Can Rock-Rubble Groynes Support Similar Intertidal Ecological Communities to Natural Rocky Shores?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
2.2. Data Analysis
3. Results
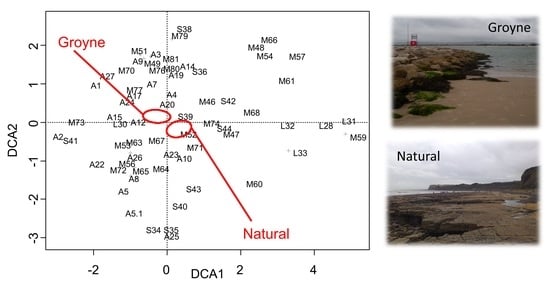
3.1. Species Richness and Abundance
3.2. Species- and Community-Level Analysis
3.3. Species Ranges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Holloway, P.; Miller, J.A.; Gillings, S. Incorporating movement in species distribution models: How do simulations of dispersal affect the accuracy and uncertainty of projections? Int. J. Geogr. Inf. Sci. 2016, 30, 1–25. [Google Scholar] [CrossRef]
- Crowe, T.; Thompson, R.C.; Bray, S.; Hawkins, S. Impacts of anthropogenic stress on rocky intertidal communities. J. Aquat. Ecosyst. Stress Recover. 2000, 7, 273–297. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Helmuth, B. Local- and regional-scale effects of wave exposure, thermal stress, and absolute versus effective shore level on patterns of intertidal zonation. Limnol. Oceanogr. 2003, 48, 1498–1508. [Google Scholar] [CrossRef]
- Lees, F.; Baillie, M.; Gettinby, G.; Revie, C.W. The Efficacy of Emamectin Benzoate against infestations of Lepeoptheirus salmonis on Farmed Atlantic Salmon (Salmo salar L.) in Scotland, 2002–2006. PLoS ONE 2008, 3, e1549. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Birchenough, S.N.R.; Mieszkowska, N.; Robinson, L.A.; Simpson, S.; Burrows, M.T.; Capasso, E.; Cleall-Harding, P.; Crummy, J.; Duck, C.; et al. Temporal change in UK marine communities: Trends or regime shifts? Mar. Ecol. 2011, 32, 10–24. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Davidson, V.E.; Franklin, M.C.; Benes, K.M.; Doellman, M.M.; Etter, R.J.; Hannigan, R.; Lubchenco, J.; Menge, B.A. Long-term declines in an intertidal foundation species parallel shifts in community composition. Glob. Chang. Biol. 2016, 23, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obst, M.; Vicario, S.; Lundin, K.; Berggren, M.; Karlsson, A.; Haines, R.; Williams, A.; Goble, C.; Mathew, C.; Güntsch, A. Marine long-term biodiversity assessment suggests loss of rare species in the Skagerrak and Kattegat region. Mar. Biodivers. 2017, 48, 2165–2176. [Google Scholar] [CrossRef]
- Hillebrand, H.; Brey, T.; Gutt, J.; Hagen, W.; Metfies, K.; Meyer, B.; Lewandowska, A.M. Climate Change: Warming Impacts on Marine Biodiversity. In Handbook on Marine Environment Protection; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 353–373. [Google Scholar]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Neumann, B.; Vafeidis, A.; Zimmermann, J.; Nicholls, R. Future Coastal Population Growth and Exposure to Sea-Level Rise and Coastal Flooding—A Global Assessment. PLoS ONE 2015, 10, e0118571. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Fragkias, M.; Güneralp, B.; Reilly, M.K. A Meta-Analysis of Global Urban Land Expansion. PLoS ONE 2011, 6, e23777. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.; Chapman, M. Mitigating against the loss of species by adding artificial intertidal pools to existing seawalls. Mar. Ecol. Prog. Ser. 2014, 497, 119–129. [Google Scholar] [CrossRef]
- Bulleri, F.; Chapman, M.G. The introduction of coastal infrastructure as a driver of change in marine environments. J. Appl. Ecol. 2010, 47, 26–35. [Google Scholar] [CrossRef]
- Nordstrom, K.F. Living with shore protection structures: A review. Estuar. Coast. Shelf Sci. 2014, 150, 11–23. [Google Scholar] [CrossRef]
- Perkins, M.J.; Ng, T.P.; Dudgeon, D.; Bonebrake, T.C.; Leung, K.M. Conserving intertidal habitats: What is the potential of ecological engineering to mitigate impacts of coastal structures? Estuar. Coast. Shelf Sci. 2015, 167, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.; Bulleri, F. Intertidal seawalls—New features of landscape in intertidal environments. Landsc. Urban Plan. 2003, 62, 159–172. [Google Scholar] [CrossRef]
- Moschella, P.; Abbiati, M.; Aberg, P.; Airoldi, L.; Anderson, J.; Bacchiocchi, F.; Bulleri, F.; Dinesen, G.; Frost, M.; Gacia, E.; et al. Low-crested coastal defence structures as artificial habitats for marine life: Using ecological criteria in design. Coast. Eng. 2005, 52, 1053–1071. [Google Scholar] [CrossRef]
- Pinn, E.H.; Mitchell, K.; Corkill, J. The assemblages of groynes in relation to substratum age, aspect and microhabitat. Estuar. Coast. Shelf Sci. 2005, 62, 271–282. [Google Scholar] [CrossRef]
- Lam, N.W.; Huang, R.; Chan, B.K. Variations in Intertidal assemblages and zonation patterns between vertical artificial seawalls and natural rocky shores: A case study from Victoria Harbour, Hong Kong. Zool. Stud. 2009, 48, 184–195. [Google Scholar]
- Firth, L.B.; Thompson, R.C.; White, F.J.; Schofield, M.; Skov, M.W.; Hoggart, S.P.G.; Jackson, J.; Knights, A.M.; Hawkins, S.J. The importance of water-retaining features for biodiversity on artificial intertidal coastal defence structures. Divers. Distrib. 2013, 19, 1275–1283. [Google Scholar] [CrossRef] [Green Version]
- Firth, L.B.; Thompson, R.C.; Bohn, K.; Abbiati, M.; Airoldi, L.; Bouma, T.; Bozzeda, F.; Ceccherelli, V.; Colangelo, M.; Evans, A.; et al. Between a rock and a hard place: Environmental and engineering considerations when designing coastal defence structures. Coast. Eng. 2014, 87, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, M.A.; Broitman, B.R.; Thiel, M. Spatial variability in community composition on a granite breakwater versus natural rocky shores: Lack of microhabitats suppresses intertidal biodiversity. Mar. Pollut. Bull. 2014, 87, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Fernandez, J.A.; Lazzari, N.; Riera, R.; Becerro, M.A. Building up marine biodiversity loss: Artificial substrates hold lower number and abundance of low occupancy benthic and sessile species. Mar. Environ. Res. 2018, 140, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Chapman, M.G.; Underwood, A.J. Intertidal assemblages on seawalls and vertical rocky shores in Sydney Harbour, Australia. Austral. Ecol. 2005, 30, 655–667. [Google Scholar] [CrossRef]
- Bacchiocchi, F.; Airoldi, L. Distribution and dynamics of epibiota on hard structures for coastal protection. Estuar. Coast. Shelf Sci. 2003, 56, 1157–1166. [Google Scholar] [CrossRef]
- Pister, B. Urban marine ecology in southern California: The ability of riprap structures to serve as rocky intertidal habitat. Mar. Biol. 2009, 156, 861–873. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.G. Paucity of mobile species on constructed sea walls: Effects of urbanisation on biodiversity. Mar. Ecol. Prog. Ser. 2003, 264, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Clynick, B.G. Assemblages of fish associated with coastal marinas in north-western Italy. J. Mar. Biol. Assoc. U. K. 2006, 86, 847–852. [Google Scholar] [CrossRef]
- Pinn, E.H.; Rodgers, M. The influence of visitors on intertidal biodiversity. J. Mar. Biol. Assoc. U. K. 2005, 85, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Bishop, M.J.; Mayer-Pinto, M.; Airoldi, L.; Firth, L.B.; Morris, R.L.; Loke, L.H.L.; Hawkins, S.; Naylor, L.; Coleman, R.A.; Chee, S.Y.; et al. Effects of ocean sprawl on ecological connectivity: Impacts and solutions. J. Exp. Mar. Biol. Ecol. 2017, 492, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Bulleri, F.; Airoldi, L. Artificial marine structures facilitate the spread of a non-indigenous green alga, Codium fragile spp tomentosoides, in the north Adriatic Sea. J. Appl. Ecol. 2005, 42, 1063–1072. [Google Scholar] [CrossRef]
- Airoldi, L.; Turon, X.; Perkol-Finkel, S.; Rius, M. Corridors for aliens but not for natives: Effects of marine urban sprawl at a regional scale. Divers. Distrib. 2015, 21, 755–768. [Google Scholar] [CrossRef] [Green Version]
- Holloway, M.G.; Keough, M.J. An introduced polychaete affects recruitment and larval abundance of sessile invertebrates. Ecol. Appl. 2002, 12, 1803–1823. [Google Scholar] [CrossRef]
- Bulleri, F.; Abbiati, M.; Airoldi, L. The colonization of artificial human-made structures by the invasive alga Codium fragile ssp. tomentosoides in the north Adriatic Sea (NE Mediterranean). Hydrobiologia 2006, 555, 263–269. [Google Scholar] [CrossRef]
- Glasby, T.M.; Connell, S.D.; Holloway, M.G.; Hewitt, C. Nonindigenous biota on artificial structures: Could habitat creation facilitate biological invasions? Mar. Biol. 2006, 151, 887–895. [Google Scholar] [CrossRef]
- Airoldi, L.; Bulleri, F. Anthropogenic Disturbance Can Determine the Magnitude of Opportunistic Species Responses on Marine Urban Infrastructures. PLoS ONE 2011, 6, e22985. [Google Scholar] [CrossRef] [Green Version]
- Vaselli, S.; Bulleri, F.; Benedetti-Cecchi, L. Hard coastal-defence structures as habitats for native and exotic rocky-bottom species. Mar. Environ. Res. 2008, 66, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.; Ebert, C.; Fox, H.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, S.J.; Jones, H.D. Marine Field Course Guide: Rocky Shores; IMMEL Publishing: London, UK, 1992. [Google Scholar]
- Lohrer, A.M.; Fukui, Y.; Wada, K.; Whitlatch, R.B. Structural complexity and vertical zonation of intertidal crabs, with focus on habitat requirements of the invasive Asian shore crab, Hemigrapsus sanguineus (de Haan). J. Exp. Mar. Biol. Ecol. 2000, 244, 203–217. [Google Scholar] [CrossRef]
- Walker, S.J.; Schlacher, T.A.; Thompson, L.M. Habitat modification in a dynamic environment: The influence of a small artificial groyne on macrofaunal assemblages of a sandy beach. Estuar. Coast. Shelf Sci. 2008, 79, 24–34. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Low impact of disturbance on ecological success of invasive tree and shrub species in temperate forests. Plant Ecol. 2018, 219, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package version 2.5-5; R Core Team: Vienna, Austria, 2019; Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 June 2019).
- Ter Braak, C.; Smilauer, P. Topics in constrained and unconstrained ordination. Plant Ecol. 2014, 216, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.W. Putting Things in Even Better Order: The Advantages of Canonical Correspondence Analysis. Ecology 1993, 74, 2215–2230. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 19 August 2011).
- Gibson, R.; Hextall, B.; Rogers, A. Photographic Guide to the Sea and Shore Life of Britain and North-West Europe; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- MarLIN (Marine Life Information Network). Marine Life Information Network; Marine Biological Association of the United Kingdom: Plymouth, UK, 2016; Available online: www.marlin.ac.uk (accessed on 1 January 2009).
- Connor, D.W.; Allen, J.H.; Golding, N.; Howell, K.L.; Lieberknecht, L.M.; Northern, K.O.; Reker, J.B. The Marine Habitat Classification for Britain and Ireland; Version 04.05; JNCC: Peterborough, UK, 2004. [Google Scholar]
- Werner, A. Lichen Growth Rates for the Northwest Coast of Spitsbergen, Svalbard. Arct. Alp. Res. 1990, 22, 129. [Google Scholar] [CrossRef]
- Mieszkowska, N. Osilinus lineatus. Thick Top Shell. Marine Life Information Network: Biology and Sensitivity Key Information Sub-Programme; Marine Biological Association of the United Kingdom: Plymouth, UK, 2008; Available online: http://www.marlin.ac.uk/speciesfullreview.php?speciesID=3990 (accessed on 30 March 2012).
- Pizzolla, P. Melarhaphe neritoides. Small periwinkle. Marine Life Information Network: Biology and Sensitivity Key Information Sub-Programme; Marine Biological Association of the United Kingdom: Plymouth, UK, 2007; Available online: http://www.marlin.ac.uk/speciesinformation.php?speciesID=3785 (accessed on 30 March 2012).
- Avant, P. Elminius modestus. An Acorn Barnacle. Marine Life Information Network: Biology and Sensitivity Key Information Sub-Programme; Marine Biological Association of the United Kingdom: Plymouth, UK, 2007; Available online: http://www.marlin.ac.uk/speciesinformation.php?speciesID=3252 (accessed on 30 March 2012).
- Ji, Y.; Tanaka, J. Effect of desiccation on the photosynthesis of seaweeds from the intertidal zone in Honshu, Japan. Phycol. Res. 2002, 50, 145–153. [Google Scholar] [CrossRef]
- Baugh, T.M.; Yarish, C.; Kirkman, H. Seaweeds: Their Environment, Biogeography, and Ecophysiology. Revised Translation of (Meersbotanik: Verbreitung, ökophysiologie und Nutzung der Marinen Makroalgen), K. Lüning (1985). Estuaries 1992, 15, 255. [Google Scholar] [CrossRef]
- Moreira, J.; Chapman, M.; Underwood, A. Maintenance of chitons on seawalls using crevices on sandstone blocks as habitat in Sydney Harbour, Australia. J. Exp. Mar. Biol. Ecol. 2007, 347, 134–143. [Google Scholar] [CrossRef]
- Chapman, M.G.; Blockley, D.J. Engineering novel habitats on urban infrastructure to increase intertidal biodiversity. Oecologia 2009, 161, 625–635. [Google Scholar] [CrossRef]
- Firth, L.B.; Browne, K.A.; Knights, A.M.; Hawkins, S.J.; Nash, R. Eco-engineered rock pools: A concrete solution to biodiversity loss and urban sprawl in the marine environment. Environ. Res. Lett. 2016, 11, 94015. [Google Scholar] [CrossRef]
- Strain, E.M.A.; Olabarria, C.; Mayer-Pinto, M.; Cumbo, V.; Morris, R.L.; Bugnot, A.; Dafforn, K.; Heery, E.; Firth, L.B.; Brooks, P.; et al. Eco-engineering urban infrastructure for marine and coastal biodiversity: Which interventions have the greatest ecological benefit? J. Appl. Ecol. 2017, 55, 426–441. [Google Scholar] [CrossRef]
- Evans, S.; Finnie, M.; Manica, A. Shoaling preferences in decapod crustacea. Anim. Behav. 2007, 74, 1691–1696. [Google Scholar] [CrossRef]
- Martins, G.M.; Hawkins, S.; Thompson, R.C.; Jenkins, S.R. Community structure and functioning in intertidal rock pools: Effects of pool size and shore height at different successional stages. Mar. Ecol. Prog. Ser. 2007, 329, 43–55. [Google Scholar] [CrossRef]
- Moksnes, P.O.; Pihl, L.; van Montfrans, J. Predation on postlarvae and juveniles of the shorecrab Carcinus maenus: Importance of shelter, size, and cannibalism. Mar. Ecol. Prog. Ser. 1998, 166, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Pugh, D.T. Changing Sea Levels: Effects of Tide, Weather, and Climate; Cambridge University Press: New York, NY, USA, 2004. [Google Scholar]
- Humphreys, J. Salinity and Tides in Poole Harbour: Estuary or Lagoon? In The Ecology of Poole Harbour; Humphreys, J., May, V., Eds.; Elsevier: Oxford, UK, 2005. [Google Scholar]
- Bromley, R.G.; Heinberg, C. Attachment strategies of organisms on hard substrates: A palaeontological view. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 232, 429–453. [Google Scholar] [CrossRef]
- Coombes, M.A.; La Marca, E.C.; Naylor, L.A.; Thompson, R.C. Getting into the goove: Opportunities to enhance the ecological value of hard coastal infrastructure using fine-scale surface textures. Ecol. Eng. 2015, 77, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, R.L.; Callow, M.E. The settlement, attachment and establishment of marine algal spores. Br. Phycol. J. 1992, 27, 303–329. [Google Scholar] [CrossRef] [Green Version]
- Underwood, A.; Chapman, M.; Crowe, T. Identifying and understanding ecological preferences for habitat or prey. J. Exp. Mar. Biol. Ecol. 2004, 300, 161–187. [Google Scholar] [CrossRef]
- Jones, C.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Ramsey, J.; Osorio-Olvera, L. Co-occurrence Networks do not Support Identification of Biotic Interactions. Biodivers. Inform. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Dormann, C.F.; Bobrowski, M.; Dehling, D.M.; Harris, D.J.; Hartig, F.; Lischke, H.; Moretti, M.D.; Pagel, J.; Pinkert, S.; Schleuning, M.; et al. Biotic interactions in species distribution modelling: 10 questions to guide interpretation and avoid false conclusions. Glob. Ecol. Biogeogr. 2018, 27, 1004–1016. [Google Scholar] [CrossRef] [Green Version]
- Bracewell, S.A.; Robinson, L.A.; Firth, L.B.; Knights, A.M. Predicting Free-Space Occupancy on Novel Artificial Structures by an Invasive Intertidal Barnacle Using a Removal Experiment. PLoS ONE 2013, 8, e74457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, J.S.; Ding, L.; Stokes, J.; Kavouras, J.H.; Rittschof, D. Substratum/bacterial interactions and larval attachment: Films and exopolysaccharides ofHalomonas marina (ATCC 25374) and their effect on barnacle cyprid larvae, Balanus amphitriteDarwin. Biofouling 2000, 16, 159–170. [Google Scholar] [CrossRef]
- Miron, G.; Boudrea, B.; Bourget, E. Intertidal barnacle distribution:a case study using multiple working hypotheses. Mar. Ecol. Prog. Ser. 1999, 189, 205–219. [Google Scholar] [CrossRef]
- Crothers, J.H. Common topshells: An introduction to the biology of Osilinus lineatus with notes on other species in the genus. Field Stud. 2001, 10, 115–160. [Google Scholar]
- Riel Patrick, V.; Breugelmans, K.; De Wolf, H.; Mikhailova, N.; Backeljau, T. Analysis of mitochondrial DNA variation via PCR-SSCP revels micro- and macrogeographic genetic heterogeneity in the planktonic developing periwinkle, Melaraphe neriotoides (Caenogastropoda, Littorinidae). Vlis Spec. Publ. 2004, 17, 76. [Google Scholar]
- Wang, W.; Wang, J.; Choi, F.M.P.; Ding, P.; Li, X.; Han, G.; Ding, M.; Guo, M.; Huang, X.; Duan, W.; et al. Global warming and artificial shorelines reshape seashore biogeography. Glob. Ecol. Biogeogr. 2019, 29, 220–231. [Google Scholar] [CrossRef]
- Chu, F.J.; Seaward, M.R.D.; Hodgkiss, I.J. Effects of Wave Exposure and Aspect on Hong Kong Supralittoral Lichens. Lichenologist 2000, 32, 155–170. [Google Scholar] [CrossRef]
- Firth, L.B.; White, F.J.; Schofield, M.; Hanley, M.E.; Burrows, M.T.; Thompson, R.C.; Skov, M.W.; Evans, A.; Moore, P.J.; Hawkins, S.J. Facing the future: The importance of substratum features for ecological engineering of artificial habitats in the rocky intertidal. Mar. Freshw. Res. 2016, 67, 131. [Google Scholar] [CrossRef]




| Response Variable | Type | Level | County | Type * Level | Type * County | Level * County | Type * Level * County | |
|---|---|---|---|---|---|---|---|---|
| df: | 1 | 2 | 2 | 2 | 2 | 4 | 4 | |
| total: Rock | F: | 14.76 ** | 50.85 ** | 9.18 ** | 5.09 ** | 1.00 | 3.48 ** | 4.88 ** |
| algae: Rock | F: | 0.56 | 37.45 ** | 41.17 ** | 4.76 ** | 8.19 ** | 3.26 * | 2.12 |
| lichen: Rock | F: | 58.89 ** | 50.06 ** | 13.79 ** | 33.92 ** | 9.32 ** | 8.49 ** | 5.07 ** |
| sessile: Rock | F: | 17.75 ** | 36.02 ** | 12.45 ** | 3.05 * | 0.83 | 7.12 ** | 7.31 ** |
| mobile: Rock | F: | 24.27 ** | 14.71 ** | 22.98 ** | 7.18 ** | 8.24 ** | 6.11 ** | 9.15 ** |
| total: Pool | F: | 58.74 ** | 5.55 ** | 2.72 | 1.40 | 1.46 | 5.46 ** | 2.68 |
| algae: Pool | F: | 22.45 ** | 3.96 * | 3.85 * | 3.23 | 7.20 ** | 4.03 * | 0.22 |
| sessile: Pool | F: | 0.007 | 8.90 ** | 3.15 | 1.10 | 5.48 * | 0.45 | 1.77 |
| mobile: Pool | F: | 56.97 ** | 0.81 | 2.52 | 0.54 | 0.41 | 3.23 * | 3.21 * |
| Response Variable | Type | Level | County | Type * Level | Type * County | Level * County | Type * Level * County | |
|---|---|---|---|---|---|---|---|---|
| df: | 1 | 2 | 2 | 2 | 2 | 4 | 4 | |
| algae: Rock (sqrt) | F: | 0.02 | 11.37 ** | 31.90 ** | 10.32 ** | 12.47 ** | 3.13 * | 3.81 ** |
| lichen: Rock (sqrt) | F: | 57.52 ** | 47.30 ** | 12.74 ** | 31.16 ** | 8.53 ** | 8.90 ** | 5.53 ** |
| sessile: Rock (sqrt) | F: | 52.21 ** | 79.60 ** | 15.27 ** | 20.44 ** | 15.65 ** | 3.53 ** | 14.62 ** |
| mobile: Rock (sqrt) | F: | 1.05 | 17.13 ** | 53.42 ** | 16.90 ** | 8.60 ** | 8.59 ** | 2.16 |
| algae: Pool (sqrt) | F: | 11.25 ** | 1.88 | 0.01 | 8.03 ** | 5.56 ** | 3.16 * | 1.60 |
| sessile: Pool (sqrt) | F: | 0.97 | 7.37 ** | 5.42 * | 1.51 | 10.80 ** | 0.29 | 3.22 * |
| mobile: Pool (sqrt) | F: | 54.02 ** | 2.49 | 3.80 * | 3.98 * | 9.13 ** | 2.67 | 1.66 |
| Species | ||||
|---|---|---|---|---|
| Padina Pavonica | Nucella Lapillus | Pomatoschistus Minutus | ||
| Marine and Conservation Conventions and Legislation | UK BAP | OSPAR | Bern Convention | |
| Structure Type | Habitat | |||
| Groyne | Rock | X | 102.3 | X |
| Groyne | Pool | X | 26.5 | 0.3 |
| Natural | Rock | X | 1.0 | X |
| Natural | Pool | 14.3 | 1.0 | 11.7 |
| Site | County | Structure Type | Shore Level | ||
|---|---|---|---|---|---|
| High | Mid | Low | |||
| Sidmouth | Devon | Groyne | 5.9 | 49.8 | 60.8 |
| Stokes | Devon | Natural | 13.9 | 31.8 | 29.9 |
| West Runton | Norfolk | Natural | 0 | 2.3 | 0 |
| Groynes | Natural | |
|---|---|---|
| High shore | ||
| All counties | 0.9 | 9.7 |
| Devon | 1.0 ±1.0 | 0.7 ±0.6 |
| Dorset | 2.0 ±1.7 | 7.3 ±1.2 |
| Norfolk | 0.3 ±0.6 | 18.7 ±7.6 |
| Mid shore | ||
| All counties | 2.3 | 14.8 |
| Devon | 1.0 ±1.0 | 6.7 ±1.5 |
| Dorset | 3.3 ±0.6 | 13.7 ±5.1 |
| Norfolk | 3.0 ±1.0 | 26.3 ±3.2 |
| Low shore | ||
| All counties | 3.8 | 13.7 |
| Devon | 0.3 ±0.6 | 8.7 ±2.5 |
| Dorset | 5.0 ±1.0 | 20.0 ±1.7 |
| Norfolk | 4.7 ±0.6 | 12.3 ±2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holloway, P.; Field, R. Can Rock-Rubble Groynes Support Similar Intertidal Ecological Communities to Natural Rocky Shores? Land 2020, 9, 131. https://doi.org/10.3390/land9050131
Holloway P, Field R. Can Rock-Rubble Groynes Support Similar Intertidal Ecological Communities to Natural Rocky Shores? Land. 2020; 9(5):131. https://doi.org/10.3390/land9050131
Chicago/Turabian StyleHolloway, Paul, and Richard Field. 2020. "Can Rock-Rubble Groynes Support Similar Intertidal Ecological Communities to Natural Rocky Shores?" Land 9, no. 5: 131. https://doi.org/10.3390/land9050131
APA StyleHolloway, P., & Field, R. (2020). Can Rock-Rubble Groynes Support Similar Intertidal Ecological Communities to Natural Rocky Shores? Land, 9(5), 131. https://doi.org/10.3390/land9050131