The Impact of a Ski Piste on the Stock and Stoichiometry of Soil Carbon, Nitrogen, and Phosphorus: A Case Study on a Forest Area in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sample Collection and Processing
2.4. Data Analysis and Processing
3. Results
3.1. Concentrations and Stocks of Soil C, N, and P
3.2. Stoichiometry of Soil Carbon, N, and P
3.3. Interdependence of Soil C with Soil Water Content and Bulk Density
4. Discussion
4.1. Impacts of Ski Piste on Soil C, N and P
4.2. Variations in Ecological Stoichiometry of Soil C, N, and P
4.3. Methodological Consideration of Disturbance Effects on Soil C and Nutrients
4.4. Synergistic Changes in Soil C with Soil Water Content and Bulk Density
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Carbon |
| N | Nitrogen |
| P | Phosphorus |
| SP | Ski piste |
| NP | Natural forest |
| SWC | Soil water content on a dry mass basis |
| BD | Bulk density |
| C:N | C-to-N mass ratio |
| C:P | C-to-P mass ratio |
| N:P | N-to-P mass ratio |
| SMA | Standard major axis |
| FDM | Fixed depth method |
| ESM | Equivalent soil mass method |
References
- Gillett, N.; Kirchmeier-Young, M.; Ribes, A.; Shiogama, H.; Hegerl, G.; Knutti, R.; Gastineau, G.; John, J.; Li, L.; Nazarenko, L.; et al. Constraining human contributions to observed warming since the pre-industrial period. Nat. Clim. Change 2021, 11, 1–6. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Rocci, K.S.; Lehmann, A.; Rillig, M.C. Nitrogen increases soil organic carbon accrual and alters its functionality. Glob. Change Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Feng, X.; Dai, G.; Liu, T.; Jia, J.; Zhu, E.; Liu, C.; Zhao, Y.; Wang, Y.; Kang, E.; Xiao, J.; et al. Understanding the mechanisms and potential pathways of soil carbon sequestration from the biogeochemistry perspective. Sci. China-Earth Sci. 2024, 67, 3386–3396. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Luetzow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Liess, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Mgelwa, A.S.; Ngaba, M.J.Y.; Hu, B.; Gurmesa, G.A.; Mwakaje, A.G.; Nyemeck, M.P.B.; Zhu, F.; Qiu, Q.; Song, L.; Wang, Y.; et al. Meta-analysis of 21st century studies shows that deforestation induces profound changes in soil characteristics, particularly soil organic carbon accumulation. For. Ecosyst. 2025, 12, 100257. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Xiang, W.; Ouyang, S.; Zhang, T.; Zhang, X.; Zeng, Y.; Hu, Y.; Luo, G.; Kuzyakov, Y. Forest conversion to plantations: A meta-analysis of consequences for soil and microbial properties and functions. Glob. Change Biol. 2021, 27, 5643–5656. [Google Scholar] [CrossRef]
- Hobley, E.; Baldock, J.; Hua, Q.; Wilson, B. Land-use contrasts reveal instability of subsoil organic carbon. Glob. Change Biol. 2017, 23, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Vernal nitrogen and phosphorus retention by forest understory vegetation and soil microbes. Plant Soil 2003, 256, 443–453. [Google Scholar] [CrossRef]
- Gao, Y.; He, N.; Yu, G.; Chen, W.; Wang, Q. Long-term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: A case study in China. Ecol. Eng. 2014, 67, 171–181. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Ma, C.; Xiang, Y.; Wu, J. Restoring farmland to forest increases phosphorus limitation based on microbial and soil C:N:P stoichiometry-a synthesis across China. For. Ecol. Manag. 2024, 556, 121745. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, J.; Ren, C.; Kang, D.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Land use change influences soil C, N, and P stoichiometry under ’Grain-to-Green Program’ in China. Sci. Rep. 2015, 5, 10195. [Google Scholar] [CrossRef]
- Bing, H.; Wu, Y.; Zhou, J.; Sun, H.; Luo, J.; Wang, J.; Yu, D. Stoichiometric variation of carbon, nitrogen, and phosphorus in soils and its implication for nutrient limitation in alpine ecosystem of Eastern Tibetan Plateau. J. Soils Sediments 2016, 16, 405–416. [Google Scholar] [CrossRef]
- Hume, A.M.; Chen, H.Y.H.; Taylor, A.R. Intensive forest harvesting increases susceptibility of northern forest soils to carbon, nitrogen and phosphorus loss. J. Appl. Ecol. 2018, 55, 246–255. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Petrenko, C.L.; Friedland, A.J. Mineral soil carbon pool responses to forest clearing in Northeastern hardwood forests. Glob. Change Biol. Bioenergy 2015, 7, 1283–1293. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.; Augusto, L.; Cécillon, L.; Ferreira, G.W.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Nazari, M.; Arthur, E.; Lamandé, M.; Keller, T.; Bilyera, N.; Bickel, S. A meta-analysis of soil susceptibility to machinery-induced compaction in forest ecosystems across global climatic zones. Curr. For. Rep. 2023, 9, 370–381. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Qin, J.; Liu, X.; Mayer, M. A global meta-analysis of forest harvesting effects on soil respiration, its components, and temperature sensitivity. Agric. For. Meteorol. 2024, 358, 110259. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Rovira, P.; Sauras-Yera, T.; Romanya, J. Equivalent-mass versus fixed-depth as criteria for quantifying soil carbon sequestration: How relevant is the difference? Catena 2022, 214, 106283. [Google Scholar] [CrossRef]
- von Haden, A.C.; Yang, W.H.; DeLucia, E.H. Soils’ dirty little secret: Depth-based comparisons can be inadequate for quantifying changes in soil organic carbon and other mineral soil properties. Glob. Change Biol. 2020, 26, 3759–3770. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zeng, R.; Han, Y. Coupling coordination evaluation of economic development, ecological environment, and the tourism industry in ski resorts. J. Clean. Prod. 2023, 426, 139076. [Google Scholar] [CrossRef]
- Wipf, S.; Rixen, C.; Fischer, M.; Schmid, B.; Stoeckli, V. Effects of ski piste preparation on alpine vegetation. J. Appl. Ecol. 2005, 42, 306–316. [Google Scholar] [CrossRef]
- Rolando, A.; Caprio, E.; Rinaldi, E.; Ellena, I. The impact of high-altitude ski-runs on alpine grassland bird communities. J. Appl. Ecol. 2007, 44, 210–219. [Google Scholar] [CrossRef]
- Allegrezza, M.; Cocco, S.; Pesaresi, S.; Courchesne, F.; Corti, G. Effect of snowpack management on grassland biodiversity and soil properties at a ski resort in the Mediterranean basin (central Italy). Plant Biosyst. 2017, 151, 1101–1110. [Google Scholar] [CrossRef]
- Ciarkowska, K. Assessment of heavy metal pollution risks and enzyme activity of meadow soils in urban area under tourism load: A case study from Zakopane (Poland). Environ. Sci. Pollut. Res. 2018, 25, 13709–13718. [Google Scholar] [CrossRef] [PubMed]
- Kangas, K.; Tolvanen, A.; Kalkaja, T.; Siikamaki, P. Ecological impacts of revegetation and management practices of ski slopes in northern Finland. Environ. Manag. 2009, 44, 408–419. [Google Scholar] [CrossRef]
- Lee, J.; Hopmans, J.W.; Rolston, D.E.; Baer, S.G.; Six, J. Determining soil carbon stock changes: Simple bulk density corrections fail. Agric. Ecosyst. Environ. 2009, 134, 251–256. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. 2006, 81, 259–291. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.; Sanchez-Maranon, M.; Martin-Garcia, J.M.; Aranda, V.; Serrano-Bernardo, F.; Rosua, J.L. Impact of ski pistes on soil properties: A case study from a mountainous area in the Mediterranean region. Soil Use Manag. 2007, 23, 269–277. [Google Scholar] [CrossRef]
- Zummo, L.M.; Friedland, A.J. Soil carbon release along a gradient of physical disturbance in a harvested northern hardwood forest. For. Ecol. Manag. 2011, 261, 1016–1026. [Google Scholar] [CrossRef]
- Leppälammi-Kujansuu, J.; Aro, L.; Salemaa, M.; Hansson, K.; Kleja, D.B.; Helmisaari, H.-S. Fine root longevity and carbon input into soil from below-and aboveground litter in climatically contrasting forests. For. Ecol. Manag. 2014, 326, 79–90. [Google Scholar] [CrossRef]
- Mayer, M.; Baltensweiler, A.; James, J.; Rigling, A.; Hagedorn, F. A global synthesis and conceptualization of the magnitude and duration of soil carbon losses in response to forest disturbances. Glob. Ecol. Biogeogr. 2024, 33, 141–150. [Google Scholar] [CrossRef]
- Kong, Q. Effect of Detritus Removal on Soil Carbon Concentration and Mineralization in a Deciduous Forest in the Maoershan Mountain. Mater Thesis, Northeast Forestry University, Harbin, China, 2018. [Google Scholar]
- James, J.; Harrison, R. The effect of harvest on forest soil carbon: A meta-analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Harvest impacts on soil carbon storage in temperate forests. For. Ecol. Manag. 2010, 259, 857–866. [Google Scholar] [CrossRef]
- Mushinski, R.M.; Boutton, T.W.; Scott, D.A. Decadal-scale changes in forest soil carbon and nitrogen storage are influenced by organic matter removal during timber harvest. J. Geophys. Res. Biogeosciences 2017, 122, 846–862. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Z.; Zhang, S.; Jin, D.; Yang, C.; Melnikov, A.; Jinbang, Z. Self-weighting of the overlying soil horizon catalyzed by freeze–thaw cycles leads to silt particle enrichment in the soil profile. Catena 2024, 237, 107815. [Google Scholar] [CrossRef]
- Jackson, R.; Schenk, H.; Jobbagy, E.; Canadell, J.; Colello, G.; Dickinson, R.; Field, C.; Friedlingstein, P.; Heimann, M.; Hibbard, K. Belowground consequences of vegetation change and their treatment in models. Ecol. Appl. 2000, 10, 470–483. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Ding, Z.; Tang, M.; Zhu, B. Changes in soil total, microbial and enzymatic C-N-P contents and stoichiometry with depth and latitude in forest ecosystems. Sci. Total Environ. 2022, 816, 151583. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Reynolds, L.L.; Lajtha, K.; Bowden, R.D.; Tfaily, M.M.; Johnson, B.R.; Bridgham, S.D. The path from litter to soil: Insights into soil C cycling from long-term input manipulation and high-resolution mass spectrometry. J. Geophys. Res. Biogeosciences 2018, 123, 1486–1497. [Google Scholar] [CrossRef]
- Barberis, D.; Lombardi, G.; Enri, S.R.; Pittarello, M.; Viglietti, D.; Freppaz, M.; Lonati, M. Nitrogen fertilizer enhances vegetation establishment of a high-altitude machine-graded ski slope. Restor. Ecol. 2023, 31, e13777. [Google Scholar] [CrossRef]
- Zhang, H.-S.; Zha, T.-G.; Yu, Y.; Ji, X.-D.; Jesus, R.-C. Assessing vegetation community distribution characteristics and succession stages in mountainous areas hosting coming Winter Olympics Games. J. Mt. Sci. 2021, 18, 2870–2887. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Godbold, D.L. Stump harvesting for bioenergy—A review of the environmental impacts. Forestry 2010, 83, 17–38. [Google Scholar] [CrossRef]
- James, J.; Page-Dumroese, D.; Busse, M.; Palik, B.; Zhang, J.; Eaton, B.; Slesak, R.; Tirocke, J.; Kwon, H. Effects of forest harvesting and biomass removal on soil carbon and nitrogen: Two complementary meta-analyses. For. Ecol. Manag. 2021, 485, 118935. [Google Scholar] [CrossRef]
- Han, S.-K.; Han, H.-S.; Page-Dumroese, D.S.; Johnson, L.R. Soil compaction associated with cut-to-length and whole-tree harvesting of a coniferous forest. Can. J. For. Res. 2009, 39, 976–989. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.-J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Mariani, L.; Chang, S.X.; Kabzems, R. Effects of tree harvesting, forest floor removal, and compaction on soil microbial biomass, microbial respiration, and N availability in a boreal aspen forest in British Columbia. Soil Biol. Biochem. 2006, 38, 1734–1744. [Google Scholar] [CrossRef]
- Allman, M.; Jankovský, M.; Messingerová, V.; Allmanová, Z. Soil moisture content as a predictor of soil disturbance caused by wheeled forest harvesting machines on soils of the Western Carpathians. J. For. Res. 2017, 28, 283–289. [Google Scholar] [CrossRef]
- Wang, C.; Han, Y.; Chen, J.; Wang, X.; Zhang, Q.; Bond-Lamberty, B. Seasonality of soil CO2 efflux in a temperate forest: Biophysical effects of snowpack and spring freeze–thaw cycles. Agric. For. Meteorol. 2013, 177, 83–92. [Google Scholar] [CrossRef]

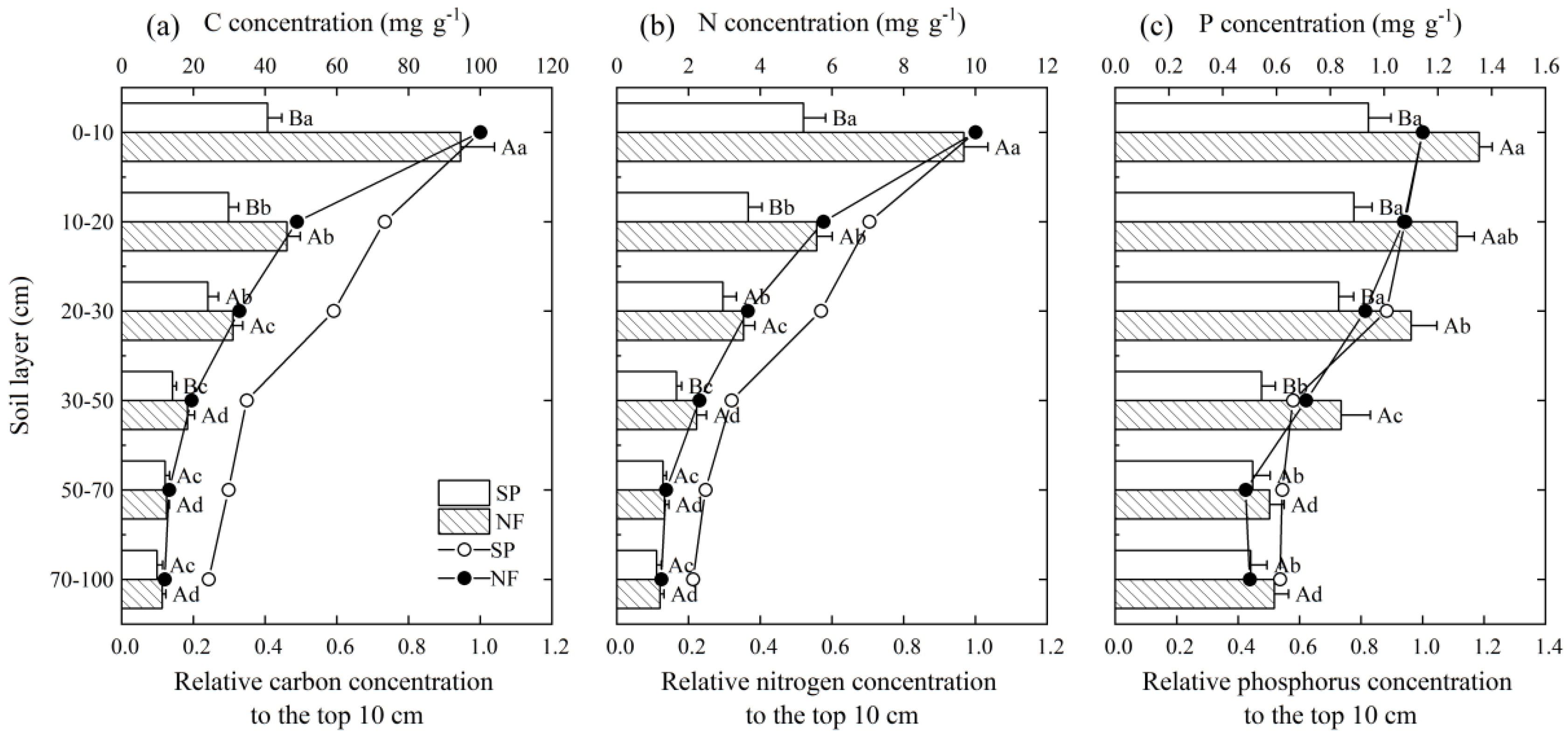

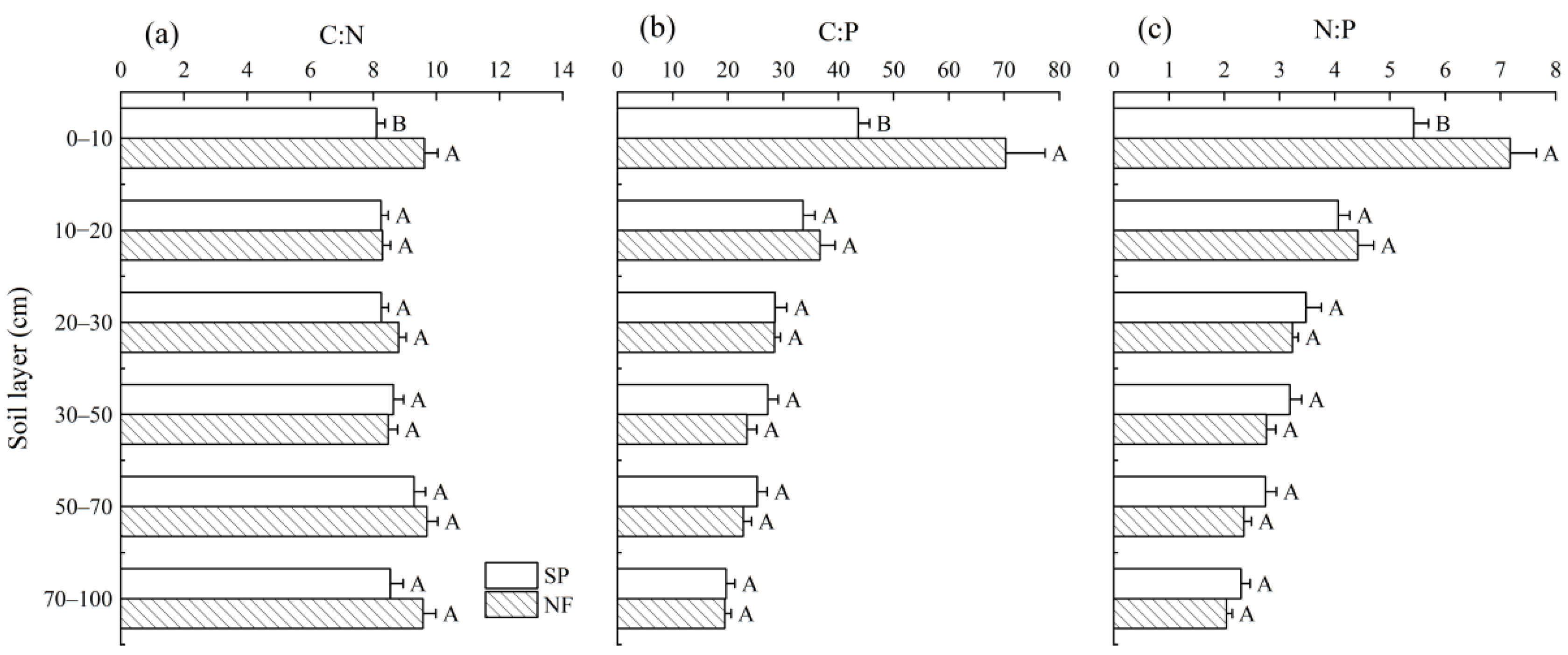


| Treatment | C density (h ta−1) | N density (h ta−1) | P density (h ta−1) |
|---|---|---|---|
| NF | 220.39 ± 12.11 Aa | 24.53 ± 1.27 Aa | 7.72 ± 0.41 Aa |
| SP based on FDM | 168.62 ± 8.17 B | 20.23 ± 1.16 B | 6.02 ± 0.37 B |
| SP based on ESM | 160.09 ± 6.36 b | 19.32 ± 0.99 b | 5.60 ± 0.29 b |
| Y | X | Treatment | R2 | Slope [95% CI] | LRT | p | Intercept |
|---|---|---|---|---|---|---|---|
| C | N | Natural forest (NF) | 0.763 | 91.01 a [81.05, 102.19] | −5.446 | ||
| Ski piste (SP) | 0.918 | 48.38 b [45.18, 51.81] | 66.064 | 0.001 | 5.968 | ||
| C | P | NF | 0.439 | 161.82 b [135.49, 193.28] | 45.87 | ||
| SP | 0.616 | 69.68 a [60.14, 80.74] | 46.565 | 0.001 | 35.28 | ||
| N | P | NF | 0.559 | 16.08 a [13.74, 18.83] | 4.942 | ||
| SP | 0.592 | 9.416 b [8.09, 10.96] | 22.158 | 0.001 | 4.471 |
| X | Soil Layer (cm) | Treatment | R2 | p | Slope [95% CI] | LRT | p | Intercept | Wald | p |
|---|---|---|---|---|---|---|---|---|---|---|
| SWC | 0–10 cm | Ski piste (SP) | 0.927 | <0.001 | −1.71 b [−2.06, −1.41] | 13.99 | 0.001 | 156.9 | — | — |
| Natural forest (NF) | 0.365 | 0.038 | −5.71 a [−9.75, −3.34] | 431.1 | ||||||
| 10–20 cm | SP | 0.798 | <0.001 | −1.95 a [−2.66, −1.42] | 2.95 | 0.092 | 172.3 b | 11.65 | 0.001 | |
| NF | 0.521 | 0.008 | −3.13 a [−5.01, −1.95] | 267.4 a | ||||||
| 20–30 cm | SP | 0.625 | 0.002 | −1.38 a [−2.10, −0.91] | 0.45 | 0.500 | 125.4 a | 6.69 | 0.010 | |
| NF | 0.318 | 0.056 | −1.11 a [−1.92, −0.64] | 114.0 b | ||||||
| 30–50 cm | SP | 0.765 | <0.001 | −1.53 a [−2.14, −1.09] | 0.34 | 0.559 | 134.3 a | 2.09 | 0.149 | |
| NF | 0.403 | 0.027 | −1.28 a [−2.16, −0.76] | 118.1 a | ||||||
| 50–70 cm | SP | 0.001 | 0.938 | −1.34 a [−2.58, −0.70] | 1.69 | 0.201 | 126.1 a | 0.58 | 0.448 | |
| NF | 0.540 | 0.007 | −0.80 a [−1.27, −0.50] | 79.2 a | ||||||
| 70–100 cm | SP | 0.139 | 0.233 | −2.65 a [−4.90, −1.43] | 6.93 | 0.007 | 242.4 | — | — | |
| NF | 0.204 | 0.140 | −0.84 b [−1.53, −0.47] | 83.6 | ||||||
| BD | 0–10 cm | SP | 0.810 | <0.001 | −69.60 a [−94.21, −51.42] | 4.13 | 0.054 | 107.0 b | 6.02 | 0.014 |
| NF | 0.151 | 0.212 | −139.51 a [−256.92, −75.76] | 175.9 a | ||||||
| 10–20 cm | SP | 0.762 | <0.001 | −68.68 a [−96.24, −49.02] | 1.47 | 0.218 | 108.9 b | 4.60 | 0.032 | |
| NF | 0.490 | 0.011 | −97.01 a [−157.41, −59.79] | 124.7 a | ||||||
| 20–30 cm | SP | 0.641 | 0.002 | −45.85 a [−69.12, −30.41] | 0.18 | 0.676 | 78.8 a | 0.002 | 0.969 | |
| NF | 0.658 | 0.001 | −40.90 a [−61.08, −27.38] | 73.3 a | ||||||
| 30–50 cm | SP | 0.517 | 0.008 | −39.40 a [−63.16, −24.57] | 0.20 | 0.644 | 66.8 a | 0.74 | 0.391 | |
| NF | 0.635 | 0.002 | −34.49 a [−52.17, −22.80] | 61.8 a | ||||||
| 50–70 cm | SP | 0.126 | 0.258 | 30.92 a [16.65, 57.39] | 4.23 | 0.048 | −37.5 | — | — | |
| NF | 0.742 | <0.001 | −14.85 b [−21.09, −10.46] | 34.31 | ||||||
| 70–100 cm | SP | 0.006 | 0.809 | −56.06 a [107.88, −29.13] | 8.79 | 0.006 | 20.5 | — | — | |
| NF | 0.484 | 0.012 | −16.17 b [−26.31, -9.94] | 37.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Duan, Y.; Zhao, H.; Zhang, L.; Wang, X.; Zhang, H. The Impact of a Ski Piste on the Stock and Stoichiometry of Soil Carbon, Nitrogen, and Phosphorus: A Case Study on a Forest Area in Northeast China. Land 2025, 14, 846. https://doi.org/10.3390/land14040846
Han Y, Duan Y, Zhao H, Zhang L, Wang X, Zhang H. The Impact of a Ski Piste on the Stock and Stoichiometry of Soil Carbon, Nitrogen, and Phosphorus: A Case Study on a Forest Area in Northeast China. Land. 2025; 14(4):846. https://doi.org/10.3390/land14040846
Chicago/Turabian StyleHan, Yongjie, Yichen Duan, Huabin Zhao, Luna Zhang, Xingchang Wang, and Haiyan Zhang. 2025. "The Impact of a Ski Piste on the Stock and Stoichiometry of Soil Carbon, Nitrogen, and Phosphorus: A Case Study on a Forest Area in Northeast China" Land 14, no. 4: 846. https://doi.org/10.3390/land14040846
APA StyleHan, Y., Duan, Y., Zhao, H., Zhang, L., Wang, X., & Zhang, H. (2025). The Impact of a Ski Piste on the Stock and Stoichiometry of Soil Carbon, Nitrogen, and Phosphorus: A Case Study on a Forest Area in Northeast China. Land, 14(4), 846. https://doi.org/10.3390/land14040846






