Monitoring the Soil Copper of Urban Land with Visible and Near-Infrared Spectroscopy: Comparing Spectral, Compositional, and Spatial Similarities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Spectral Measurement and Chemical Analysis
2.4. Soil Similarity
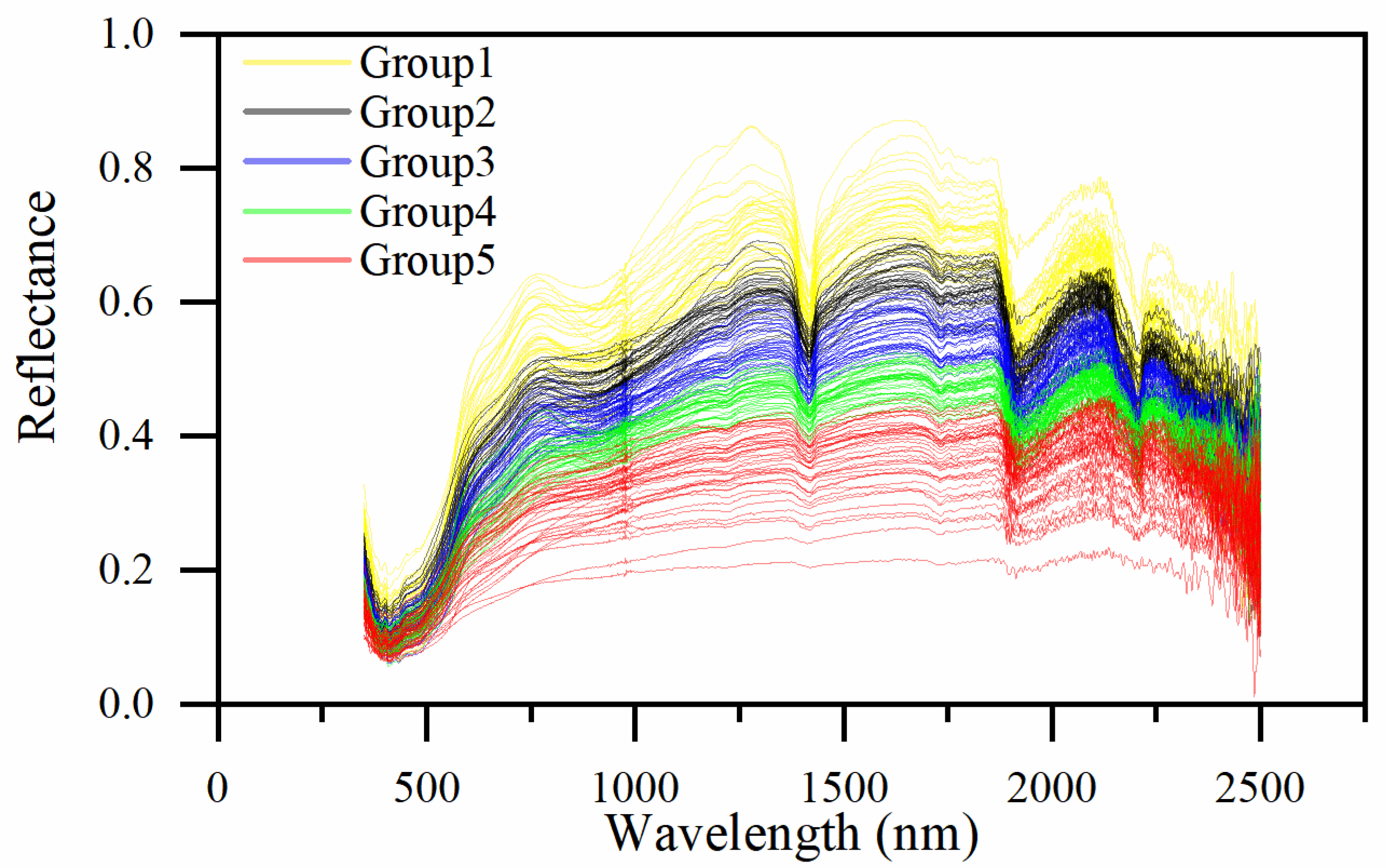
2.4.1. Spectral Similarity
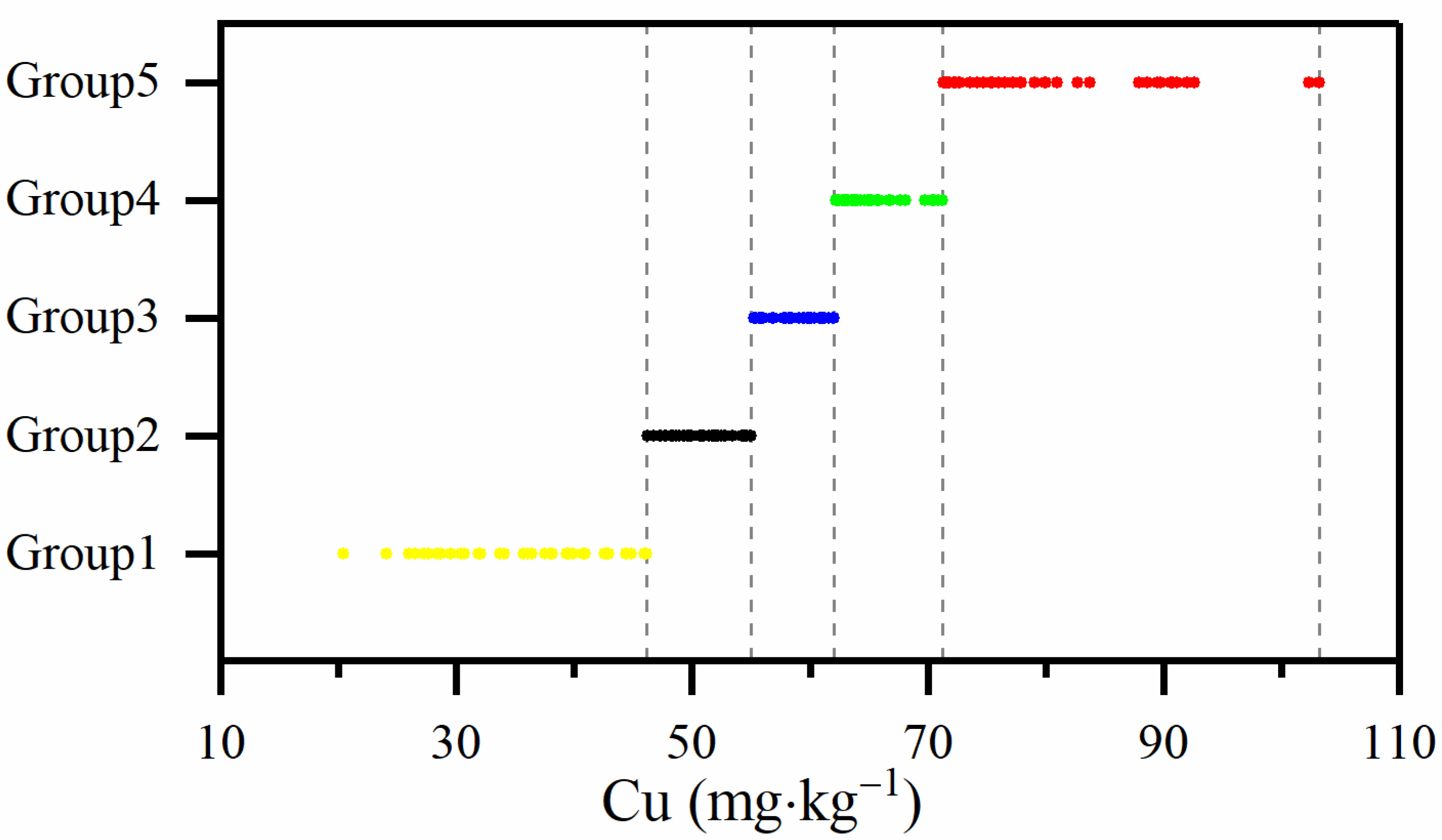
2.4.2. Compositional Similarity
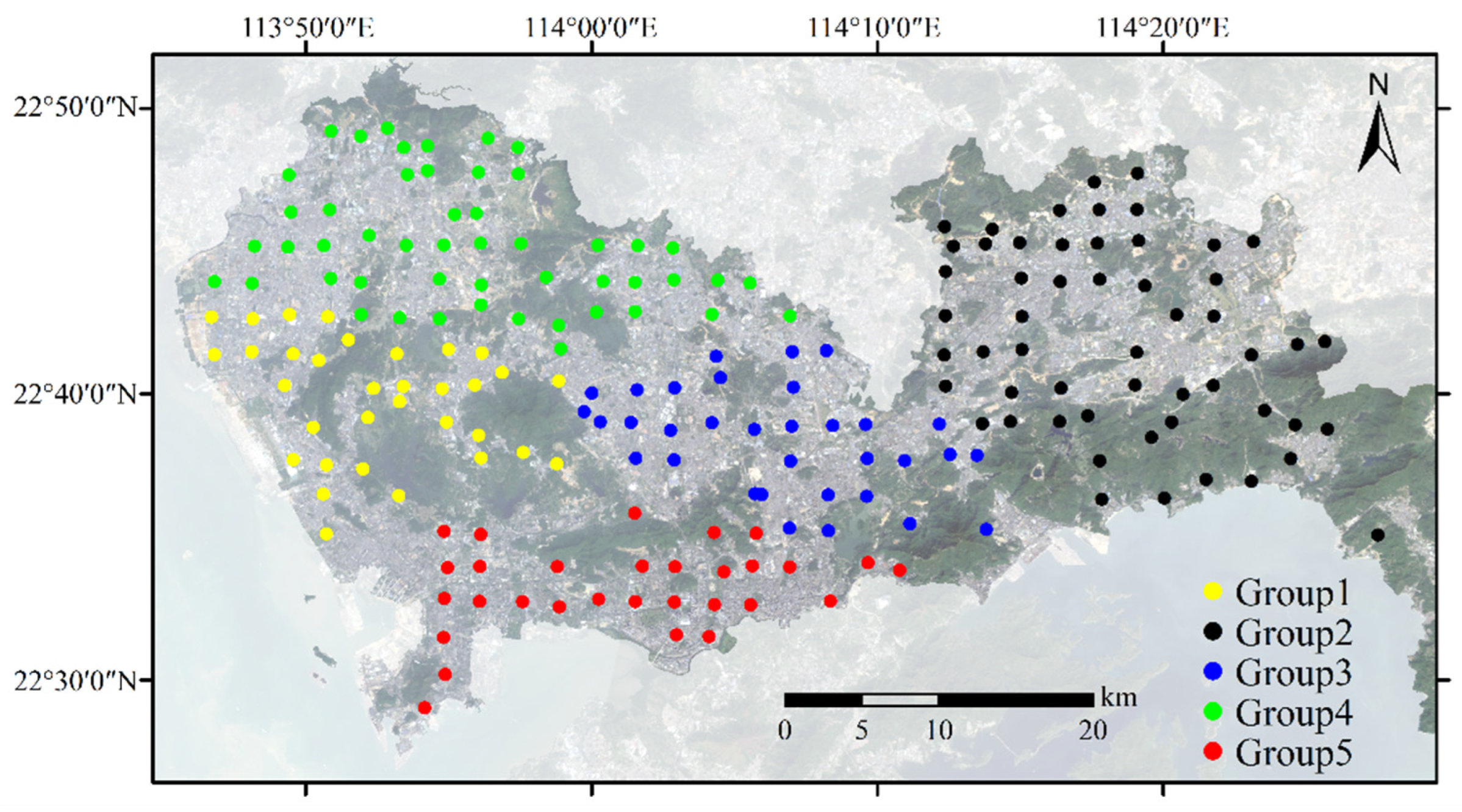
2.4.3. Spatial Similarity
2.5. Model Calibration
2.6. Performance of Models
3. Results
3.1. Descriptive of Statistics of Soil Samples
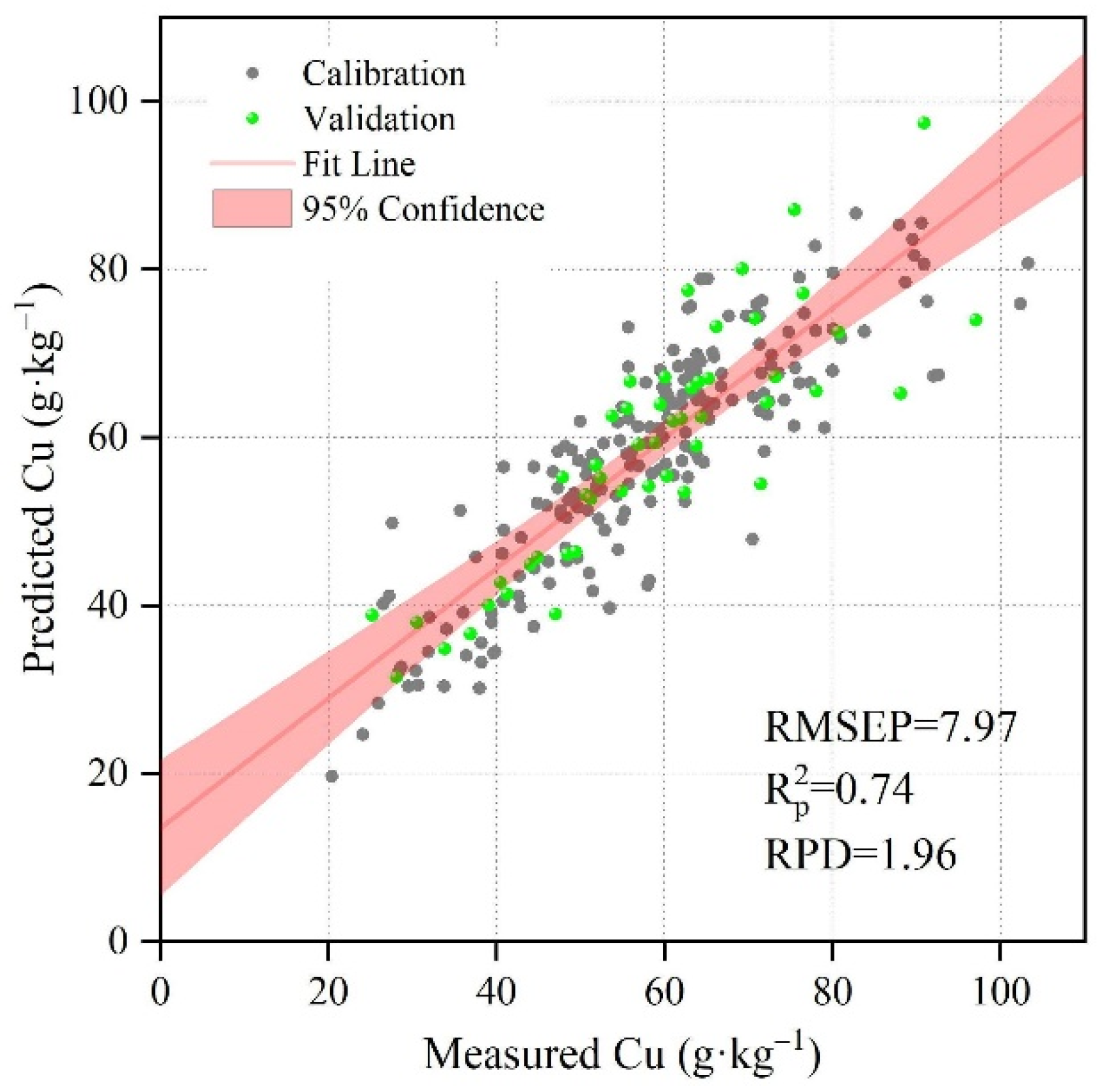
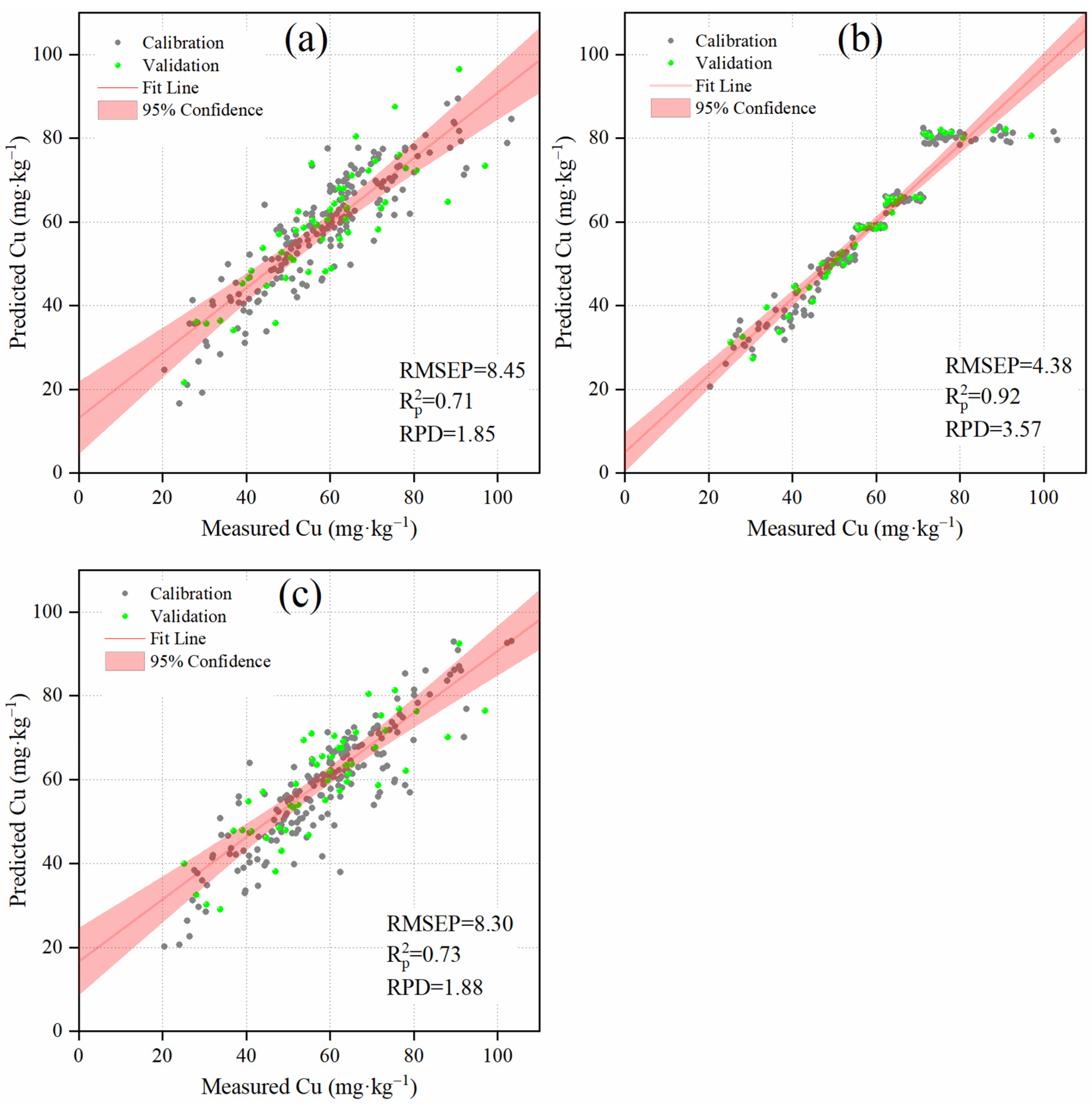
3.2. Measurement Accuracy of Cu Models without Considering Similarity
3.3. Measurement Accuracy of Cu Models when Considering Similarity
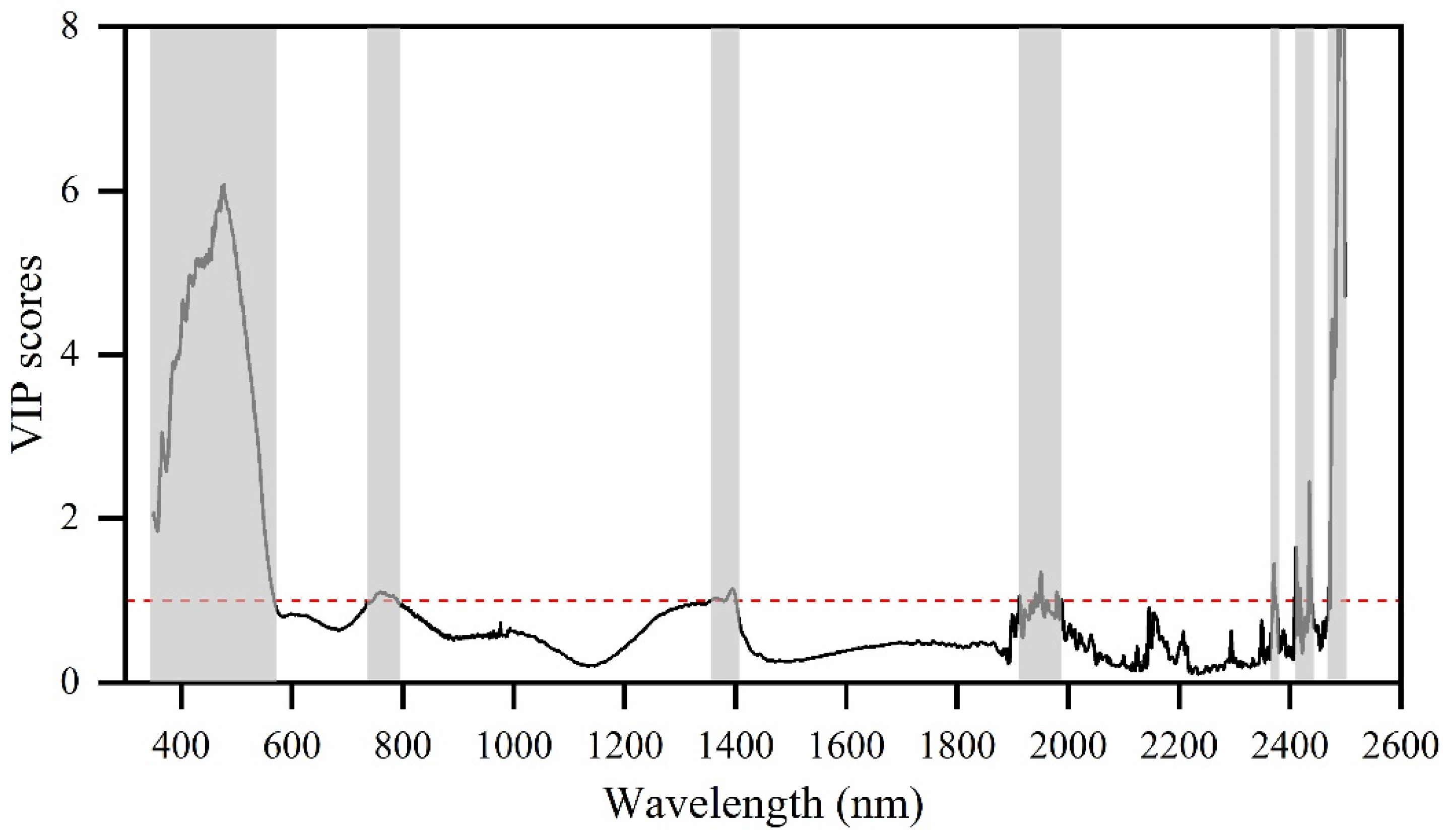
3.3.1. Spectral Similarity
3.3.2. Compositional Similarity
3.3.3. Spatial Similarity
3.3.4. Comparing Spectral, Compositional, and Spatial Similarities
4. Discussion
4.1. Performance of Estimating Cu in a Megacity by Vis-NIR Spectroscopy
4.2. Spectral Similarity vs. Compositional Similarity vs. Spatial Similarity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Cai, L.-M.; Wen, H.-H.; Luo, J.; Wang, Q.-S.; Liu, X. Spatial distribution and source apportionment of heavy metals in soil from a typical county-level city of Guangdong Province, China. Sci. Total Environ. 2019, 655, 92–101. [Google Scholar] [CrossRef]
- Liu, L.L.; Liu, Q.Y.; Ma, J.; Wu, H.W.; Qu, Y.J.; Gong, Y.W.; Yang, S.H.; An, Y.F.; Zhou, Y.Z. Heavy metal(loid)s in the topsoil of urban parks in Beijing, China: Concentrations, potential sources, and risk assessment. Environ. Pollut. 2020, 260, 114083. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Xu, D.Y.; Chen, S.C.; Xu, H.Y.; Wang, N.; Zhou, Y.; Shi, Z. Data fusion for the measurement of potentially toxic elements in soil using portable spectrometers. Environ. Pollut. 2020, 263, 114649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, L.; Liu, J.; Zhang, Z.; Tan, C. Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility. Appl. Sci. 2019, 9, 4213. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.K.; Yang, L.X.; Wang, Z.Q.; Dong, G.C.; Huang, H.Y.; Wang, Y.L. Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere 2006, 62, 602–607. [Google Scholar] [CrossRef]
- Sun, G.L.; Reynolds, E.E.; Belcher, A.M. Designing yeast as plant-like hyperaccumulators for heavy metals. Nat. Commun. 2019, 10, 5080. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, W.; Li, D.; Gao, W.; Zeng, R.; Zheng, G.; Cai, K.; Zeng, Y.; Jiang, C. Can fusion of vis-NIR and MIR spectra at three levels improve the prediction accuracy of soil nutrients? Geoderma 2024, 441, 116754. [Google Scholar] [CrossRef]
- Krzebietke, S.; Daszykowski, M.; Czarnik-Matusewicz, H.; Stanimirova, I.; Pieszczek, L.; Sienkiewicz, S.; Wierzbowska, J. Monitoring the concentrations of Cd, Cu, Pb, Ni, Cr, Zn, Mn and Fe in cultivated Haplic Luvisol soils using near-infrared reflectance spectroscopy and chemometrics. Talanta 2023, 251, 123749. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Yuan, X.; Browne, P.; Chen, L.; Ji, J. The influences of soil properties on Cu and Zn availability in soil and their transfer to wheat t (Triticum aestivum L.) in the Yangtze River delta region, China. Geoderma 2013, 193, 131–139. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, B.; Su, Y.; Zhang, D.; Wang, Y.; Bian, Y.; Suo, L.; Guo, X.; Bai, H. Retrieving zinc concentrations in topsoil with reflectance spectroscopy at Opencast Coal Mine sites. Sci. Rep. 2021, 11, 19909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, T.; Lan, Z.; Guo, K.; Zhuang, D.; Zhang, X.; Liang, X.; Qiu, T.; Zhang, S.; Chen, Y. Estimating the Soil Copper Content of Urban Land in a Megacity Using Piecewise Spectral Pretreatment. Land 2024, 13, 517. [Google Scholar] [CrossRef]
- Shi, Z.; Ji, W.; Viscarra Rossel, R.A.; Chen, S.; Zhou, Y. Prediction of soil organic matter using a spatially constrained local partial least squares regression and the Chinese vis–NIR spectral library. Eur. J. Soil Sci. 2015, 66, 679–687. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Chen, Y.; Zhang, Y.; Shi, T.; Wang, J.; Hong, Y.; Fei, T. The Influence of Spectral Pretreatment on the Selection of Representative Calibration Samples for Soil Organic Matter Estimation Using Vis-NIR Reflectance Spectroscopy. Remote Sens. 2019, 11, 450. [Google Scholar] [CrossRef]
- Dor, E.B.; Granot, A.; Wallach, R.; Francos, N.; Pearlstein, D.H.; Efrati, B.; Boruvka, L.; Gholizadeh, A.; Schmid, T. Exploitation of the SoilPRO® (SP) apparatus to measure soil surface reflectance in the field: Five case studies. Geoderma 2023, 438, 17. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Lobsey, C.R.; Sharman, C.; Flick, P.; McLachlan, G. Novel soil profile sensing to monitor organic C stocks and condition. Environ. Sci. Technol. 2017, 51, 5630–5641. [Google Scholar] [CrossRef] [PubMed]
- Viscarra Rossel, R.A.; Behrens, T.; Ben-Dor, E.; Chabrillat, S.; Dematte, J.A.M.; Ge, Y.; Gomez, C.; Guerrero, C.; Peng, Y.; Ramirez-Lopez, L.; et al. Diffuse reflectance spectroscopy for estimating soil properties: A technology for the 21st century. Eur. J. Soil Sci. 2022, 73, e13271. [Google Scholar] [CrossRef]
- Hong, Y.; Shen, R.; Cheng, H.; Chen, S.; Chen, Y.; Guo, L.; He, J.; Liu, Y.; Yu, L.; Liu, Y. Cadmium concentration estimation in peri-urban agricultural soils: Using reflectance spectroscopy, soil auxiliary information, or a combination of both? Geoderma 2019, 354, 113875. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Borůvka, L.; Saberioon, M.M.; Kozák, J.; Vašát, R.; Němeček, K. Comparing different data preprocessing methods for monitoring soil heavy metals based on soil spectral features. Soil Water Res. 2015, 10, 218–227. [Google Scholar] [CrossRef]
- Shi, T.; Chen, Y.; Liu, Y.; Wu, G. Visible and near-infrared reflectance spectroscopy—An alternative for monitoring soil contamination by heavy metals. J. Hazard. Mater. 2014, 265, 166–176. [Google Scholar] [CrossRef]
- Qi, Y.; Qie, X.; Qin, Q.; Shukla, M.K. Prediction of soil calcium carbonate with soil visible-near-infrared reflection (Vis-NIR) spectral in Shaanxi province, China: Soil groups vs. spectral groups. Int. J. Remote Sens. 2021, 42, 2502–2516. [Google Scholar] [CrossRef]
- Shoshany, M.; Roitberg, E.; Goldshleger, N.; Kizel, F. Universal quadratic soil spectral reflectance line and its deviation patterns’ relationships with chemical and textural properties: A global data base analysis. Remote Sens. Environ. 2022, 280, 113182. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Jiang, X. Sample selection method using near-infrared spectral information entropy as similarity criterion for constructing and updating peach firmness and soluble solids content prediction models. J. Chemom. 2024, 38, e3528. [Google Scholar] [CrossRef]
- Wei, C.; Zhao, Y.; Li, D.; Zhang, G.; Wu, D.; Chen, J. Prediction of soil organic matter and cation exchange capacity based on spectral similarity measuring. Trans. Chin. Soc. Agric. Eng. 2014, 30, 81–88. [Google Scholar]
- Liu, Y.; Shi, Z.; Zhang, G.; Chen, Y.; Li, S.; Hong, Y.; Shi, T.; Wang, J.; Liu, Y. Application of Spectrally Derived Soil Type as Ancillary Data to Improve the Estimation of Soil Organic Carbon by Using the Chinese Soil Vis-NIR Spectral Library. Remote Sens. 2018, 10, 1747. [Google Scholar] [CrossRef]
- Tziolas, N.; Tsakiridis, N.; Ben-Dor, E.; Theocharis, J.; Zalidis, G. A memory-based learning approach utilizing combined spectral sources and geographical proximity for improved VIS-NIR-SWIR soil properties estimation. Geoderma 2019, 340, 11–24. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.; Behrens, T.; Schmidt, K.; Rossel, R.V.; Demattê, J.; Scholten, T. Distance and similarity-search metrics for use with soil vis–NIR spectra. Geoderma 2013, 199, 43–53. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, J.P.; Cai, K.; Gao, W.C.; Pan, W.J.; Jiang, C.Y.; Zhang, P.Y.; Wu, B.W.; Wang, C.H.; Jin, X.Y.; et al. How similar is “similar”, or what is the best measure of soil spectral and physiochemical similarity? PLoS ONE 2021, 16, e0247028. [Google Scholar] [CrossRef]
- Zeng, R.; Rossiter, D.G.; Zhao, Y.; Li, D.; Liu, F.; Zheng, G.; Zhang, G. The choice of spectral similarity algorithms influences suspected soil sample provenance. Forensic Sci. Int. 2023, 347, 111688. [Google Scholar] [CrossRef]
- Spiers, R.C.; Norby, C.; Kalivas, J.H. Physicochemical Responsive Integrated Similarity Measure (PRISM) for a Comprehensive Quantitative Perspective of Sample Similarity Dynamically Assessed with NIR Spectra. Anal. Chem. 2023, 95, 12776–12784. [Google Scholar] [CrossRef] [PubMed]
- Wadoux, A.M.J.C.; Malone, B.; Minasny, B.; Fajardo, M.; McBratney, A.B. Similarity Between Spectra and the Detection of Outliers. In Soil Spectral Inference with R: Analysing Digital Soil Spectra Using the R Programming Environment; Wadoux, A.M.J.C., Malone, B., Minasny, B., Fajardo, M., McBratney, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 115–141. [Google Scholar]
- Hong, Y.; Yu, L.; Chen, Y.; Liu, Y.; Liu, Y.; Liu, Y.; Cheng, H. Prediction of soil organic matter by vis–NIR spectroscopy using normalized soil moisture index as a proxy of soil moisture. Remote Sens. 2017, 10, 28. [Google Scholar] [CrossRef]
- Peng, Y.; Knadel, M.; Gislum, R.; Deng, F.; Norgaard, T.; de Jonge, L.W.; Moldrup, P.; Greve, M.H. Predicting soil organic carbon at field scale using a national soil spectral library. J. Near Infrared Spectrosc. 2013, 21, 213–222. [Google Scholar] [CrossRef]
- McDowell, M.L.; Bruland, G.L.; Deenik, J.L.; Grunwald, S. Effects of subsetting by carbon content, soil order, and spectral classification on prediction of soil total carbon with diffuse reflectance spectroscopy. Appl. Environ. Soil Sci. 2012, 2012, 294121. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.; Behrens, T.; Schmidt, K.; Stevens, A.; Demattê, J.A.M.; Scholten, T. The spectrum-based learner: A new local approach for modeling soil vis–NIR spectra of complex datasets. Geoderma 2013, 195, 268–279. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Zou, B.; Wu, T. Exploring the Potential of Spectral Classification in Estimation of Soil Contaminant Elements. Remote Sens. 2017, 9, 632. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; Toth, G.; Panagos, P.; van Wesemael, B.; Montanarella, L. Prediction of soil organic carbon content by diffuse reflectance spectroscopy using a local partial least square regression approach. Soil Biol. Biochem. 2014, 68, 337–347. [Google Scholar] [CrossRef]
- Jaconi, A.; Don, A.; Freibauer, A. Prediction of soil organic carbon at the country scale: Stratification strategies for near-infrared data. Eur. J. Soil Sci. 2017, 68, 919–929. [Google Scholar] [CrossRef]
- Madari, B.E.; Reeves III, J.B.; Coelho, M.R.; Machado, P.L.; De-Polli, H.; Coelho, R.M.; Benites, V.M.; Souza, L.F.; McCarty, G.W. Mid-and Near-Infrared Spectroscopic Determination of Carbon in a Diverse Set of Soils from the Brazilian National Soil Collection. Spectrosc. Lett. 2005, 38, 721–740. [Google Scholar] [CrossRef]
- Udelhoven, T.; Emmerling, C.; Jarmer, T. Quantitative analysis of soil chemical properties with diffuse reflectance spectrometry and partial least-square regression: A feasibility study. Plant Soil 2003, 251, 319–329. [Google Scholar] [CrossRef]
- Vasques, G.M.; Grunwald, S.; Harris, W.G. Spectroscopic models of soil organic carbon in Florida, USA. J. Environ. Qual. 2010, 39, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Udelhoven, T.; Denis, A.; Tychon, B.; Lioy, R.; Hoffmann, L.; Van Wesemael, B. Measuring soil organic carbon in croplands at regional scale using airborne imaging spectroscopy. Geoderma 2010, 158, 32–45. [Google Scholar] [CrossRef]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Chapter five-visible and near infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Zhao, P.Z.; Fallu, D.J.; Pears, B.; Allonsius, C.; Lembrechts, J.J.; van de Vondel, S.; Meysman, F.J.R.; Cucchiaro, S.; Tarolli, P.; Shi, P.; et al. Quantifying soil properties relevant to soil organic carbon biogeochemical cycles by infrared spectroscopy: The importance of compositional data analysis. Soil Tillage Res. 2023, 231, 105718. [Google Scholar] [CrossRef]
- Li, J.K.; Xu, H.; Song, Y.P.; Tang, L.L.; Gong, Y.B.; Yu, R.L.; Shen, L.; Wu, X.L.; Liu, Y.D.; Zeng, W.M. Geography Plays a More Important Role than Soil Composition on Structuring Genetic Variation of Pseudometallophyte Commelina communis. Front. Plant Sci. 2016, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, S.H.; Xi, X.P.; Yang, K.; Luo, F. Environmental Background Values of Heavy Metals and Physicochemical Properties in Different Soils in Shenzhen. Environ. Sci. 2021, 42, 3518–3526. [Google Scholar]
- Zhang, W.; Xu, A.; Zhang, R.; Ji, H. Review of Soil Classification and Revision of China Soil Classification System. Sci. Agric. Sin. 2014, 47, 3214–3230. [Google Scholar]
- Mousavi, F.; Abdi, E.; Ghalandarzadeh, A.; Bahrami, H.A.; Majnounian, B.; Ziadi, N. Diffuse reflectance spectroscopy for rapid estimation of soil Atterberg limits. Geoderma 2020, 361, 114083. [Google Scholar] [CrossRef]
- Vohland, M.; Ludwig, B.; Seidel, M.; Hutengs, C. Quantification of soil organic carbon at regional scale: Benefits of fusing vis-NIR and MIR diffuse reflectance data are greater for in situ than for laboratory-based modelling approaches. Geoderma 2022, 405, 115426. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Guo, L.; Liu, L. Construction of the Calibration Set through Multivariate Analysis in Visible and Near-Infrared Prediction Model for Estimating Soil Organic Matter. Remote Sens. 2017, 9, 201. [Google Scholar] [CrossRef]
- Guerrero, C.; Zornoza, R.; Gómez, I.; Mataix-Beneyto, J. Spiking of NIR regional models using samples from target sites: Effect of model size on prediction accuracy. Geoderma 2010, 158, 66–77. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Peng, J.; Ji, W.; Liu, H.; Li, X.; Viscarra Rossel, R.A. Development of a national VNIR soil-spectral library for soil classification and prediction of organic matter concentrations. Sci. China Earth Sci. 2014, 57, 1671–1680. [Google Scholar] [CrossRef]
- Asa, G.; Nimrod, C.; Ale?, K.; Eyal, B.D.; Lubo?, B.V. Agricultural Soil Spectral Response and Properties Assessment: Effects of Measurement Protocol and Data Mining Technique. Remote Sens. 2017, 9, 1078. [Google Scholar] [CrossRef]
- Mancini, M.; Andrade, R.; Silva, S.H.G.; Rafael, R.B.A.; Mukhopadhyay, S.; Li, B.; Chakraborty, S.; Guilherme, L.R.G.; Acree, A.; Weindorf, D.C.; et al. Multinational prediction of soil organic carbon and texture via proximal sensors. Soil Sci. Soc. Am. J. 2024, 88, 8–26. [Google Scholar] [CrossRef]
- Li, S.; Rossel, R.A.V.; Webster, R. The cost-effectiveness of reflectance spectroscopy for estimating soil organic carbon. Eur. J. Soil Sci. 2022, 73, e13202. [Google Scholar] [CrossRef]
- Goodarzi, M.; Sharma, S.; Ramon, H.; Saeys, W. Multivariate calibration of NIR spectroscopic sensors for continuous glucose monitoring. TrAC Trends Anal. Chem. 2015, 67, 147–158. [Google Scholar] [CrossRef]
- Cheng, H.X.; Li, K.; Li, M.; Yang, K.; Liu, F.; Cheng, X. Geochemical background and baseline value of chemical elements in urban soil in China. Earth Sci. Front. 2014, 21, 265–306. [Google Scholar]
- Gozukara, G.; Acar, M.; Ozlu, E.; Dengiz, O.; Hartemink, A.E.; Zhang, Y. A soil quality index using Vis-NIR and pXRF spectra of a soil profile. Catena 2022, 211, 105954. [Google Scholar] [CrossRef]
- Pyo, J.; Hong, S.M.; Kwon, Y.S.; Kim, M.S.; Cho, K.H. Estimation of heavy metals using deep neural network with visible and infrared spectroscopy of soil. Sci. Total Environ. 2020, 741, 140162. [Google Scholar] [CrossRef]
- Nawar, S.; Mohamed, E.S.; Sayed, S.E.E.; Mohamed, W.S.; Rebouh, N.Y.; Hammam, A.A. Estimation of key potentially toxic elements in arid agricultural soils using Vis-NIR spectroscopy with variable selection and PLSR algorithms. Front. Environ. Sci. 2023, 11, 1222871. [Google Scholar] [CrossRef]
- Cheng, H.; Shen, R.; Chen, Y.; Wan, Q.; Shi, T.; Wang, J.; Wan, Y.; Hong, Y.; Li, X. Estimating heavy metal concentrations in suburban soils with reflectance spectroscopy. Geoderma 2019, 336, 59–67. [Google Scholar] [CrossRef]
- Riedel, F.; Denk, M.; Müller, I.; Barth, N.; Glässer, C. Prediction of soil parameters using the spectral range between 350 and 15,000 nm: A case study based on the Permanent Soil Monitoring Program in Saxony, Germany. Geoderma 2018, 315, 188–198. [Google Scholar] [CrossRef]
- Trap, J.; Bureau, F.; Perez, G.; Aubert, M. PLS-regressions highlight litter quality as the major predictor of humus form shift along forest maturation. Soil Biol. Biochem. 2013, 57, 969–971. [Google Scholar] [CrossRef]
- Xie, X.L.; Pan, X.Z.; Sun, B. Visible and Near-Infrared Diffuse Reflectance Spectroscopy for Prediction of Soil Properties near a Copper Smelter. Pedosphere 2012, 22, 351–366. [Google Scholar] [CrossRef]
- Lian, S.; Ji, J.; De-Jun, T.; Hong-Bing, X.; Zhen-Fu, L.; Bo, G. Estimate of heavy metals in soil and streams using combined geochemistry and field spectroscopy in Wan-sheng mining area, Chongqing, China. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 1–9. [Google Scholar]
- Zhang, X.; Huang, C.P.; Liu, B.; Tong, Q.X. Inversion of soil Cu concentration based on band selection of hyperspetral data. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Honolulu, HI, USA, 25–30 June 2010; pp. 3680–3683. [Google Scholar]

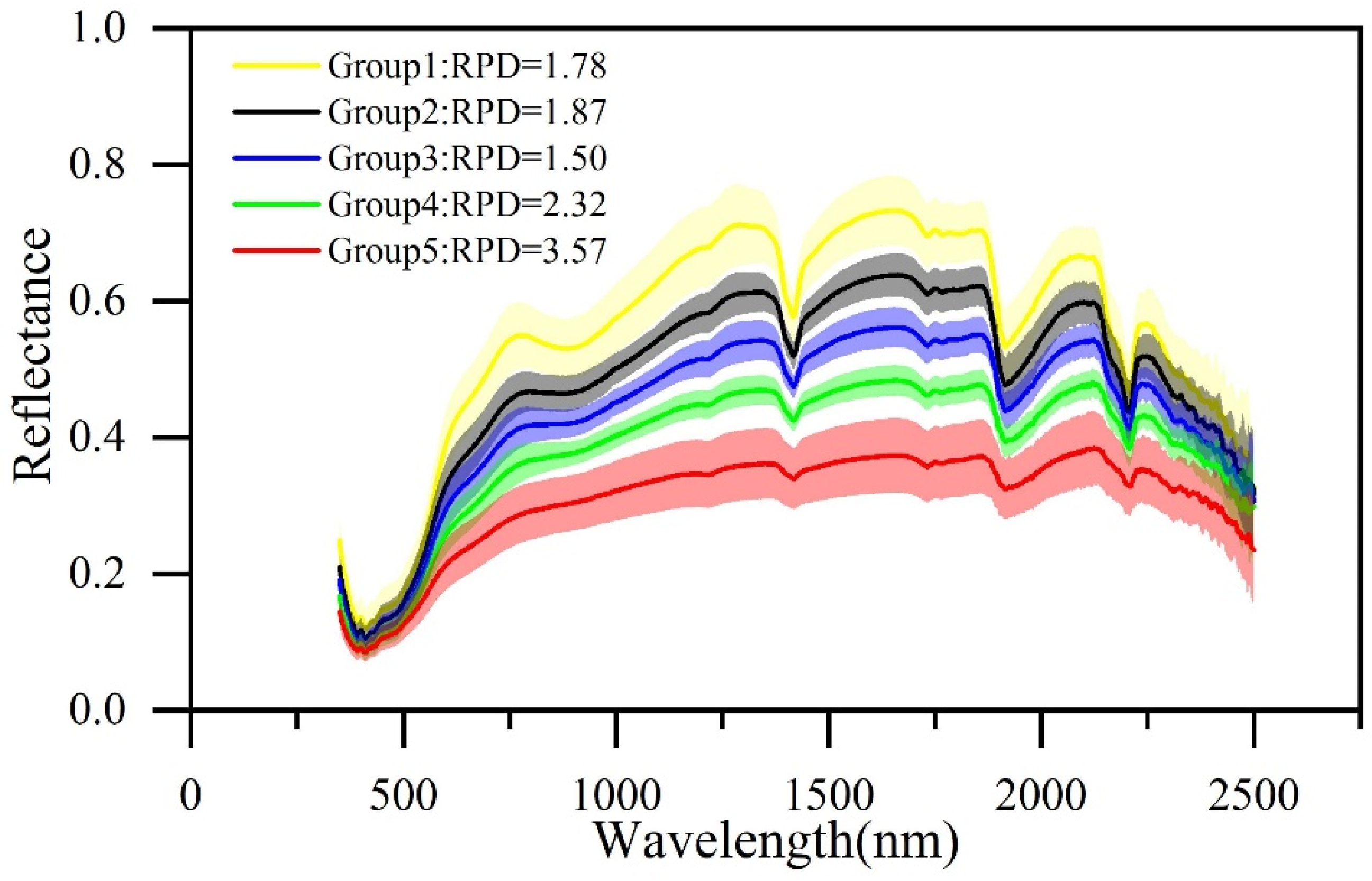
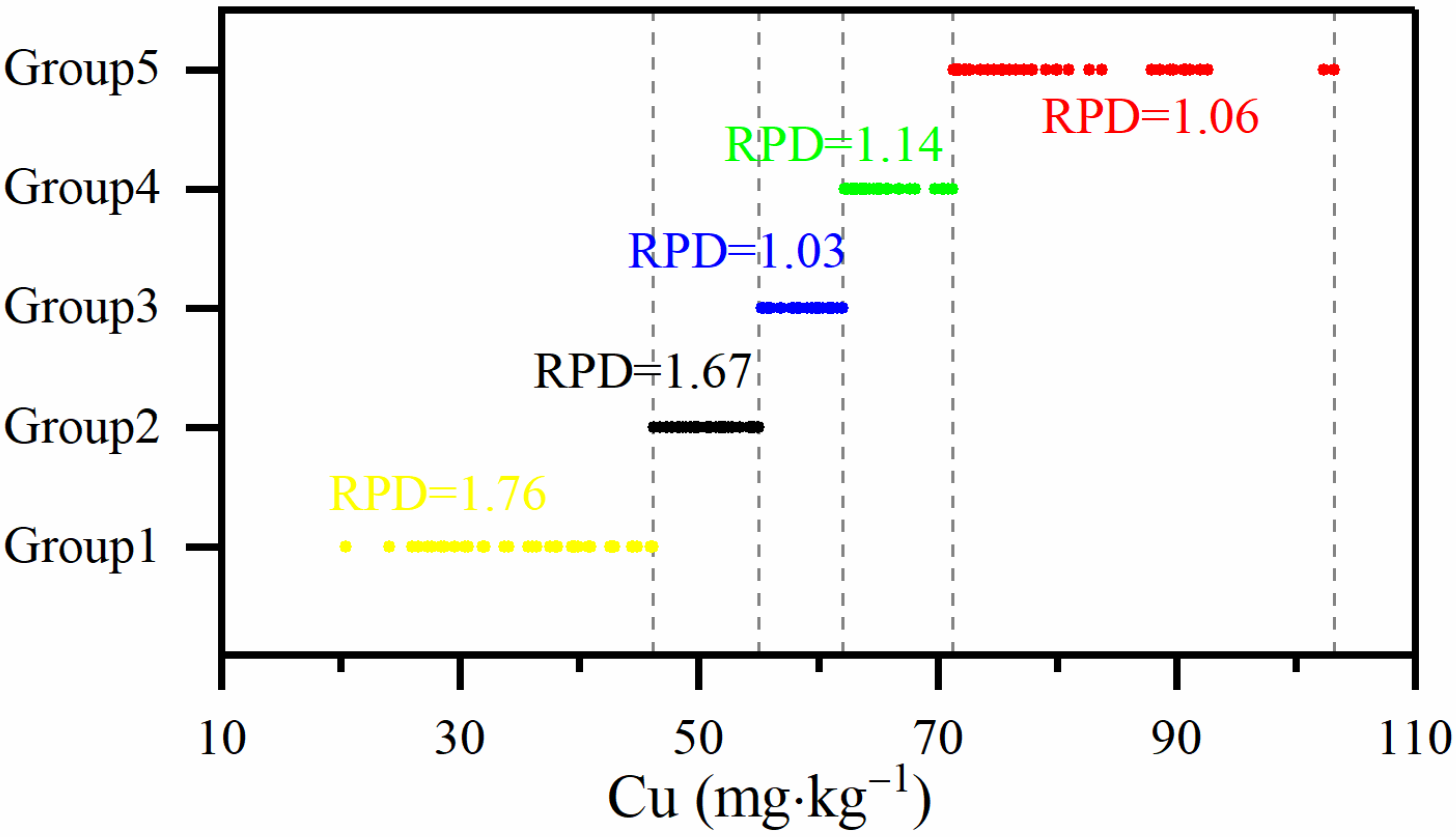
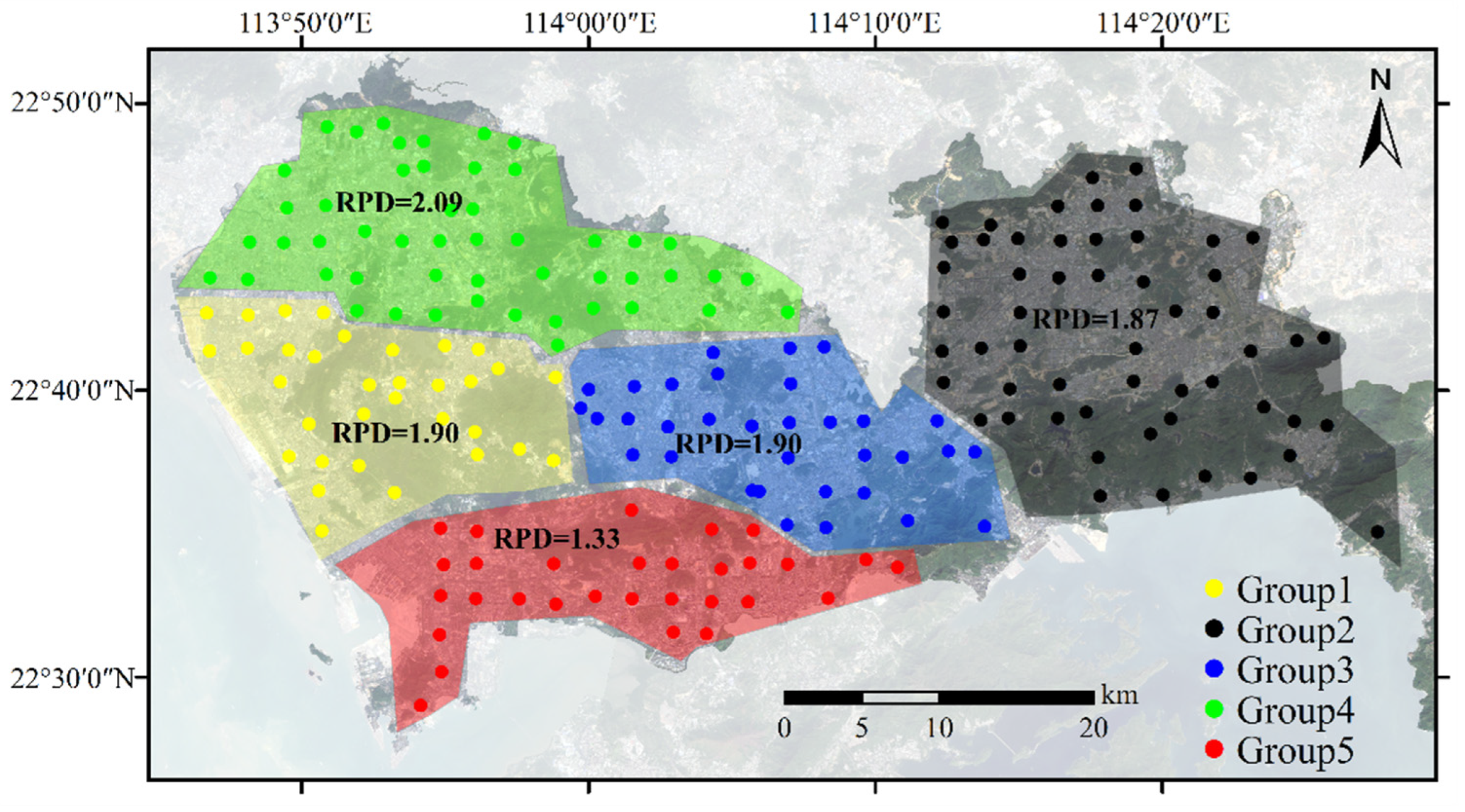

| Spectral Similarity | Sample Set | Count | Cu (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range 1 | Min | Max | Mean | Std 2 | Skewness | Kurtosis | CV 3 | |||
| Group 1 | All | 53 | 81.90 | 20.45 | 102.35 | 57.19 | 17.71 | 0.40 | 0.09 | 0.31 |
| Calibration | 40 | 81.90 | 20.45 | 102.35 | 56.49 | 17.98 | 0.38 | 0.30 | 0.32 | |
| Validation | 13 | 57.01 | 33.83 | 90.84 | 59.35 | 17.37 | 0.58 | −0.36 | 0.29 | |
| Group 2 | All | 49 | 68.57 | 24.05 | 92.62 | 57.50 | 15.88 | 0.32 | −0.63 | 0.28 |
| Calibration | 40 | 68.57 | 24.05 | 92.62 | 56.68 | 16.12 | 0.48 | −0.36 | 0.28 | |
| Validation | 9 | 41.19 | 36.88 | 78.07 | 61.13 | 15.08 | −0.49 | −1.36 | 0.25 | |
| Group 3 | All | 50 | 70.55 | 26.51 | 97.06 | 56.09 | 15.10 | 0.05 | 0.63 | 0.27 |
| Calibration | 40 | 63.22 | 26.51 | 89.73 | 55.15 | 15.00 | −0.31 | −0.02 | 0.27 | |
| Validation | 10 | 55.76 | 41.3 | 97.06 | 59.84 | 15.70 | 1.61 | 3.21 | 0.26 | |
| Group 4 | All | 50 | 55.71 | 28.08 | 83.79 | 55.91 | 13.96 | −0.25 | −0.43 | 0.25 |
| Calibration | 40 | 55.13 | 28.66 | 83.79 | 56.59 | 13.33 | −0.22 | −0.32 | 0.24 | |
| Validation | 10 | 52.64 | 28.08 | 80.72 | 53.19 | 16.76 | −0.16 | −0.69 | 0.32 | |
| Group 5 | All | 48 | 78.03 | 25.21 | 103.24 | 65.07 | 13.39 | 0.17 | 1.90 | 0.21 |
| Calibration | 40 | 62.46 | 40.78 | 103.24 | 66.51 | 12.93 | 0.68 | 0.88 | 0.19 | |
| Validation | 8 | 45.49 | 25.21 | 70.70 | 57.90 | 14.22 | −2.06 | 5.05 | 0.25 | |
| Total | All | 250 | 82.79 | 20.45 | 103.24 | 58.29 | 15.57 | 0.13 | 0.12 | 0.27 |
| Calibration | 200 | 82.79 | 20.45 | 103.24 | 58.29 | 15.60 | 0.13 | 0.15 | 0.27 | |
| Validation | 50 | 71.85 | 25.21 | 97.06 | 58.30 | 15.63 | 0.13 | 0.12 | 0.27 | |
| Compositional Similarity | Sample Set | Count | Cu (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range 1 | Min | Max | Mean | Std 2 | Skewness | Kurtosis | CV 3 | |||
| Group 1 | All | 53 | 25.67 | 20.45 | 46.12 | 36.37 | 6.74 | −0.46 | −0.85 | 0.19 |
| Calibration | 40 | 25.67 | 20.45 | 46.12 | 36.35 | 6.81 | −0.48 | −0.77 | 0.19 | |
| Validation | 10 | 19.66 | 25.21 | 44.87 | 36.42 | 6.78 | −0.43 | −1.11 | 0.19 | |
| Group 2 | All | 50 | 8.7 | 46.26 | 54.96 | 50.72 | 2.46 | 0.1 | −1.01 | 0.05 |
| Calibration | 40 | 8.7 | 46.26 | 54.96 | 50.71 | 2.47 | 0.1 | −0.99 | 0.05 | |
| Validation | 10 | 7.91 | 47.01 | 54.92 | 50.74 | 2.57 | 0.13 | −0.93 | 0.05 | |
| Group 3 | All | 50 | 6.85 | 55.23 | 62.08 | 58.77 | 2.06 | −0.32 | −1.15 | 0.04 |
| Calibration | 40 | 6.85 | 55.23 | 62.08 | 58.78 | 2.06 | −0.3 | −1.15 | 0.04 | |
| Validation | 10 | 6.46 | 55.52 | 61.98 | 58.81 | 2.17 | −0.28 | −1.06 | 0.04 | |
| Group 4 | All | 50 | 9.06 | 62.22 | 71.28 | 65.2 | 2.7 | 1.09 | 0.05 | 0.04 |
| Calibration | 40 | 9.06 | 62.22 | 71.28 | 65.2 | 2.68 | 1.07 | −0.02 | 0.04 | |
| Validation | 10 | 8.37 | 62.33 | 70.7 | 65.19 | 2.78 | 1.2 | 0.49 | 0.04 | |
| Group 5 | All | 50 | 31.93 | 71.31 | 103.24 | 80.39 | 8.64 | 1.03 | 0.32 | 0.11 |
| Calibration | 40 | 31.93 | 71.31 | 103.24 | 80.38 | 8.57 | 0.98 | 0.1 | 0.11 | |
| Validation | 10 | 25.59 | 71.47 | 97.06 | 80.35 | 8.76 | 0.93 | −0.34 | 0.11 | |
| Total | All | 250 | 82.79 | 20.45 | 103.24 | 58.29 | 15.57 | 0.13 | 0.12 | 0.27 |
| Calibration | 200 | 82.79 | 20.45 | 103.24 | 58.29 | 15.60 | 0.13 | 0.15 | 0.27 | |
| Validation | 50 | 71.85 | 25.21 | 97.06 | 58.30 | 15.63 | 0.13 | 0.12 | 0.27 | |
| Spatial Similarity | Sample Set | Count | Cu (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range 1 | Min | Max | Mean | Std 2 | Skewness | Kurtosis | CV 3 | |||
| Group 1 | All | 43 | 61.87 | 40.48 | 102.35 | 64.51 | 13.69 | 0.5 | 0.43 | 0.21 |
| Calibration | 33 | 61.51 | 40.84 | 102.35 | 64.32 | 13.59 | 0.75 | 0.8 | 0.21 | |
| Validation | 10 | 50.36 | 40.48 | 90.84 | 65.15 | 14.77 | −0.24 | 0.37 | 0.23 | |
| Group 2 | All | 69 | 76.61 | 20.45 | 97.06 | 53.72 | 16.24 | 0.17 | 0.22 | 0.3 |
| Calibration | 54 | 70.39 | 20.45 | 90.84 | 53.37 | 16.28 | 0.07 | −0.2 | 0.31 | |
| Validation | 15 | 71.85 | 25.21 | 97.06 | 54.96 | 16.61 | 0.56 | 2.7 | 0.3 | |
| Group 3 | All | 40 | 49.52 | 29.54 | 79.06 | 54.84 | 13.6 | −0.08 | −1.07 | 0.25 |
| Calibration | 33 | 49.52 | 29.54 | 79.06 | 55.41 | 13.73 | −0.14 | −0.95 | 0.25 | |
| Validation | 7 | 34.59 | 36.88 | 71.47 | 52.18 | 13.67 | 0.24 | −1.81 | 0.26 | |
| Group 4 | All | 61 | 79.19 | 24.05 | 103.24 | 59.56 | 15.94 | 0.27 | 0.53 | 0.27 |
| Calibration | 50 | 79.19 | 24.05 | 103.24 | 60.42 | 15.72 | 0.28 | 0.85 | 0.26 | |
| Validation | 11 | 57.58 | 30.5 | 88.08 | 55.61 | 17.08 | 0.37 | −0.04 | 0.31 | |
| Group 5 | All | 37 | 58.26 | 33.73 | 91.99 | 61.22 | 15.02 | 0.1 | −0.55 | 0.25 |
| Calibration | 30 | 58.26 | 33.73 | 91.99 | 60.1 | 15.76 | 0.21 | −0.59 | 0.26 | |
| Validation | 7 | 25.8 | 54.92 | 80.72 | 66.03 | 10.91 | 0.44 | −2.19 | 0.17 | |
| Total | All | 250 | 82.79 | 20.45 | 103.24 | 58.29 | 15.57 | 0.13 | 0.12 | 0.27 |
| Calibration | 200 | 82.79 | 20.45 | 103.24 | 58.29 | 15.60 | 0.13 | 0.15 | 0.27 | |
| Validation | 50 | 71.85 | 25.21 | 97.06 | 58.30 | 15.63 | 0.13 | 0.12 | 0.27 | |
| Spectral Similarity | Calibration | Validation | LVs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Std | RMSEP | RPD | ||||||
| No Group | Not Croup | 200 | 0.67 | 8.90 | 50 | 15.63 | 0.74 | 7.97 | 1.96 | 6 |
| Group | Group 1 | 40 | 0.56 | 12.05 | 13 | 17.37 | 0.70 | 9.78 | 1.78 | 5 |
| Group 2 | 40 | 0.53 | 11.07 | 9 | 15.08 | 0.90 | 8.05 | 1.87 | 4 | |
| Group 3 | 40 | 0.48 | 10.76 | 10 | 15.70 | 0.54 | 10.47 | 1.50 | 4 | |
| Group 4 | 40 | 0.47 | 9.85 | 10 | 16.76 | 0.82 | 7.22 | 2.32 | 4 | |
| Group 5 | 40 | 0.10 | 13.04 | 8 | 14.22 | 0.99 | 3.98 | 3.57 | 5 | |
| Overall | 200 | 0.48 | 11.41 | 50 | 15.63 | 0.71 | 8.45 | 1.85 | - | |
| Compositional Similarity | Calibration | Validation | LVs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Std | RMSEP | RPD | ||||||
| No Group | Not Croup | 200 | 0.67 | 8.90 | 50 | 15.63 | 0.74 | 7.97 | 1.96 | 6 |
| Group | Group 1 | 40 | 0.20 | 6.55 | 10 | 6.78 | 0.67 | 3.85 | 1.76 | 5 |
| Group 2 | 40 | 0.09 | 3.79 | 10 | 2.57 | 0.61 | 1.54 | 1.67 | 5 | |
| Group 3 | 40 | 0.07 | 2.12 | 10 | 2.17 | 0.04 | 2.03 | 1.07 | 1 | |
| Group 4 | 40 | 0.03 | 3.02 | 10 | 2.78 | 0.15 | 2.43 | 1.14 | 3 | |
| Group 5 | 40 | 0.08 | 8.93 | 10 | 8.76 | 0.01 | 8.30 | 1.06 | 1 | |
| Overall | 200 | 0.88 | 5.49 | 50 | 15.63 | 0.92 | 4.38 | 3.57 | - | |
| Spatial Similarity | Calibration | Validation | LVs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Std | RMSEP | RPD | ||||||
| No Group | Not Croup | 200 | 0.67 | 8.90 | 50 | 15.63 | 0.74 | 7.97 | 1.96 | 6 |
| Group | Group 1 | 33 | 0.61 | 8.44 | 10 | 14.77 | 0.81 | 7.78 | 1.90 | 6 |
| Group 2 | 54 | 0.51 | 11.76 | 15 | 16.61 | 0.72 | 8.88 | 1.87 | 7 | |
| Group 3 | 33 | 0.22 | 12.53 | 7 | 13.67 | 0.79 | 8.04 | 1.70 | 3 | |
| Group 4 | 50 | 0.58 | 10.28 | 11 | 17.08 | 0.77 | 8.16 | 2.09 | 6 | |
| Group 5 | 30 | 0.37 | 12.32 | 7 | 10.91 | 0.41 | 8.19 | 1.33 | 2 | |
| Overall | 200 | 0.51 | 11.14 | 50 | 15.63 | 0.73 | 8.30 | 1.88 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shi, T.; Chen, Y.; Lan, Z.; Guo, K.; Zhuang, D.; Yang, C.; Zhang, W. Monitoring the Soil Copper of Urban Land with Visible and Near-Infrared Spectroscopy: Comparing Spectral, Compositional, and Spatial Similarities. Land 2024, 13, 1279. https://doi.org/10.3390/land13081279
Liu Y, Shi T, Chen Y, Lan Z, Guo K, Zhuang D, Yang C, Zhang W. Monitoring the Soil Copper of Urban Land with Visible and Near-Infrared Spectroscopy: Comparing Spectral, Compositional, and Spatial Similarities. Land. 2024; 13(8):1279. https://doi.org/10.3390/land13081279
Chicago/Turabian StyleLiu, Yi, Tiezhu Shi, Yiyun Chen, Zeying Lan, Kai Guo, Dachang Zhuang, Chao Yang, and Wenyi Zhang. 2024. "Monitoring the Soil Copper of Urban Land with Visible and Near-Infrared Spectroscopy: Comparing Spectral, Compositional, and Spatial Similarities" Land 13, no. 8: 1279. https://doi.org/10.3390/land13081279







