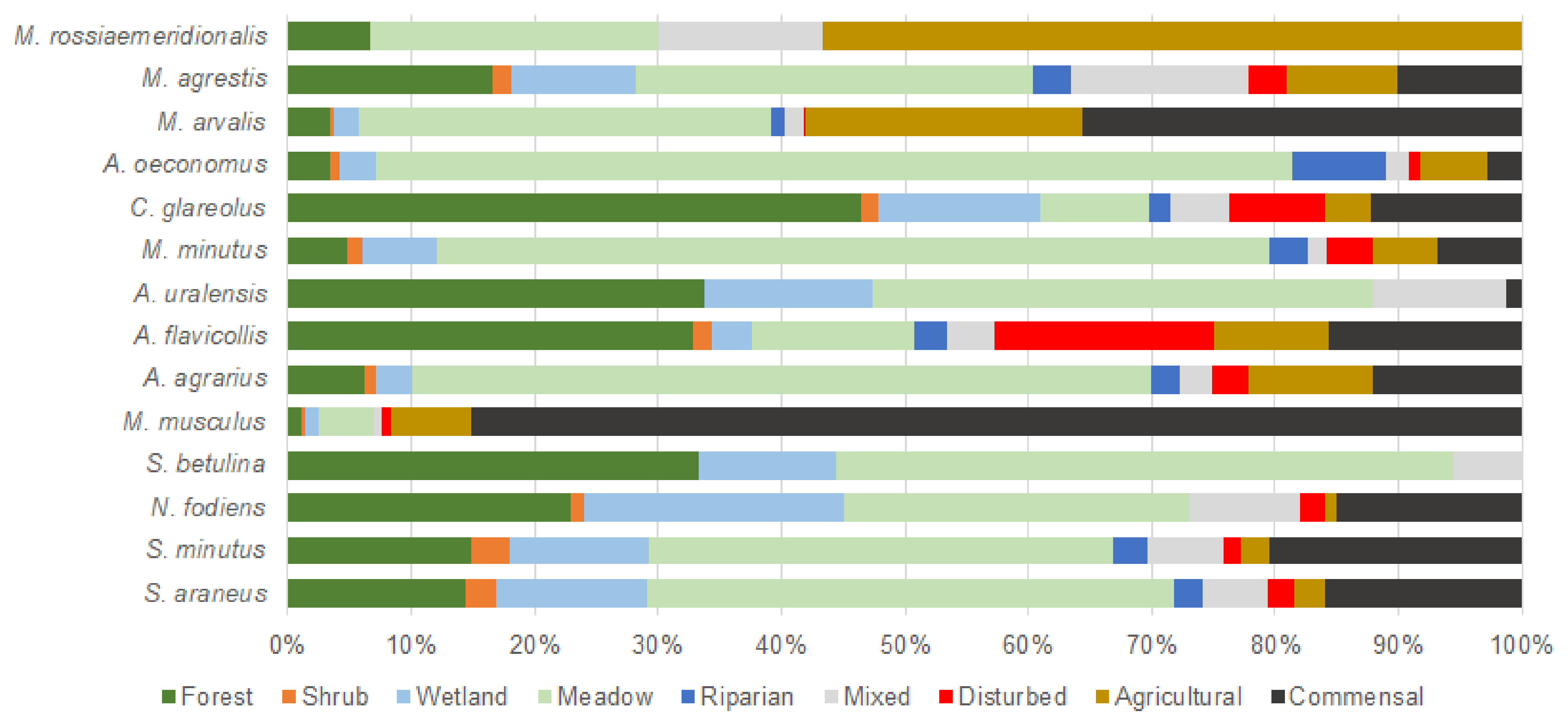
3.1. Habitat Use by Small Mammals Based on Their Catches
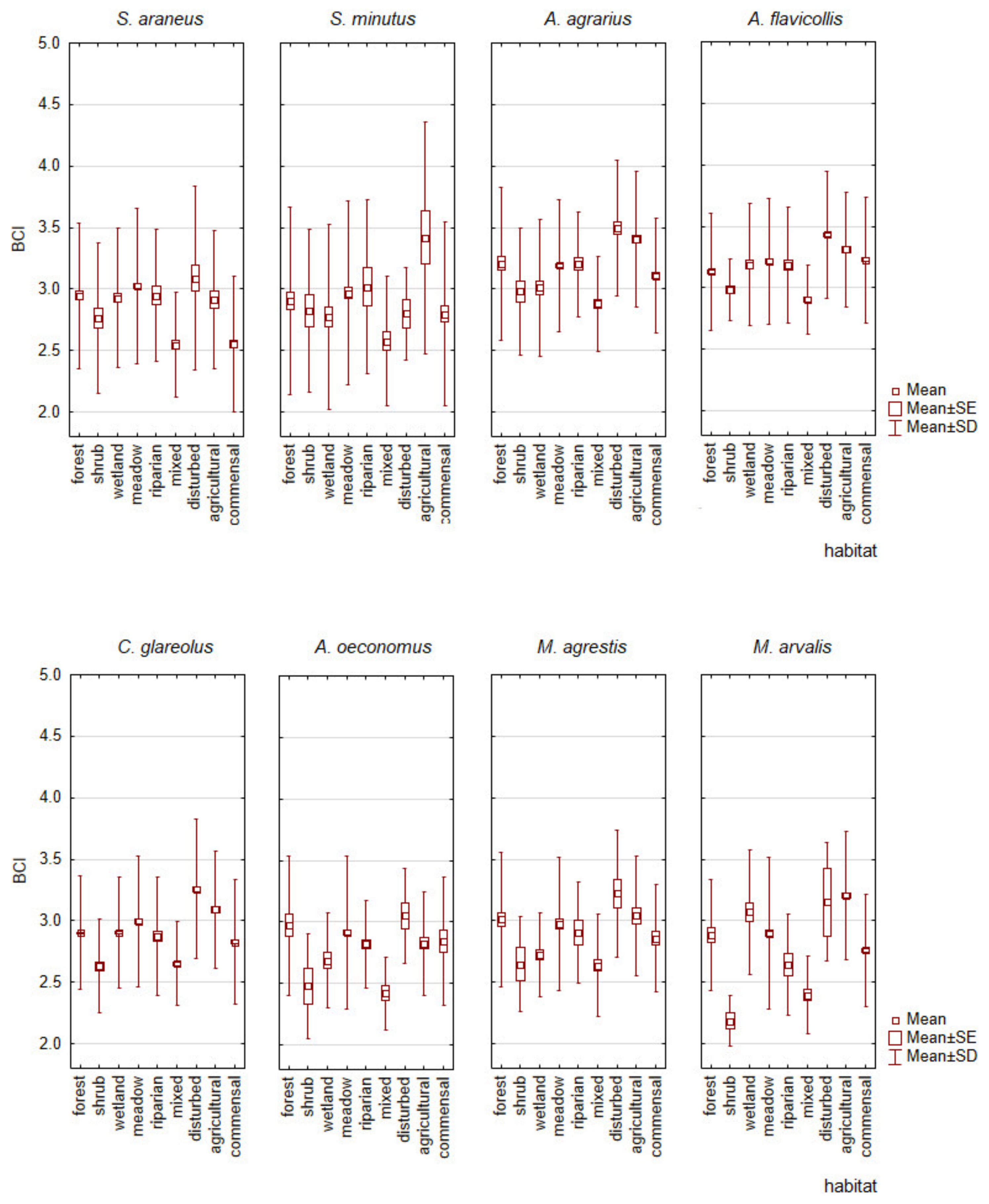
3.2. Analysis of Factors Affecting Body Condition in Relation to Habitat
3.3. The Influence of Habitat on the Body Condition Index of Small Mammal Species
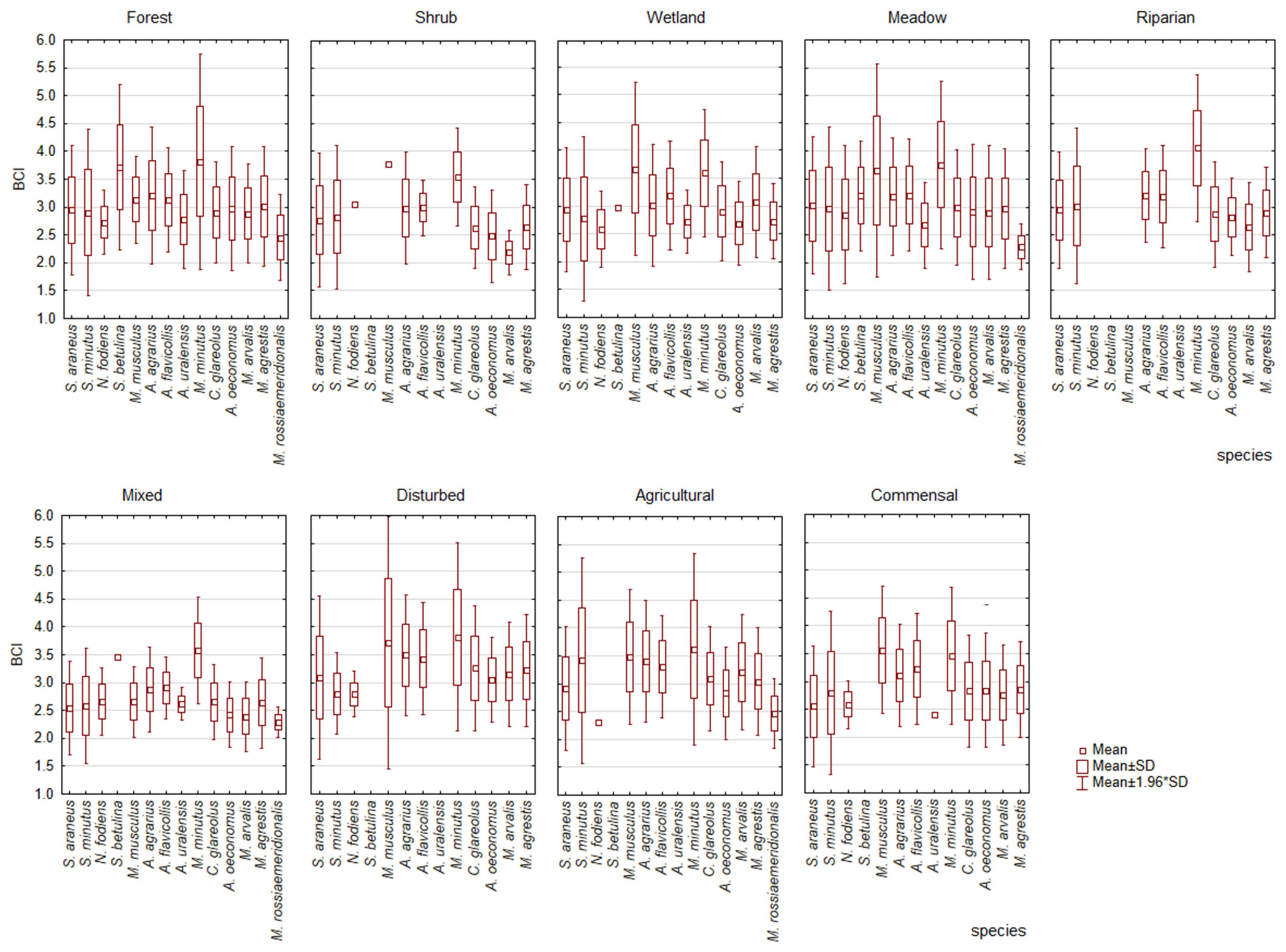
3.4. Variations in Body Condition Index of Small Mammal Species across Habitats
3.5. Body Condition Variability among Species in Different Habitats
4. Discussion
5. Conclusions
- We found that the representation of small mammal species was habitat-dependent, with certain species dominating certain habitats. The most suitable habitat was meadows, where 17 small mammal species were found, with nine species being the most abundant.
- The BCI variation across habitats was species-specific, indicating habitats and species with the highest and lowest average BCIs.
- No correlation was found between the proportion of a species in a habitat and its BCI. Higher BCI values were found to be characteristic of non-dominant species.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rondinini, C.; Di Marco, M.; Chiozza, F.; Santulli, G.; Baisero, D.; Visconti, P.; Hoffmann, M.; Schipper, J.; Stuart, S.N.; Tognelli, M.F.; et al. Global habitat suitability models of terrestrial mammals. Philos. Trans. R. Soc. B. 2011, 366, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Mitchell-Jones, A.J.; Mitchell, J.; Amori, G.; Bogdanowicz, W.; Kryštufek, B.; Reijnders, P.H.; Spitzenberger, F.; Stubbe, M.; Thissen, J.; Vohralík, V.; et al. (Eds.) The Atlas of European Mammals; Academic Press: London, UK, 1999; pp. 1–484. [Google Scholar]
- Smith, R.J.; Verissimo, D.; Leader-Williams, N.; Cowling, R.M.; Knight, A.T. Let the locals lead. Nature 2009, 462, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Dahal, P.R.; Butchart, S.H.M.; Donald, P.F.; De Lamo, X.; Lesiv, M.; Kapos, V.; Rondinini, C.; Visconti, P. A global map of terrestrial habitat types. Sci. Data 2020, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Orrock, J.L.; Pagels, J.F.; McShea, W.J.; Harper, E.K. Predicting presence and abundance of a small mammal species: The effect of scale and resolution. Ecol. Appl. 2000, 10, 1356–1366. [Google Scholar] [CrossRef]
- Habitats Classification Scheme (Version 3.1). Available online: https://www.iucnredlist.org/resources/habitat-classification-scheme (accessed on 12 May 2024).
- CORINE Land Cover. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 12 May 2024).
- European Nature Information System. Available online: https://eunis.eea.europa.eu/ (accessed on 14 May 2024).
- Baisero, D.; Visconti, P.; Pacifici, M.; Cimatti, M.; Rondinini, C. Projected global loss of mammal habitat due to land-use and climate change. One Earth 2020, 2, 578–585. [Google Scholar] [CrossRef]
- Ecke, F.; Löfgren, O.; Hörnfeldt, B.; Eklund, U.; Ericsson, P.; Sörlin, D. Abundance and diversity of small mammals in relation to structural habitat factors. Ecol. Bull. 2001, 165–171. [Google Scholar]
- Morris, D.W.; MacEachern, J.T. Active density-dependent habitat selection in a controlled population of small mammals. Ecology 2010, 91, 3131–3137. [Google Scholar] [CrossRef] [PubMed]
- Goldingay, R.L. General or local habitat preferences? Unravelling geographically consistent patterns of habitat preference in gliding mammals. For. Ecol. Manag. 2021, 491, 119204. [Google Scholar] [CrossRef]
- Varner, J.; Dearing, M.D. The importance of biologically relevant microclimates in habitat suitability assessments. PLoS ONE 2014, 9, e104648. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.I.; Encarnação, J.A. Silvicolous on a Small Scale: Possibilities and Limitations of Habitat Suitability Models for Small, Elusive Mammals in Conservation Management and Landscape Planning. PLoS ONE 2015, 10, e0120562. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Ferreira, E.; Godinho, S.; Pita, R.; Mira, A.; Fernandes, N.; Santos, S.M. Predicting microhabitat suitability for an endangered small mammal using sentinel-2 data. Remote Sens. 2020, 12, 562. [Google Scholar] [CrossRef]
- Moudrý, V.; Cord, A.F.; Gábor, L.; Laurin, G.V.; Barták, V.; Gdulová, K.; Malavasi, M.; Rocchini, D.; Stereńczak, K.; Prošek, J.; et al. Vegetation structure derived from airborne laser scanning to assess species distribution and habitat suitability: The way forward. Divers. Distrib. 2023, 29, 39–50. [Google Scholar] [CrossRef]
- Appleby, S.M.; Bebre, I.; Riebl, H.; Balkenhol, N.; Seidel, D. Linking small mammal capture probability with understory structural complexity using a mobile laser scanning-derived metric: A case study. Ecol. Res. 2024, 39, 360–367. [Google Scholar] [CrossRef]
- Klenovsek, T.; Novak, T.; Cas, M.; Trilar, T.; Janžekovič, F. Feeding ecology of three sympatric Sorex shrew species in montane forests of Slovenia. Folia Zool. 2013, 62, 193–199. [Google Scholar] [CrossRef]
- Brouard, M.J.; Knowles, S.C.L.; Dressen, S.; Coulson, T.; Malo, A.F. Factors affecting woodland rodent growth. J. Zool. 2020, 312, 174–182. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I.; Lazăr, A. Responses of small mammals to habitat characteristics in Southern Carpathian forests. Sci. Rep. 2021, 11, 12031. [Google Scholar] [CrossRef] [PubMed]
- Čepelka, L.; Suchomel, J.; Purchart, L.; Heroldová, M. Small Mammal Communities in Central European Forests—A Case Study from the Czech Republic. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4442095 (accessed on 18 May 2024).
- Díaz, M.; Morán-López, T. Forest Fragmentation and Developmental Stability of Wood Mice Apodemus sylvaticus: A Food-Mediated Effect? Diversity 2023, 15, 423. [Google Scholar] [CrossRef]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat suitability for small mammals in Mediterranean landscapes: How and why shrubs matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- Rowland-Schaefer, E.G.; Koehn, O.; Jones, H.P. Small mammal associations with habitat composition, configuration, and management in tallgrass prairies: A review. Mammal Rev. 2024, 54, 178–192. [Google Scholar] [CrossRef]
- Bence, S.L.; Stander, K.; Griffiths, M. Habitat characteristics of harvest mouse nests on arable farmland. Agric. Ecosyst. Environ. 2003, 99, 179–186. [Google Scholar] [CrossRef]
- Benedek, A.M.; Lazăr, A.; Cic, N.V.; Cocîrlea, M.D.; Sîrbu, I. Effects of Long-Term Habitat Protection on Montane Small Mammals: Are Sorex araneus and S. minutus More Sensitive Than Previously Considered? Diversity 2022, 14, 38. [Google Scholar] [CrossRef]
- Haberl, W.; Kryštufek, B. Spatial distribution and population density of the harvest mouse Micromys minutus in a habitat mosaic at Lake Neusiedl, Austria. Mammalia 2003, 57, 355–565. [Google Scholar] [CrossRef]
- Canova, L.; Fasola, M. Communities of small mammals in six biotopes of northern Italy. Acta Theriol. 1991, 36, 73–86. [Google Scholar] [CrossRef]
- Churchfield, S.; Rychlik, L. Diets and coexistence in Neomys and Sorex shrews in Białowieża forest, eastern Poland. J. Zool. 2006, 269, 381–390. [Google Scholar] [CrossRef]
- Ivanter, E.V. The Ecology of Sibling Species of the Common Vole (Microtus arvalis Pall. and Microtus rossiaemeridionalis Ognev) at the Northern Limit of Its Range. Biol. Bull. Russ. Acad. Sci. 2023, 50, 1286–1293. [Google Scholar] [CrossRef]
- Harris, S. History, distribution, status and habitat requirements of the Harvest mouse (Micromys minutus) in Britain. Mammal Rev. 1979, 9, 159–171. [Google Scholar] [CrossRef]
- Darinot, F. Improving detectability of the harvest mouse (Micromys minutus Pallas, 1771) by above ground live-trapping. Mammalia 2019, 84, 239–245. [Google Scholar] [CrossRef]
- Fuentes-Montemayor, E.; Ferryman, M.; Watts, K.; Macgregor, N.A.; Hambly, N.; Brennan, S.; Coxon, R.; Langridge, H.; Park, K.J. Small mammal responses to long-term large-scale woodland creation: The influence of local and landscape-level attributes. Ecol. Appl. 2020, 30, e02028. [Google Scholar] [CrossRef] [PubMed]
- Caminero-Saldaña, C.; Correa-Cuadros, J.P.; Baños-Herrero, A.; Riquelme, C.; Pallavicini, Y.; Fernández-Villán, M.; Plaza, J.; Pérez-Sánchez, R.; Sánchez, N.; Mougeot, F.; et al. Exploring the influence of density-dependence and weather on the spatial and temporal variation in common vole (Microtus arvalis) abundance in Castilla y León, NW Spain. Pest Manag. Sci, 2023; early view. [Google Scholar] [CrossRef]
- Torre, I.; Díaz, M. Assessing the effects of landscape change on the occupancy dynamics of the Greater white-toothed shrew Crocidura russula. Life 2022, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Kitchings, J.T.; Levy, D.J. Habitat patterns in a small mammal community. J. Mammal. 1981, 62, 814–820. [Google Scholar] [CrossRef]
- Väli, Ü.; Tõnisalu, G. Community-and species-level habitat associations of small mammals in a hemiboreal forest–farmland landscape. Ann. Zool. Fenn. 2020, 58, 1–11. [Google Scholar] [CrossRef]
- McCarthy, A.; Caravaggi, A.; Fernández-Bellon, D.; Irwin, S.; Lusby, J.; O’Halloran, J. Bird and small mammal community composition and abundance in upland open habitats and early conifer forests. Eur. J. Wildl. Res. 2021, 67, 26. [Google Scholar] [CrossRef]
- Schweiger, B.R.; Frey, J.K.; Cain, J.W., III. A case for multiscale habitat selection studies of small mammals. J. Mammal. 2021, 102, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.O. Cost, effort and outcome of mammal rediscovery: Neglect of small species. Biol. Conserv. 2011, 144, 1712–1718. [Google Scholar] [CrossRef]
- Peck, M.R.; Maddock, S.T.; Morales, J.N.; Oñate, H.; Mafla-Endara, P.; Peñafiel, V.A.; Torres-Carvajal, O.; Pozo-Rivera, W.E.; Cueva-Arroyo, X.A.; Tolhurst, B.A. Cost-effectiveness of using small vertebrates as indicators of disturbance. Conserv. Biol. 2014, 28, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.H.S. The foods eaten by wood mice (Apodemus sylvaticus) and bank voles (Clethrionomys glareolus) in Wytham Woods, Berkshire. J. Anim. Ecol. 1968, 37, 25. [Google Scholar] [CrossRef]
- Ware, R.L.; Booker, A.L.; Allaby, F.R.; Allaby, R.G. Habitat selection drives dietary specialisation in Sorex minutus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lukyanova, L.E.; Ukhova, N.L.; Ukhova, O.V.; Gorodilova, Y.V. Common Shrew (Sorex araneus, Eulipotyphla) Population and the Food Supply of Its Habitats in Ecologically Contrasting Environments. Russ. J. Ecol. 2021, 52, 316–328. [Google Scholar] [CrossRef]
- Hansson, L. Population growth and habitat distribution in cyclic small rodents: To expand or to change? Oecologia 1997, 112, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Surmacki, A.; Goldyn, B.; Tryjanowski, P. Location and habitat characteristics of the breeding nests of the harvest mouse (Micromys minutus) in the reed-beds of an intensively used farmland. Mammalia 2005, 69, 5–9. [Google Scholar] [CrossRef]
- Ivanter, E.V. The Reproductive Ecology of the Bank Vole Myodes (Clethrionomys) glareolus Schreb. in North Periphery of Its Areal: I. Sex Cycles, Course, Dates, and Intensive Reproduction. Biol. Bull. Russ. Acad. Sci. 2020, 47, 535–547. [Google Scholar] [CrossRef]
- Koskela, P.; Viro, P. The abundance, autumn migration, population structure and body dimensions of the Harvest mouse in Northern Finland. Acta Theriol. 1976, 21, 375–387. [Google Scholar] [CrossRef]
- Gehring, T.M.; Swihart, R.K. Body size, niche breadth, and ecologically scaled responses to habitat fragmentation: Mammalian predators in an agricultural landscape. Biol. Conserv. 2003, 109, 283–295. [Google Scholar] [CrossRef]
- Prevedello, J.A.; Forero-Medina, G.; Vieira, M.V. Movement behaviour within and beyond perceptual ranges in three small mammals: Effects of matrix type and body mass. J. Anim. Ecol. 2010, 79, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.T.; Anholt, B.; Volpe, J.P. Body mass explains characteristic scales of habitat selection in terrestrial mammals. Ecol. Evol. 2011, 1, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Battisti, C.; Fanelli, G.; Cerfolli, F.; Amori, G.; Luiselli, L. Body mass and trophic level variations in relation to habitat disturbance in a set of mammal species. Life Environ. 2019, 69, 147–152. [Google Scholar]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Wilder, S.M.; Raubenheimer, D.; Simpson, S.J. Moving beyond body condition indices as an estimate of fitness in ecological and evolutionary studies. Funct. Ecol. 2016, 30, 108–115. [Google Scholar] [CrossRef]
- Moya-Laraño, J.; Macías-Ordóñez, R.; Blanckenhorn, W.U.; Fernández-Montraveta, C. Analysing body condition: Mass, volume or density? J. Anim. Ecol. 2008, 77, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Wishart, A.E.; Guerrero-Chacón, A.L.; Smith, R.; Hawkshaw, D.M.; McAdam, A.G.; Dantzer, B.; Boutin, S.; Lane, J.E. Inferring condition in wild mammals: Body condition indices confer no benefit over measuring body mass across ecological contexts. Oecologia 2024, 204, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ergon, T.; Speakman, J.R.; Scantlebury, M.; Cavanagh, R.; Lambin, X. Optimal body size and energy expenditure during winter: Why are voles smaller in declining populations? Am. Nat. 2004, 163, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.C.; Grapputo, A.; Jokinen, I.; Koskela, E.; Mappes, T.; Poikonen, T. Fitness trade-offs mediated by immunosuppression costs in a small mammal. Evolution 2010, 64, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Hostedde, A.I.; Zinner, B.; Millar, J.S.; Hickling, G.J. Restitution of mass–size residuals: Validating body condition indices. Ecology 2005, 86, 155–163. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Insight into Body Condition Variability in Small Mammals. Animals 2024, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Balčiauskienė, L. Small Mammal Diversity Changes in a Baltic Country, 1975–2021: A Review. Life 2022, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Balčiauskienė, L. Long-Term Stability of Harvest Mouse Population. Diversity 2023, 15, 1102. [Google Scholar] [CrossRef]
- CORINE Land Cover. Available online: https://www.eea.europa.eu/en/datahub/datahubitem-view/a5144888-ee2a-4e5d-a7b0-2bbf21656348 (accessed on 17 May 2024).
- Moors, P.J. Norway rats (Rattus norvegicus) on the Noises and Motukawao islands, Hauraki Gulf, New Zealand. N. Z. J. Ecol. 1985, 8, 37–54. [Google Scholar]
- Sibly, R.M.; Brown, J.H. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl. Acad. Sci. USA 2007, 104, 17707–17712. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.J.; Singleton, G.R. Indexes of condition for small mammals. Aust. J. Zool. 1993, 41, 317–323. [Google Scholar] [CrossRef]
- Past 4—The Past of the Future. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 1 January 2024).
- TIBCO Software Inc. Data Science Textbook. Available online: https://docs.tibco.com/data-science/textbook (accessed on 15 January 2024).
- Pulliam, H.R.; Danielson, B.J. Sources, sinks, and habitat selection: A landscape perspective on population dynamics. Am. Nat. 1991, 137, 50–66. [Google Scholar] [CrossRef]
- Kristan, W.B., III. The role of habitat selection behavior in population dynamics: Source–sink systems and ecological traps. Oikos 2003, 103, 457–468. [Google Scholar] [CrossRef]
- Corbalán, V.; Tabeni, S.; Ojeda, R.A. Assessment of habitat quality for four small mammal species of the Monte Desert, Argentina. Mamm. Biol. 2006, 71, 227–237. [Google Scholar] [CrossRef]
- Morris, D.W. Sex-dependent habitat selection modulates risk management by meadow voles. Ecosphere 2023, 14, e4378. [Google Scholar] [CrossRef]
- Escalante, M.A.; Horníková, M.; Marková, S.; Kotlík, P. Niche differentiation in a postglacial colonizer, the bank vole Clethrionomys glareolus. Ecol. Evol. 2021, 11, 8054–8070. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.; Eichholz, M.W. Habitat associations of small mammal communities in a restored prairie system in southern Illinois. J. Mammal. 2021, 102, 789–801. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Jasiulionis, M.; Balčiauskienė, L.; Remeikis, V. Immediate increase in isotopic enrichment in small mammals following the expansion of a great cormorant colony. Biogeosciences 2018, 15, 3883–3891. [Google Scholar] [CrossRef]
- Fischer, C.; Thies, C.; Tscharntke, T. Small mammals in agricultural landscapes: Opposing responses to farming practices and landscape complexity. Biol. Conserv. 2011, 144, 1130–1136. [Google Scholar] [CrossRef]
- Delciellos, A.C.; Vieira, M.V.; Grelle, C.E.; Cobra, P.; Cerqueira, R. Habitat quality versus spatial variables as determinants of small mammal assemblages in Atlantic Forest fragments. J. Mammal. 2016, 97, 253–265. [Google Scholar] [CrossRef]
- Mortelliti, A.; Amori, G.; Boitani, L. The role of habitat quality in fragmented landscapes: A conceptual overview and prospectus for future research. Oecologia 2010, 163, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.J.; Bennett, A.F. Habitat fragmentation disrupts the demography of a widespread native mammal. Ecography 2010, 33, 841–853. [Google Scholar] [CrossRef]
- Delciellos, A.C.; Barros, C.D.S.D.; Prevedello, J.A.; Ferreira, M.S.; Cerqueira, R.; Vieira, M.V. Habitat fragmentation affects individual condition: Evidence from small mammals of the Brazilian Atlantic Forest. J. Mammal. 2018, 99, 936–945. [Google Scholar] [CrossRef]
- Sozio, G.; Mortelliti, A. Empirical evaluation of the strength of interspecific competition in shaping small mammal communities in fragmented landscapes. Landsc. Ecol. 2016, 31, 775–789. [Google Scholar] [CrossRef]
- Van Apeldoorn, R.C.; Oostenbrink, W.T.; Van Winden, A.; Van Der Zee, F.F. Effects of habitat fragmentation on the bank vole, Clethrionomys glareolus, in an agricultural landscape. Oikos 1992, 65, 265–274. [Google Scholar] [CrossRef]
- Mazurkiewicz, M. Factors influencing the distribution of the bank vole in forest habitats. Acta Theriol. 1994, 39, 113–126. [Google Scholar] [CrossRef]
- Linderholm, H.W. Growing season changes in the last century. Agric. For. Meteorol. 2006, 137, 1–14. [Google Scholar] [CrossRef]
- Balčiauskienė, L.; Balčiauskas, L.; Čepukienė, A. Winter growth depression of common vole (Microtus arvalis). Acta Zool. Litu. 2009, 19, 85–92. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Stirkė, V.; Garbaras, A.; Balčiauskienė, L. Shrews Under-Represented in Fruit Farms and Homesteads. Animals 2023, 13, 1028. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, L. Habitat preferences of four sympatric species of shrews. Acta Theriol. 2000, 45, 173–190. [Google Scholar] [CrossRef]
- Hanski, I.; Kaikusalo, A. Distribution and habitat selection of shrews in Finland. Ann. Zool. Fenn. 1989, 26, 339–348. [Google Scholar]
- Lázaro, J.; Nováková, L.; Hertel, M.; Taylor, J.R.; Muturi, M.; Zub, K.; Dechmann, D.K. Geographic patterns in seasonal changes of body mass, skull, and brain size of common shrews. Ecol. Evol. 2021, 11, 2431–2448. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, F.; Mohallal, E.; Gilfillan, G.D.; Lowe, A.; Reader, T. Seasonal patterns in habitat use by the harvest mouse (Micromys minutus) and other small mammals. Mammalia 2021, 85, 325–335. [Google Scholar] [CrossRef]
- Hilderbrand, G.V.; Gustine, D.D.; Mangipane, B.A.; Joly, K.; Leacock, W.; Mangipane, L.S.; Erlenbach, J.; Sorum, M.S.; Cameron, M.D.; Belant, J.L.; et al. Body size and lean mass of brown bears across and within four diverse ecosystems. J. Zool. 2018, 305, 53–62. [Google Scholar] [CrossRef]
- Ross, J.G.B.; Newman, C.; Buesching, C.D.; Connolly, E.; Nakagawa, S.; Macdonald, D.W. A fat chance of survival: Body condition provides life-history dependent buffering of environmental change in a wild mammal population. Clim. Change Ecol. 2021, 2, 100022. [Google Scholar] [CrossRef]
- Reynolds, D.S.; Sullivan, J.C.; Kunz, T.H. Evaluation of total body electrical conductivity to estimate body composition of a small mammal. J. Wildl. Manag. 2009, 73, 1197–1206. [Google Scholar] [CrossRef]
- Stevenson, R.D.; Woods, W.A., Jr. Condition indices for conservation: New uses for evolving tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, K.J.; Hilbers, J.P.; Garcia-Ulloa, J.; Graae, B.J.; May, R.; Verones, F.; Huijbregts, M.A.; Schipper, A.M. Habitat fragmentation amplifies threats from habitat loss to mammal diversity across the world’s terrestrial ecoregions. One Earth 2021, 4, 1505–1513. [Google Scholar] [CrossRef]

| Habitat | 1990 | 2018 | Trend | ||
|---|---|---|---|---|---|
| Area, ha | % | Area, ha | % | ||
| Forest | 1,919,872 | 29.57 | 1,944,747 | 29.96 | increasing |
| Shrub | 162,653 | 2.51 | 310,362 | 4.78 | increasing |
| Wetland | 57,229 | 0.88 | 56,412 | 0.87 | stable |
| Meadow | 489,966 | 7.55 | 455,858 | 7.02 | decreasing |
| Mixed | 524,740 | 8.08 | 459,971 | 7.09 | decreasing |
| Disturbed | 9357 | 0.14 | 6389 | 0.10 | stable |
| Agricultural | 2,989,978 | 46.06 | 2,905,695 | 44.76 | decreasing |
| Commensal | 147,286 | 2.27 | 157,742 | 2.43 | increasing |
| Habitat | N | S |
|---|---|---|
| Forest | 7657 | 15 |
| Shrub | 392 | 11 |
| Wetland | 2271 | 15 |
| Meadow | 7683 | 17 |
| Riparian | 647 | 9 |
| Mixed | 1181 | 15 |
| Disturbed | 2022 | 11 |
| Agricultural | 2139 | 13 |
| Commensal | 4575 | 14 |
| Total | 28,567 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Balčiauskienė, L. Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude. Land 2024, 13, 1214. https://doi.org/10.3390/land13081214
Balčiauskas L, Balčiauskienė L. Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude. Land. 2024; 13(8):1214. https://doi.org/10.3390/land13081214
Chicago/Turabian StyleBalčiauskas, Linas, and Laima Balčiauskienė. 2024. "Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude" Land 13, no. 8: 1214. https://doi.org/10.3390/land13081214
APA StyleBalčiauskas, L., & Balčiauskienė, L. (2024). Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude. Land, 13(8), 1214. https://doi.org/10.3390/land13081214








