The Chirality Induction and Modulation of Polymers by Circularly Polarized Light
Abstract
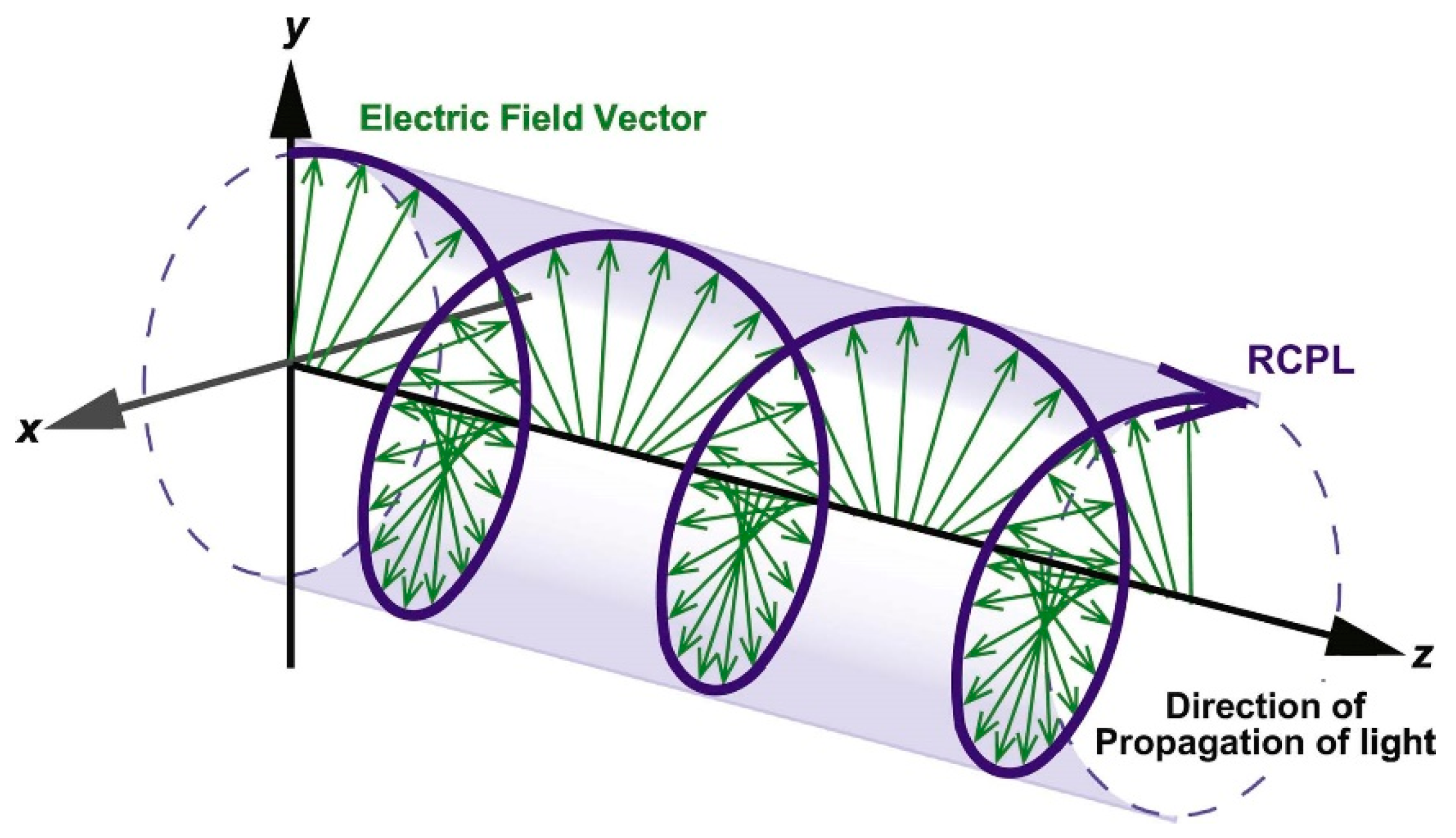
:1. Introduction
2. The Asymmetric Synthesis of Chiral Polymers Based on Circularly Polarized Light
2.1. The Asymmetric Polymerization of Diacetylene
2.2. Enantioselective Synthesis of Chiral Coordination Polymers with CPL
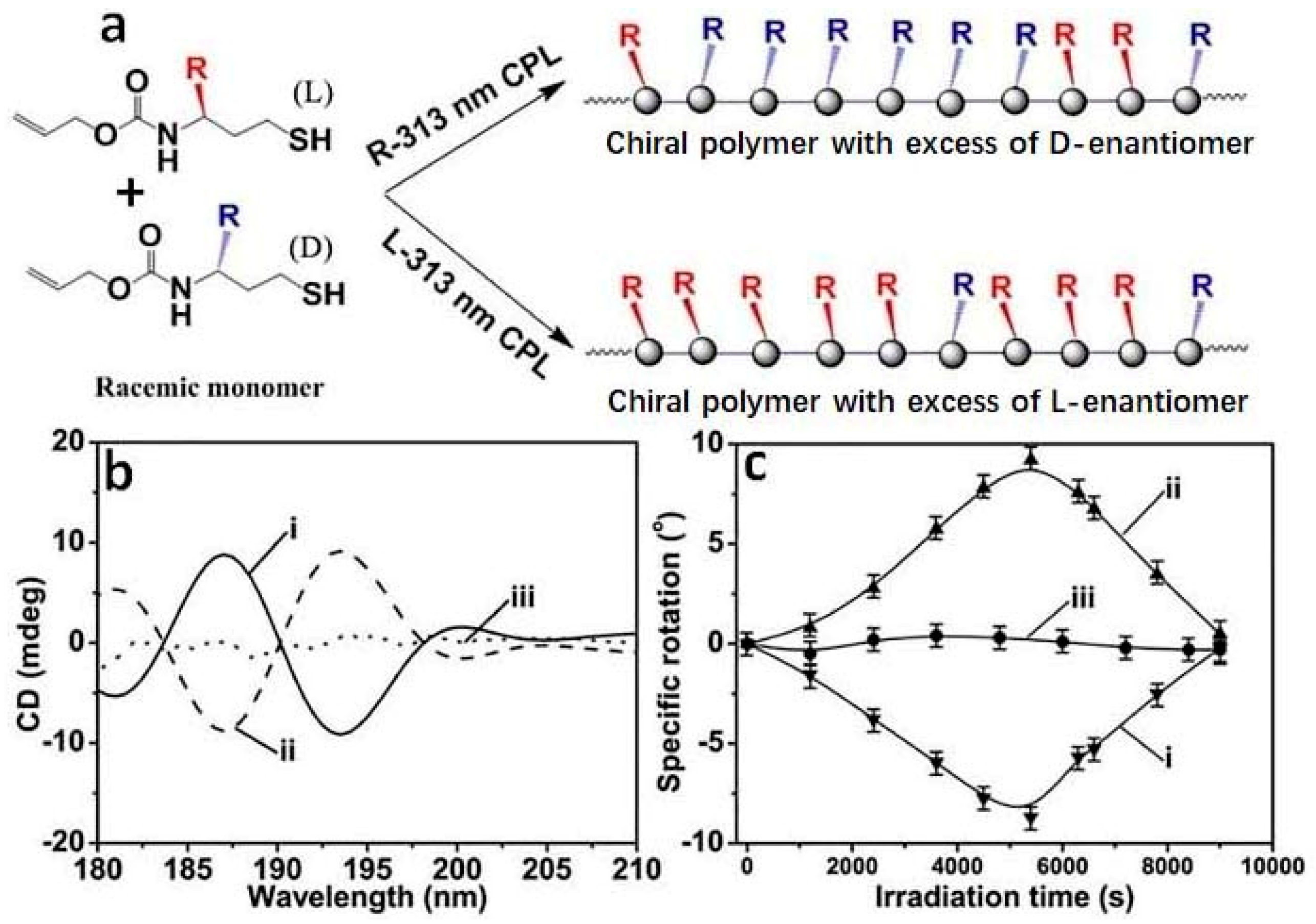
2.3. Enantioselective Thiol-ene Polymerization Reaction Triggered by CPL
3. The Asymmetric Photo-Modulation of Chirality Polymers Based on Circularly Polarized Light
3.1. Enantioselective Photo-Modulations of Azobenzene Polymers
3.2. Enantioselective Photo-Modulations of Ketone-Containing Polymers
3.3. Enantioselective Photo-Modulations of Fluorene-Based Polymers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qiu, M.; Zhang, L.; Tang, Z.X.; Jin, W.; Qiu, C.W.; Lei, D.Y. 3D Metaphotonic Nanostructures with Intrinsic Chirality. Adv. Funct. Mater. 2018, 28, 1803147–1803162. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Lynch, C.C.; Wolf, C. Optical Chirality Sensing with an Auxiliary-Free Earth-Abundant Cobalt Probe. Angew. Chem. Int. Ed. 2019, 58, 1198–1202. [Google Scholar] [CrossRef]
- Campbell, J.P.; Rajappan, S.C.; Jaynes, T.J.; Sharafi, M.; Ma, Y.T.; Li, J.N.; Schneebeli, S.T. Enantioselective Electrophilic Aromatic Nitration: AChiral Auxiliary Approach. Angew. Chem. Int. Ed. 2019, 58, 1035–1040. [Google Scholar] [CrossRef]
- Liu, G.F.; Sheng, J.H.; Teo, W.L.; Yang, G.B.; Wu, H.W.; Li, Y.X.; Zhao, Y.L. Control on Dimensions and Supramolecular Chirality of Self-Assemblies through Light and Metal Ions. J. Am. Chem. Soc. 2018, 140, 16275–16283. [Google Scholar] [CrossRef] [PubMed]
- Zor, E.; Bekar, N. Lab-in-a-syringe using gold nanoparticles for rapid colorimetric chiral discrimination of enantiomers. Biosens. Bioelectron. 2017, 91, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Kim, S.; Han, M.S. Gold nanoparticle-based colorimetric chiral discrimination of histidine: Application to determining the enantiomeric excess of histidine. Anal. Methods 2014, 6, 73–76. [Google Scholar] [CrossRef]
- Ngamdee, K.; Ngeonyae, W. Circular dichroism glucose biosensor based on chiral cadmium sulfide quantum dots. Sens. Actuators B 2018, 274, 402–411. [Google Scholar] [CrossRef]
- Copura, F.; Bekarb, N.; Zorc, E.; Alpaydind, S.; Bingold, H. Nanopaper-based photoluminescent enantioselective sensing of L-Lysine by L-Cysteine modified carbon quantum dots. Sens. Actuators B 2019, 279, 305–312. [Google Scholar] [CrossRef]
- Suzuki, N.; Wang, Y.C.; Elvati, P.; Qu, Z.B.; Kim, K.; Jiang, S.; Baumeister, E.; Lee, J.; Yeom, B.J.; Bahng, J.H.; et al. Chiral Graphene Quantum Dots. ACS Nano 2016, 10, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Li, G.H.; Yin, Y.J.; Zhang, Y.Q.; Li, H.G. Carbon quantum dot-based fluorescent vesicles and chiral hydrogels with biosurfactant and biocompatible small molecule. Soft Matter 2018, 14, 6983–6993. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Lu, X.T.; Zhong, Y.H.; Hu, Y.F.; Li, G.K.; Zhang, R.K. Carbon dot-decorated porous organic cage as fluorescenct sensor for rapid discrimination of nitrophenol isomers and alcohols. Anal. Chim. Acta 2019, 1050, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; Yang, C.N. Question of parity conservation in weak interactions. Phys. Rev. 1956, 104, 254–258. [Google Scholar] [CrossRef]
- Shukla, N.; Bartel, M.A.; Gellman, A.J. Enantioselective Separation on Chiral Au Nanoparticles. J. Am. Chem. Soc. 2010, 132, 8575–8580. [Google Scholar] [CrossRef]
- Jose BA, S.; Matsushita, S.; Akagi, K. Lyotropic Chiral Nematic Liquid Crystalline Aliphatic Conjugated Polymers Based on Disubstituted Polyacetylene Derivatives That Exhibit High Dissymmetry Factors in Circularly Polarized Luminescence. J. Am. Chem. Soc. 2012, 134, 19795–19807. [Google Scholar] [CrossRef] [PubMed]
- Spano, F.C.; Meskers SC, J.; Hennebicq, E.; Beljonne, D. Probing Excitation Delocalization in Supramolecular Chiral Stacks by Means of Circularly Polarized Light: Experiment and Modeling. J. Am. Chem. Soc. 2007, 129, 7044–7054. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Nothofer, H.G.; Scherf, U.; Šunjic’, V.; Richter, D.; Regenstein, W.; Neher, D. Chiroptical Properties of Chiral Substituted Polyfluorenes. Macromolecules 2002, 35, 6792–6798. [Google Scholar] [CrossRef]
- Gilot, B.J.; Abbel, R.; Lakhwani, G.; Meijer, E.W.; Schenning AP, H.J.; Meskers SC, J. Polymer Photovoltaic Cells Sensitive to the Circular Polarization of Light. Adv. Mater. 2010, 22, 131–134. [Google Scholar] [CrossRef]
- Wu, D.T.; Yang, J.P.; Peng, Y.G.; Yu, Y.; Zhang, J.; Guo, L.L.; Kong, Y.A.; Jiang, J.L. Highly enantioselective recognition of various acids using polymerized chiral ionic liquid as electrode modifies. Sens. Actuators B 2019, 282, 164–170. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, G.X.; Ma, Z.Y.; Xu, L.; Wang, H. Homochiral Double Helicates Based on Cyclooctatetrathiophene: Chiral Self-Sorting with the Intramolecular S···N Interaction. Chem. Eur. J. 2018, 24, 15993–15997. [Google Scholar] [CrossRef]
- Ding, J.W.; Zhang, M.; Dai, H.X.; Lin, C.M. Enantioseparation of chiral mandelic acid derivatives by supercritical fluid chromatography. Chirality 2018, 30, 1245–1256. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zhao, F.; Yu, J.; Wang, Z.K.; Song, Y.B.; Li, Q. Chiral separation and a molecular modeling study of eight azole antifungals on the cellulose tris(3,5-dichlorophenylcarbamate) chiral stationary phase. New J. Chem. 2018, 42, 13421–13429. [Google Scholar] [CrossRef]
- Bruno, R.; Marino, N.; Bartella, L.; Donna, L.D.; Munno, G.D.; Pardo, E.; Armentano, D. Highly efficient temperature-dependent chiral separation with a nucleotide-based coordination polymer. Chem. Commun. 2018, 54, 6356–6359. [Google Scholar] [CrossRef]
- Mao, B.; Mastral, M.F.; Feringa, B.L. Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev. 2017, 117, 10502–105066. [Google Scholar] [CrossRef]
- Chu, J.H.; Xu, X.H.; Kang, S.M.; Liu, N.; Wu, Z.Q. Fast Living Polymerization and Helix-Sense-Selective Polymerization of Diazoacetates Using Air-Stable Palladium (II) Catalysts. J. Am. Chem. Soc. 2018, 140, 17773–17781. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Jia, H.G.; Shi, Y.Q.; Ma, L.Q.; Yang, G.X.; Wang, Y.Z.; Xu, S.P.; Wang, J.J.; Zang, Y.; Aoki, T. [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes. Polymers 2018, 10, 1223. [Google Scholar] [CrossRef]
- Suárez-Picado, E.; Quiñoá, E.; Riguera, R.; Freire, F. Poly(phenylacetylene) Amines: A General Route to Water-Soluble Helical Polyamines. Chem. Mater. 2018, 30, 6908–6914. [Google Scholar] [CrossRef]
- Zola, R.S.; Bisoyi, H.K.; Wang, H.; Urbas, A.M.; Bunning, T.J.; Li, Q. Dynamic Control of Light Direction Enabled by Stimuli-Responsive Liquid Crystal Gratings. Adv. Mater. 2018, 31, 1806172. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Yoon, W.J.; Choi, Y.J.; Lim, S.I.; Koob, J.; Jeong, K.U. Photoresponsive chiral molecular crystal for light-directing nanostructures. J. Mater. Chem. C 2018, 6, 12314–12320. [Google Scholar] [CrossRef]
- Moran, M.J.; Magrini, M.; Walba, D.M.; Aprahamian, I. Driving a Liquid Crystal Phase Transition Using a Photochromic Hydrazone. J. Am. Chem. Soc. 2018, 140, 13623–13627. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnarao, D.; Mamonov, E.A.; Murzina, T.V.; Chandrasekar, R. Advanced Organic and Polymer Whispering-Gallery-Mode Microresonators for Enhanced Nonlinear Optical Light. Adv. Opt. Mater. 2018, 6, 1800343. [Google Scholar] [CrossRef]
- Hadj Sadok, I.B.; Hajlaoui, F.; Ayed, H.B.; Ennaceur, N.; Nasri, M.; Audebrand, N.; Bataille, T.; Zouari, N. Crystal packing, high-temperature phase transition, second-order nonlinear optical and biological activities in a hybrid material: [(S)eC7H16N2][CuBr4]. J. Mol. Struct. 2018, 1167, 316–326. [Google Scholar] [CrossRef]
- Yang, B.W.; Deng, J.P. Chiral PLLA particles with tunable morphology and lamellar structure for enantioselective crystallization. Mater. Sci. 2018, 53, 11932–11941. [Google Scholar] [CrossRef]
- Kulikov, O.V.; Siriwardane, D.A.; Budhathoki-Uprety, J.; McCandless, G.T.; Mahmood, S.F.; Novak, B.M. The secondary structures of PEG-functionalized random copolymers derived from (R)- and (S)- families of alkyne polycarbodiimides. Polym. Chem. 2018, 9, 2759–2768. [Google Scholar] [CrossRef]
- Aav, R.; Mishra, K.A. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. [Google Scholar] [CrossRef]
- Sugahara, H.; Meinert, C.; Nahon, L.; Jones, N.C.; Hoffmann, S.V.; Hamase, K.; Takano, Y.; Meierhenrich, U.J. D-Amino acids in molecular evolution in space—Absolute asymmetric photolysis and synthesis of amino acids by circularly polarized light. BBA Proteins Proteom. 2018, 1866, 743–758. [Google Scholar] [CrossRef]
- Kuhn, W.; Braun, E. Photochemische Erzeugung optisch aktiver Stoffe. Nat. Chem. 1929, 17, 227–228. [Google Scholar] [CrossRef]
- Gopalaiah, K.; Kagan, H.B. Use of Nonfunctionalized Enamides and Enecarbamates in Asymmetric Synthesis. Chem. Rev. 2011, 111, 4599–4657. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Bode, A.A.C.; van der Meijden, M.; Meekes, H.; van Etteger, A.F.; van Enckevort, W.J.P.; Christianen, P.C.M.; Kaptein, B.; Kellogg, R.M.; Rasing, T.; et al. Complete chiral symmetry breaking of an amino acid derivative directed by circularly polarized light. Nat. Chem. 2009, 1, 729–732. [Google Scholar] [CrossRef]
- Shibata, T.; Yamamoto, J.; Matsumoto, N.; Yonekubo, S.; Osanai, S.; Soai, K. Amplification of a Slight Enantiomeric Imbalance in Molecules Based on Asymmetric Autocatalysis: The First Correlation between High Enantiomeric Enrichment in a Chiral Molecule and Circularly Polarized Light. J. Am. Chem. Soc. 1998, 120, 12157–12158. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sato, M.; Ishiguro, S.; Saito, T.; Morishita, Y.; Sato, I.; Nishino, H.; Inoue, Y.; Soai, K. Enantioselective Synthesis of Near Enantiopure Compound by Asymmetric Autocatalysis Triggered by Asymmetric Photolysis with Circularly Polarized Light. J. Am. Chem. Soc. 2005, 127, 3274–3275. [Google Scholar] [CrossRef]
- Nishino, H.; Kosaka, A.; Hembury, G.A.; Aoki, F.; Miyauchi, K.; Shitomi, H.; Onuki, H.; Inoue, Y. Absolute Asymmetric Photoreactions of Aliphatic Amino Acids by Circularly Polarized Synchrotron Radiation: Critically pH-Dependent Photobehavior. J. Am. Chem. Soc. 2002, 124, 11618–11627. [Google Scholar] [CrossRef]
- Zou, G.; Jiang, H.; Kohn, H.; Manaka TIwamoto, M. Control and modulation of chirality for azobenzene-substituted polydiacetylene LB films with circularly polarized light. Chem. Commun. 2009, 15, 5627–5629. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Filippi, J.J.; Meinert, C.; Bredehöft, J.H.; Takahashi, J.I.; Nahon, L.; Jones, N.C.; Hoffmann, S.V. Circular Dichroism of Amino Acids in the Vacuum-Ultraviolet Region. Angew. Chem. Int. Ed. 2010, 49, 7799–7802. [Google Scholar] [CrossRef] [PubMed]
- Bredehöft, J.H.; Jones, N.C.; Meinert, C.; Evans, A.C.; Hoffmann, S.V.; Meierhenrich, U.J. Understanding Photochirogenesis: Solvent Effects on Circular Dichroism and Anisotropy Spectroscopy. Chirality 2014, 26, 373–378. [Google Scholar] [CrossRef]
- Meinert, C.; Hoffmann, S.V.; Chenaϊ, P.C.; Evans, A.C.; Giri, C.; Nahon, L.; Meierhenrich, U.J. Photonenergy-Controlled Symmetry Breaking with Circularly Polarized Light. Angew. Chem. Int. Ed. 2014, 53, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.; Yeom, B.; Chan, H.; Smith, K.; Medina, S.D.; Bahng, J.H.; Zhao, G.P.; Chang, S.W.; Chang, S.J.; Chuvilin, A.; et al. Chiral templating of self-assembling nanostructures by circularly polarized light. Nat. Mater. 2014, 14, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Mori, T.; Aida, T.; Miyajima, D. Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helices. Chem. Sci. 2016, 7, 6689–6694. [Google Scholar] [CrossRef]
- Wang, D.E.; Yan, J.H.; Jiang, J.J.; Liu, X.; Tian, C.; Xu, J.; Yuan, M.S.; Hana, X.; Wang, J.Y. Polydiacetylene liposomes with phenylboronic acid tags: A fluorescence turn-on sensor for sialic acid detection and cell-surface glycan imaging. Nanoscale 2018, 10, 4570–4578. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.L.; Lee, S.; Cho, Y.; Kim, M.H.; Bouffard, J.; Yoon, J. Polydiacetylene-Based Colorimetric and Fluorescent Chemosensor for the Detection of Carbon Dioxide. J. Am. Chem. Soc. 2013, 135, 17751–17754. [Google Scholar] [CrossRef]
- Xia, H.Y.; Li, J.G.; Zou, G.; Zhang, Q.J.; Jia, Q. A highly sensitive and reusable cyanide anion sensor based on spiropyran functionalized polydiacetylene vesicular receptors. J. Mater. Chem. A 2013, 1, 10713–10719. [Google Scholar] [CrossRef]
- Wu, P.J.; Kuo, S.Y.; Huang, Y.C.; Chen, C.P.; Chan, Y.H. Polydiacetylene-Enclosed Near-Infrared Fluorescent Semiconducting Polymer Dots for Bioimaging and Sensing. Anal. Chem. 2014, 86, 4831–4839. [Google Scholar] [CrossRef]
- Lee, J.; Pyo, M.; Lee, S.H.; Kim, J.; Ra, M.; Kim, W.Y.; Park, B.J.; Lee, C.W.; Kim, J.M. Hydrochromic conjugated polymers for human sweat pore mapping. Nat. Commun. 2014, 5, 3736–3745. [Google Scholar] [CrossRef]
- Park, D.H.; Hong, J.; Park, I.S.; Lee, C.W.; Kim, J.M. A Colorimetric Hydrocarbon Sensor Employing a Swelling-Induced Mechanochromic Polydiacetylene. Adv. Funct. Mater. 2014, 24, 5186–5193. [Google Scholar] [CrossRef]
- Wang, D.E.; Wang, Y.L.; Tian, C.; Zhang, L.L.; Han, X.; Tu, Q.; Yuan, M.S.; Chen, S.; Wang, J.Y. Polydiacetylene liposome-encapsulated alginate hydrogel beads for Pb2+ detection with enhanced sensitivity. J. Mater. Chem. A 2015, 3, 21690–21698. [Google Scholar] [CrossRef]
- Wang, M.W.; Wang, F.; Wang, Y.; Zhang, W.; Chen, X.Q. Polydiacetylene-based sensor for highly sensitive and selective Pb2+ detection. Dyes Pigments 2015, 120, 307–313. [Google Scholar] [CrossRef]
- Yang, G.; Hu, W.L.; Xia, H.Y.; Zou, G.; Zhang, Q.J. Highly selective and reproducible detection of picric acid in aqueous media, based on a polydiacetylene microtube optical waveguide. J. Mater. Chem. A 2014, 2, 15560–15565. [Google Scholar] [CrossRef]
- Manaka, T.; Kon, H.; Ohshima, Y.; Zou, G.; Iwamoto, M. Preparation of Chiral Polydiacetylene Film from Achiral Monomers Using Circularly Polarized Light. Chem. Lett. 2006, 35, 1028–1029. [Google Scholar] [CrossRef]
- Yang, G.; Han, L.; Jiang, H.; Zou, G.; Zhang, Q.J.; Zhang, D.G.; Wang, P.; Ming, H. Enantioselective synthesis of helical polydiacetylenes in the visible light region. Chem. Commun. 2014, 50, 2338–2340. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.L.; Xu, L.G.; Ma, W.; Wu, X.L.; Wang, L.B.; Kuang, H.; Xu, C.L. Unusual Circularly Polarized Photocatalytic Activity in Nanogapped Gold–Silver Chiroplasmonic Nanostructures. Adv. Funct. Mater. 2015, 25, 5816–5822. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, W.Y.; Kim, H.; Lee, S.; Lee, H.C.; Lee, Y.S.; Seo, M.; Kim, S.Y. Induction and control of supramolecular chirality by light in self-assembled helical nanostructures. Nat. Commun. 2015, 6, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Coppens, Z.J.; Besteuro, L.V.; Wang, W.Y.; Govorov, A.O.; Valentine, J. Circularly polarized light detection with hot electrons in chiral plasmonic metamaterials. Nat. Commun. 2015, 6, 8379–68385. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhu, L.F.; Hu, J.G.; Xia, H.Y.; Qiu, D.; Zhang, Q.J.; Zhang, D.G.; Zou, G. Near-Infrared Circularly Polarized Light Triggered Enantioselective Photopolymerization by Using Upconversion Nanophosphors. Chem. Eur. J. 2017, 23, 8032–8038. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Jiang, H.; Zhang, Q.J.; Wang, F.; Zou, G. Helical polydiacetylene prepared in the liquid crystal phase using circular polarized ultraviolet light. Chem. Commun. 2014, 50, 365–367. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Yang, G.; Kuai, Y.; Shan, S.Z.; Yang, L.; Hu, J.G.; Zhang, D.G.; Zhang, Q.J.; Zou, G. Dissymmetry enhancement in enantioselective synthesis of helical polydiacetylene by application of superchiral light. Nat. Commun. 2018, 9, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.T.; Wu, Y.R.; Kang, Q.Q.; Zhang, H.; Long, L.S.; Zheng, Z.P.; Huang, R.B.; Zheng, L.S. Chiral Symmetry Breaking by Chemically Manipulating Statistical Fluctuation in Crystallization. Angew. Chem. Int. Ed. 2007, 46, 8475–8479. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.T.; Cai, Z.W.; Ye, Q.Y.; Weng, C.H.; Huang, X.H.; Hu, X.L.; Huang, C.C.; Zhuang, N.F. Enantioselective Synthesis of a Chiral Coordination Polymer with Circularly Polarized Visible Laser. Angew. Chem. Int. Ed. 2014, 53, 12860–12864. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Kong, Z.P. A Novel 3D Framework Coordination Polymer based on Succinato bridged Helical Chains Connected by 4,4-Bipyridine: [Cu(bpy)(H2O)2(C4H4O4)] · 2H2O. Z. Anorg. Allg. Chem. 2003, 629, 1469–1471. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Y.Y.; Zhang, Z.D.; Wang, L.H.; He, X.H.; Zhang, Q.J.; Hong, C.Y.; Zou, G. Circularly polarized light triggered enantioselective thiol-ene polymerization. Chem. Commun. 2017, 53, 1735–1738. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R. Interplay of Chiral Side Chains and Helical Main Chains in Polyisocyanates reaction. Macromolecules 1996, 29, 1609–1617. [Google Scholar] [CrossRef]
- Feringa, B.L.; Jager, W.F.; Lange, B.D. Chiroptical Molecular Switch. J. Am. Chem. Soc. 1991, 13, 5468–5469. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Uchida, K.; Irie, M. Asymmetric Photocyclization of Diarylethene Derivatives. J. Am. Chem. Soc. 1997, 119, 6066–6071. [Google Scholar] [CrossRef]
- Eggers, L.; Buss, V. A Spiroindolinopyran with Switchable Optical Activity. Angew. Chem. Int. Ed. 1997, 36, 8. [Google Scholar] [CrossRef]
- Nikolova, L.; Todorov, T.; Ivanov, M.; Andruzzi, F.; Hvilsted, S.; Ramanujam, P.S. Photoinduced circular anisotropy in side-chain azobenzene polyesters. Opt. Mater. 1997, 8, 255–258. [Google Scholar] [CrossRef]
- Iftime, G.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Control of Chirality of an Azobenzene Liquid Crystalline Polymer with Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 12646–12650. [Google Scholar] [CrossRef]
- Ivanov, M.; Naydenova, I.; Todorov, T.; Nikolova, L.; Petrova, T.; Tomova, N.; Dragostinova, V. Light-induced optical activity in optically ordered amorphous side-chain azobenzene containing polymer. J. Mod. Opt. 2000, 47, 861–867. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, B.G.; Kim, J.J.; Kim, D.Y. Photoinduced Supramolecular Chirality in Amorphous Azobenzene Polymer Films. J. Am. Chem. Soc. 2002, 124, 3504–3505. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, J.; Sun, Y.Y.; Zhou, J.L.; Chen, B.; Zhang, Q.J.; Wang, K.Y. Synthesis and Chiroptical Properties of Optically Active Polymer Liquid Crystals Containing Azobenzene Chromophores. J. Polym. Sci. Part A Polym. Chem. 2010, 44, 3210–3219. [Google Scholar] [CrossRef]
- Zou, G.; Jiang, H.; Zhang, Q.J.; Kohn, H.; Manaka, T.; Iwamoto, M. Chiroptical switch based on azobenzene-substituted polydiacetylene LB films under thermal and photic stimuli. J. Mater. Chem. 2010, 20, 285–291. [Google Scholar] [CrossRef]
- Burnham, K.S.; Schuster, G.B. Transfer of Chirality from Circularly Polarized Light to a Bulk Material Property: Propagation of Photoresolution by a Liquid Crystal Transition. J. Am. Chem. Soc. 1999, 121, 10245–10246. [Google Scholar] [CrossRef]
- Li, J.; Schuster, G.B.; Cheon, K.S.; Green, M.M.; Selinger, J.V. Switching a Helical Polymer between Mirror Images Using Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 2603–2612. [Google Scholar] [CrossRef]
- Wang, Y.; Sakamoto, T.; Nakano, T. Molecular chirality induction to an achiral p-conjugated polymer by circularly polarized light. Chem. Commun. 2012, 48, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, A.; Wang, Y.; Nakano, T. Predicting the Switchable Screw Sense in Fluorene-Based Polymers. Angew. Chem. Int. Ed. 2015, 54, 2688–2692. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, M.; Yoshida, K.; Suzuki, N.; Zhang, J.; Zhang, W.; Zhu, X.L. Mirror symmetry breaking and restoration within mmsized polymer particles in optofluidic media by pumping circularly polarised light. RSC Adv. 2013, 3, 5213–5219. [Google Scholar] [CrossRef]

















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zhang, S.; Hu, J.; Fujiki, M.; Zou, G. The Chirality Induction and Modulation of Polymers by Circularly Polarized Light. Symmetry 2019, 11, 474. https://doi.org/10.3390/sym11040474
Yang G, Zhang S, Hu J, Fujiki M, Zou G. The Chirality Induction and Modulation of Polymers by Circularly Polarized Light. Symmetry. 2019; 11(4):474. https://doi.org/10.3390/sym11040474
Chicago/Turabian StyleYang, Guang, Siyu Zhang, Jingang Hu, Michiya Fujiki, and Gang Zou. 2019. "The Chirality Induction and Modulation of Polymers by Circularly Polarized Light" Symmetry 11, no. 4: 474. https://doi.org/10.3390/sym11040474
APA StyleYang, G., Zhang, S., Hu, J., Fujiki, M., & Zou, G. (2019). The Chirality Induction and Modulation of Polymers by Circularly Polarized Light. Symmetry, 11(4), 474. https://doi.org/10.3390/sym11040474






