Vibrational Properties of Benzoxaboroles and Their Interactions with Candida albicans’ LeuRS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Structure Optimization, Vibrational Properties and Preparations of Ligand Structures
2.2. IR and Raman Spectra Measurements
2.3. Samples Preparation
2.4. Docking Studies
3. Results
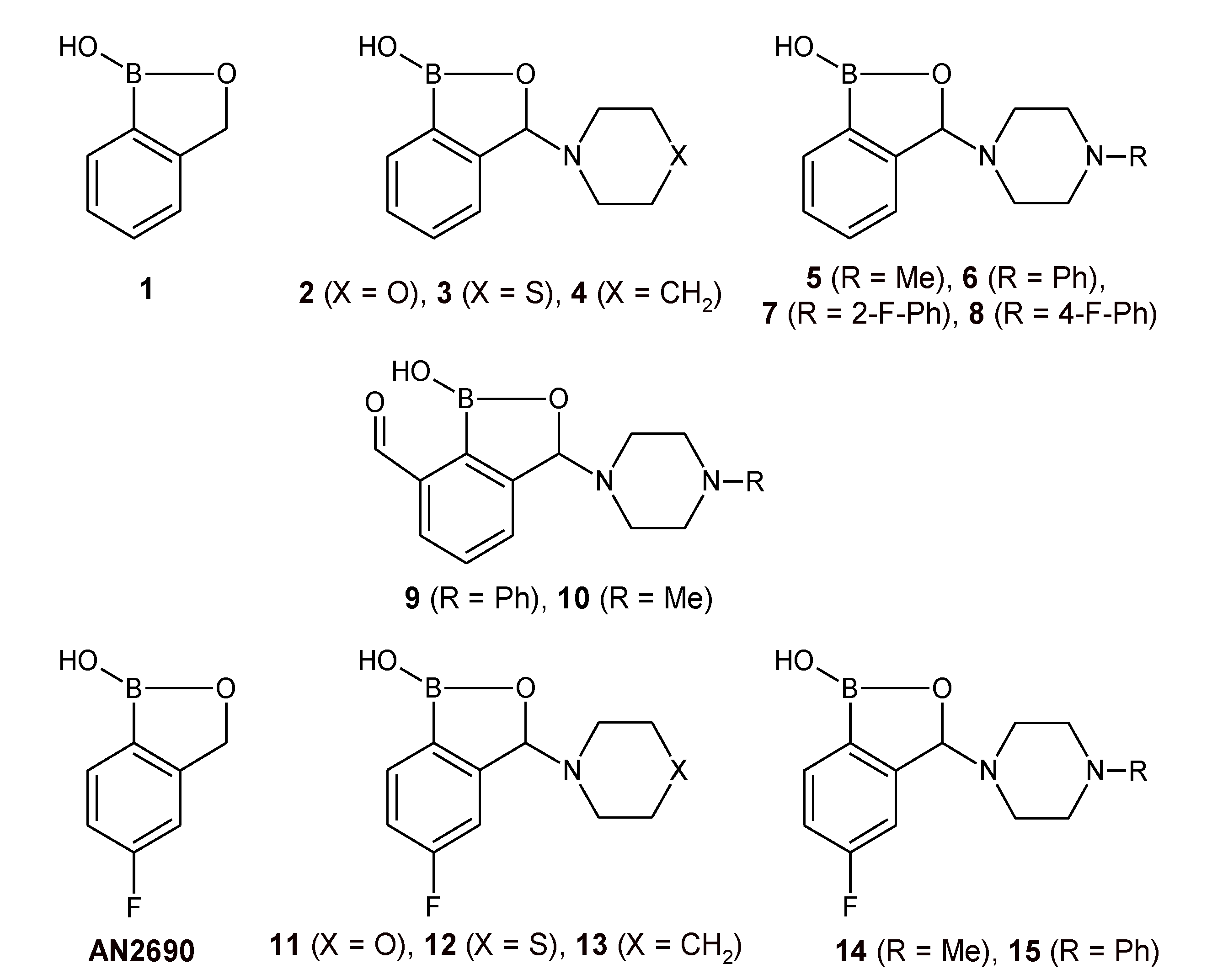
3.1. Vibrational Properties of Benzoxaboroles
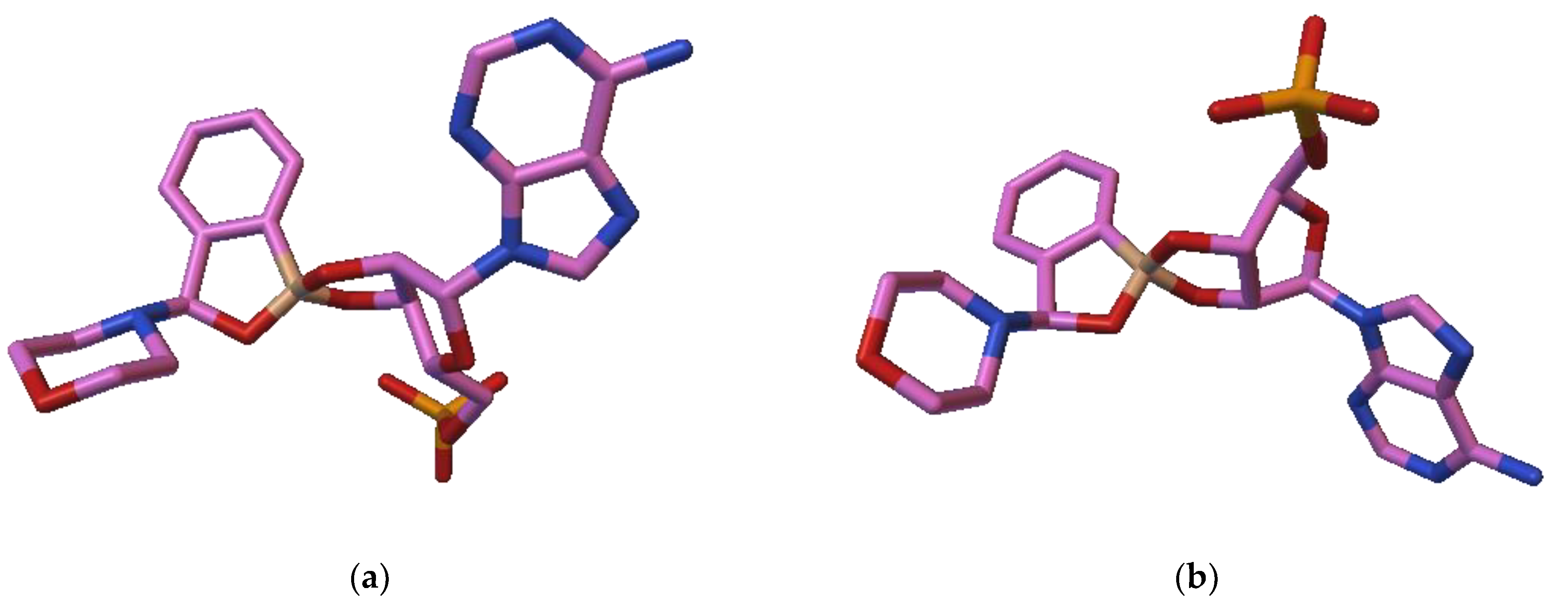
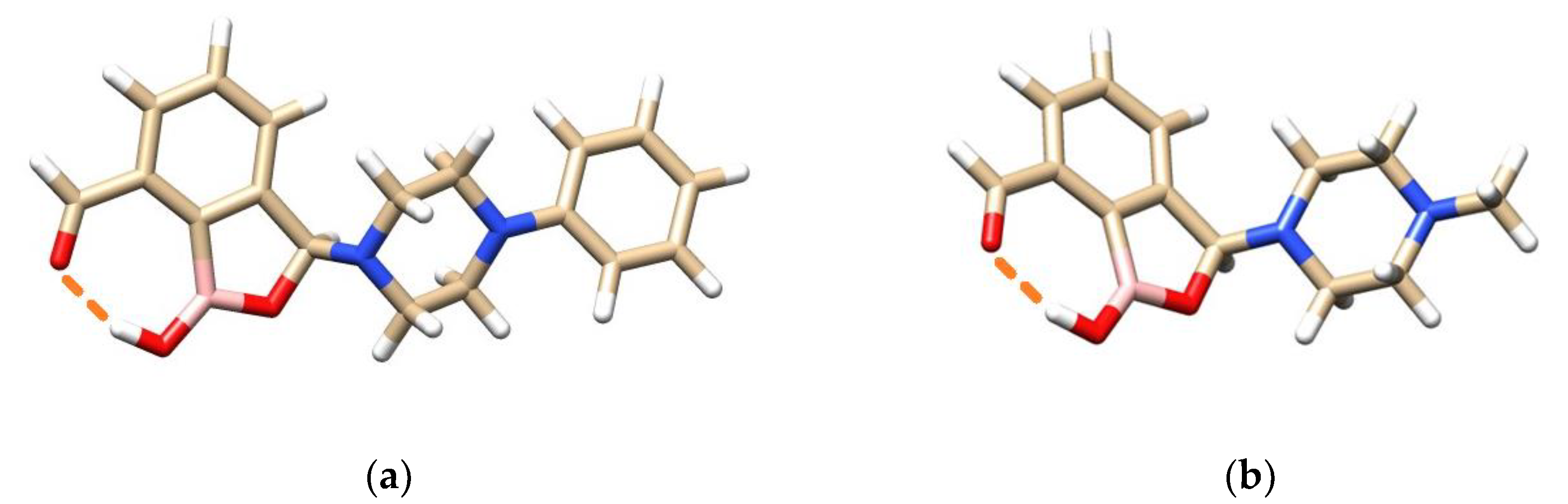
3.2. Docking Studies of Benzoxaboroles’ AMP Spiroesters with C. albicans LeuRS
4. Discussion
4.1. Vibrational Properties of Benzoxaboroles
4.2. Interactions of Benzoxaboroles’ AMP Spiroesters with C. albicans LeuRS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamczyk-Woźniak, A.; Borys, K.M.; Sporzyński, A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Mereddy, G.R.; Chakradhar, A.; Rutkoski, R.M.; Jonnalagadda, S.C. Benzoboroxoles: Synthesis and applications in medicinal chemistry. J. Organomet. Chem. 2018, 865, 12–22. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T.; Winum, J.-Y. Benzoxaborole compounds for therapeutic uses: A patent review (2010–2018). Expert Opin. Ther. Pat. 2018, 28, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Lunde, C.S.; Stebbins, E.E.; Jumani, R.S.; Hasan, M.M.; Miller, P.; Barlow, J.; Freund, Y.R.; Berry, P.; Stefanakis, R.; Gut, J.; et al. Identification of a potent benzoxaborole drug candidate for treating cryptosporidiosis. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Samaniego Lopez, C.; Martínez, J.H.; Acebedo, S.L.; Spagnuolo, C.C. Benzoxaboroles as dynamic covalent receptors for bioconjugation and transport of nucleosides and related drugs: Proof of action in HeLa cells. Bioorganic Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lapa, G.B.; Mirchink, E.P.; Isakova, E.B.; Preobrazhenskaya, M.N. Two approaches to the use of benzo[c][1,2]oxaboroles as active fragments for synthetic transformation of clarithromycin *. J. Enzyme Inhib. Med. Chem. 2017, 32, 452–456. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, M.; Zhang, J.; Zhou, H. Synthesis of biologically active boron-containing compounds. MedChemComm 2018, 9, 201–211. [Google Scholar] [CrossRef]
- Nocentini, A.; Cadoni, R.; Del Prete, S.; Capasso, C.; Dumy, P.; Gratteri, P.; Supuran, C.T.; Winum, J.-Y.Y. Benzoxaboroles as Efficient Inhibitors of the β-carbonic anhydrases from pathogenic fungi: Activity and modeling study. ACS Med. Chem. Lett. 2017, 8, 1194–1198. [Google Scholar] [CrossRef]
- Si, Y.; Basak, S.; Li, Y.; Merino, J.; Iuliano, J.; Walker, S.; Tonge, P. antibacterial activity and mode of action of a sulfonamide-based class of oxaborole leucyl-tRNA-synthetase inhibitors. ACS Infect. Dis. 2019, 5, 1231–1238. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, F.; Qiao, Z.; Zhu, M.; Zhou, H. Chalcone–benzoxaborole hybrids as novel anticancer agents. Bioorgainc Med. Chem. Lett. 2016, 26, 5797–5801. [Google Scholar] [CrossRef] [Green Version]
- Tevyashova, A.N.; Korolev, A.M.; Mirchink, E.P.; Isakova, E.B.; Osterman, I.A. Synthesis and evaluation of biological activity of benzoxaborole derivatives of azithromycin. J. Antibiot. 2019, 72, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Cadoni, R.; Esposito, D.; Vullo, D.; Di Fiore, A.; Monti, S.M.; Caporale, A.; Ruvo, M.; Sechi, M.; Dumy, P.; et al. Benzoxaborole as a new chemotype for carbonic anhydrase inhibition. Chem. Commun. 2016, 52, 11983–11986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.K.; Plattner, J.; Easom, E.; Jacobs, R.; Guo, D.; Freund, Y.; Berry, P.; Ciaravino, V.; Erve, J.; Rosenthal, P.; et al. Benzoxaborole antimalarial agents. Part 5. Lead optimization of novel amide pyrazinyloxy benzoxaboroles and identification of a preclinical candidate. J. Med. Chem. 2017, 60, 5889–5908. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Tomsho, J.W.; Benkovic, S.J. The unique chemistry of benzoxaboroles: Current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. [Google Scholar] [CrossRef]
- Xiao, Y.-C.; Chen, X.-P.; Deng, J.; Yan, Y.-H.; Zhu, K.-R.; Li, G.; Yu, J.-L.; Brem, J.; Chen, F.; Schofield, C.J.; et al. Design and enantioselective synthesis of 3-(α-acrylic acid) benzoxaboroles to combat carbapenemase resistance. Chem. Commun. 2021, 57, 7709–7712. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. [Google Scholar] [CrossRef]
- Elewski, B.; Pariser, D.; Rich, P.; Scher, R.K. Current and emerging options in the treatment of onychomycosis. Semin. Cutan. Med. Surg. 2013, 32, S9–S12. [Google Scholar] [CrossRef]
- Kubota-Ishida, N.; Takei-Masuda, N.; Kaneda, K.; Nagira, Y.; Chikada, T.; Nomoto, M.; Tabata, Y.; Takahata, S.; Maebashi, K.; Hui, X.; et al. In vitro human onychopharmacokinetic and pharmacodynamic analyses of me1111, a new topical agent for onychomycosis. Antimicrob. Agents Chemother. 2018, 62, e00779-17. [Google Scholar] [CrossRef] [Green Version]
- Ciaravino, V.; Coronado, D.; Lanphear, C.; Hoberman, A.; Chanda, S. Tavaborole, a novel boron-containing small molecule pharmaceutical agent for topical treatment of onychomycosis: I. reproductive and developmental toxicity studies. Int. J. Toxicol. 2016, 35, 530–542. [Google Scholar] [CrossRef] [Green Version]
- Wieczorek, D.; Kaczorowska, E.; Wiśniewska, M.; Madura, I.D.; Leśniak, M.; Lipok, J.; Adamczyk-Woźniak, A. Synthesis and influence of 3-amino benzoxaboroles structure on their activity against Candida albicans. Molecules 2020, 25, 5999. [Google Scholar] [CrossRef]
- Borys, K.M.; Matuszewska, A.; Wieczorek, D.; Kopczyńska, K.; Lipok, J.; Madura, I.D.; Adamczyk-Woźniak, A. Synthesis and structural elucidation of novel antifungal N-(fluorophenyl)piperazinyl benzoxaboroles and their analogues. J. Mol. Struct. 2019, 1181, 587–598. [Google Scholar] [CrossRef]
- Sporzyński, A.; Lewandowski, M.; Rogowska, P.; Cyrański, M.K. 1,3-Dihydro-1-hydroxy-3-morpholin-4-yl-2,1-benzoxaborole: Product of the reaction of o-formylphenylboronic acid with morpholine. Appl. Organomet. Chem. 2005, 19, 1202–1203. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Borys, K.M.; Madura, I.D.; Pawełko, A.; Tomecka, E.; Żukowski, K. Lewis acidity and sugar receptor activity of 3-amino-substituted benzoxaboroles and their ortho-aminomethylphenylboronic acid analogues. New J. Chem. 2013, 37, 188–194. [Google Scholar] [CrossRef]
- Madura, I.D.; Adamczyk-Woźniak, A.; Jakubczyk, M.; Sporzyński, A. 5-Fluoro-1,3-dihydro-2,1-benzoxaborol-1-ol. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o414–o415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sene, S.; Pizzoccaro, M.A.; Vezzani, J.; Reinholdt, M.; Gaveau, P.; Berthomieu, D.; Bégu, S.; Gervais, C.; Bonhomme, C.; Renaudin, G.; et al. Coordination networks based on boronate and benzoxaborolate ligands. Crystals 2016, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Sene, S.; Berthomieu, D.; Donnadieu, B.; Richeter, S.; Vezzani, J.; Granier, D.; Begu, S.; Mutin, H.; Gervais, C.; Laurencin, D. A combined experimental-computational study of benzoxaborole crystal structures. CrystEngComm 2014, 16, 4999–5011. [Google Scholar] [CrossRef]
- Al-Zoubi, R.M.; Al-Zoubi, M.S.; Jaradat, K.T.; McDonald, R. Design, synthesis and X-ray crystal structure of iodinated benzoboroxole derivatives by consecutive metal–iodine exchange of 3,4,5-triiodoanisole. Eur. J. Org. Chem. 2017, 2017, 5800–5808. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Kurach, P.; Luliński, S.; Serwatowski, J. An ortho-lithiated derivative of protected phenylboronic acid: An approach to ortho-functionalized arylboronic acids and 1,3-dihydro-1-hydroxybenzo[c][2,1]oxaboroles. Appl. Organomet. Chem. 2007, 21, 234–238. [Google Scholar] [CrossRef]
- Hazra, G.; Maity, S.; Bhowmick, S.; Ghorai, P. Organocatalytic, enantioselective synthesis of benzoxaboroles via Wittig/oxa-Michael reaction Cascade of alpha-formyl boronic acids. Chem. Sci. 2017, 8, 3026–3030. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk-Woźniak, A.; Cabaj, M.K.; Dominiak, P.M.; Gajowiec, P.; Gierczyk, B.; Lipok, J.; Popenda, Ł.; Schroeder, G.; Tomecka, E.; Urbańskia, P.; et al. The influence of fluorine position on the properties of fluorobenzoxaboroles. Bioorg. Chem. 2015, 60, 130–135. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Ejsmont, K.; Gierczyk, B.B.; Kaczorowska, E.; Matuszewska, A.; Schroeder, G.; Sporzyński, A.; Zarychta, B. Novel 2,6-disubstituted phenylboronic compounds—Synthesis, crystal structures, solution behaviour and reactivity. J. Organomet. Chem. 2015, 788, 36–41. [Google Scholar] [CrossRef]
- Czub, M.; Durka, K.; Luliński, S.; Łosiewicz, J.; Serwatowski, J.; Urban, M.; Woźniak, K. Synthesis and transformations of functionalized benzosiloxaboroles. Eur. J. Org. Chem. 2017, 2017, 818–826. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ishii, J.-I.; Nishiyama, H.; Itoh, K. Cp*RuCl-catalyzed formal intermolecular cyclotrimerization of three unsymmetrical alkynes through a boron temporary tether: Regioselective four-component coupling synthesis of phthalides. J. Am. Chem. Soc. 2005, 127, 9625–9631. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, S.; Yang, X.; Sun, X. Synthesis of 3-indolyl-substituted benzoboroxole via friedel-crafts reaction in water. Chin. J. Org. Chem. 2014, 34, 2456–2461. [Google Scholar] [CrossRef] [Green Version]
- Gunasekera, D.S.; Gerold, D.J.; Aalderks, N.S.; Chandra, J.S.; Maanu, C.A.; Kiprof, P.; Zhdankin, V.V.; Reddy, M.V.R. Practical synthesis and applications of benzoboroxoles. Tetrahedron 2007, 63, 9401–9405. [Google Scholar] [CrossRef]
- Miyamoto, S.; Matsuoka, A.; Yamada, Y.; Ishikawa, R.; Hayashida, O. Benzoxaborole catalyst for site-selective modification of polyols. Eur. J. Org. Chem. 2020, 1598–1602. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Cyrański, M.K.; Jakubczyk, M.; Klimentowska, P.; Koll, A.; Kołodziejczak, J.; Pojmaj, G.; Żubrowska, A.; Żukowska, G.Z.; Sporzyński, A. Influence of the substituents on the structure and properties of benzoxaboroles. J. Phys. Chem. A 2010, 114, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, A.; Panek, J.J.; Żukowska, G.Z.; Sporzyński, A. A combined experimental and theoretical study of benzoxaborole derivatives by Raman and IR spectroscopy, static DFT, and first-principle molecular dynamics. J. Phys. Org. Chem. 2010, 23, 451–460. [Google Scholar] [CrossRef]
- Song, Y.; Cong, Y.; Wang, B.; Zhang, N. Applications of Fourier transform infrared spectroscopy to pharmaceutical preparations. Expert Opin. Drug Deliv. 2020, 17, 551–571. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Fleschin, S. Application of fourier transform infrared spectrophotometry in pharmaceutical drugs analysis. Appl. Spectrosc. Rev. 2010, 45, 206–219. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [Green Version]
- Zirkel, J.; Klinker, H.; Kuhn, A.; Abele-Horn, M.; Tappe, D.; Turnwald, D.; Einsele, H.; Heinz, W.J. Epidemiology of Candida blood stream infections in patients with hematological malignancies or solid tumors. Med. Mycol. 2012, 50, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of direct healthcare costs of fungal diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Pappas, P.G.; Wingard, J.R. Invasive fungal pathogens: Current epidemiological trends. Clin. Infect. Dis. 2006, 43, S3–S14. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; do Socorro de Sousa Cartágenes, M.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crépin, T.; Zhou, H.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Gozdalik, J.T.; Wieczorek, D.; Madura, I.D.; Kaczorowska, E.; Brzezińska, E.; Sporzyński, A.; Lipok, J. Synthesis, properties and antimicrobial activity of 5-trifluoromethyl-2-formylphenylboronic acid. Molecules 2020, 25, 799. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk-Woźniak, A.; Gozdalik, J.T.; Kaczorowska, E.; Durka, K.; Wieczorek, D.; Zarzeczańska, D.; Sporzyński, A. (Trifluoromethoxy)phenylboronic acids: Structures, properties, and antibacterial activity. Molecules 2021, 26, 2007. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Komarovska-Porokhnyavets, O.; Misterkiewicz, B.; Novikov, V.P.; Sporzyński, A. Biological activity of selected boronic acids and their derivatives. Appl. Organomet. Chem. 2012, 26, 390–393. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C. 02; Gaussian. Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Wachters, A.J.H. Gaussian basis set for molecular wavefunctions containing third-row atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]
- Hay, P.J. Gaussian basis sets for molecular calculations. The representation of 3d orbitals in transition-metal atoms. J. Chem. Phys. 1977, 66, 4377–4384. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W. Highly correlated systems. Excitation energies of first row transition metals Sc–Cu. J. Chem. Phys. 1989, 91, 1062–1065. [Google Scholar] [CrossRef]
- Binning, R.C., Jr.; Curtiss, L.A. Compact contracted basis sets for third-row atoms: Ga–Kr. J. Comput. Chem. 1990, 11, 1206–1216. [Google Scholar] [CrossRef]
- McGrath, M.P.; Radom, L. Extension of Gaussian-1 (G1) theory to bromine-containing molecules. J. Chem. Phys. 1991, 94, 511–516. [Google Scholar] [CrossRef]
- Curtiss, L.A.; McGrath, M.P.; Blaudeau, J.; Davis, N.E.; Binning, R.C.; Radom, L. Extension of Gaussian-2 theory to molecules containing third-row atoms Ga–Kr. J. Chem. Phys. 1995, 103, 6104–6113. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Madura, I.; Pawełko, A.; Sporzyński, A.; Żubrowska, A.; Żyła, J. Amination-reduction reaction as simple protocol for potential boronic molecular receptors. Insight in supramolecular structure directed by weak interactions. Cent. Eur. J. Chem. 2011, 9, 199–205. [Google Scholar] [CrossRef]
- Otkidach, D.S.; Pletnev, I. V Conformational analysis of boron-containing compounds using Gillespie-Kepert version of molecular mechanics. J. Mol. Struct. Theochem. 2001, 536, 65–72. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesson, L.; Eisenberg, D. Atomic solvation parameters applied to molecular dynamics of proteins in solution. Protein Sci. 1992, 1, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Smith, M.K.; Northrop, B.H. Vibrational properties of boroxine anhydride and boronate ester materials: Model systems for the diagnostic characterization of covalent organic frameworks. Chem. Mater. 2014, 26, 3781–3795. [Google Scholar] [CrossRef]
- Millikan, R.C.; Pitzer, K.S. The infrared spectra of dimeric and crystalline formic acid. J. Am. Chem. Soc. 1958, 80, 3515–3521. [Google Scholar] [CrossRef]
- Adamczyk-Woźniak, A.; Madura, I.; Velders, A.H.; Sporzyński, A. Diverse reactivity of 2-formylphenylboronic acid with secondary amines: Synthesis of 3-amino-substituted benzoxaboroles. Tetrahedron Lett. 2010, 51, 6181–6185. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Cintas, P. Biochirality: Origins, Evolution and Molecular Recognition; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3642376269. [Google Scholar]
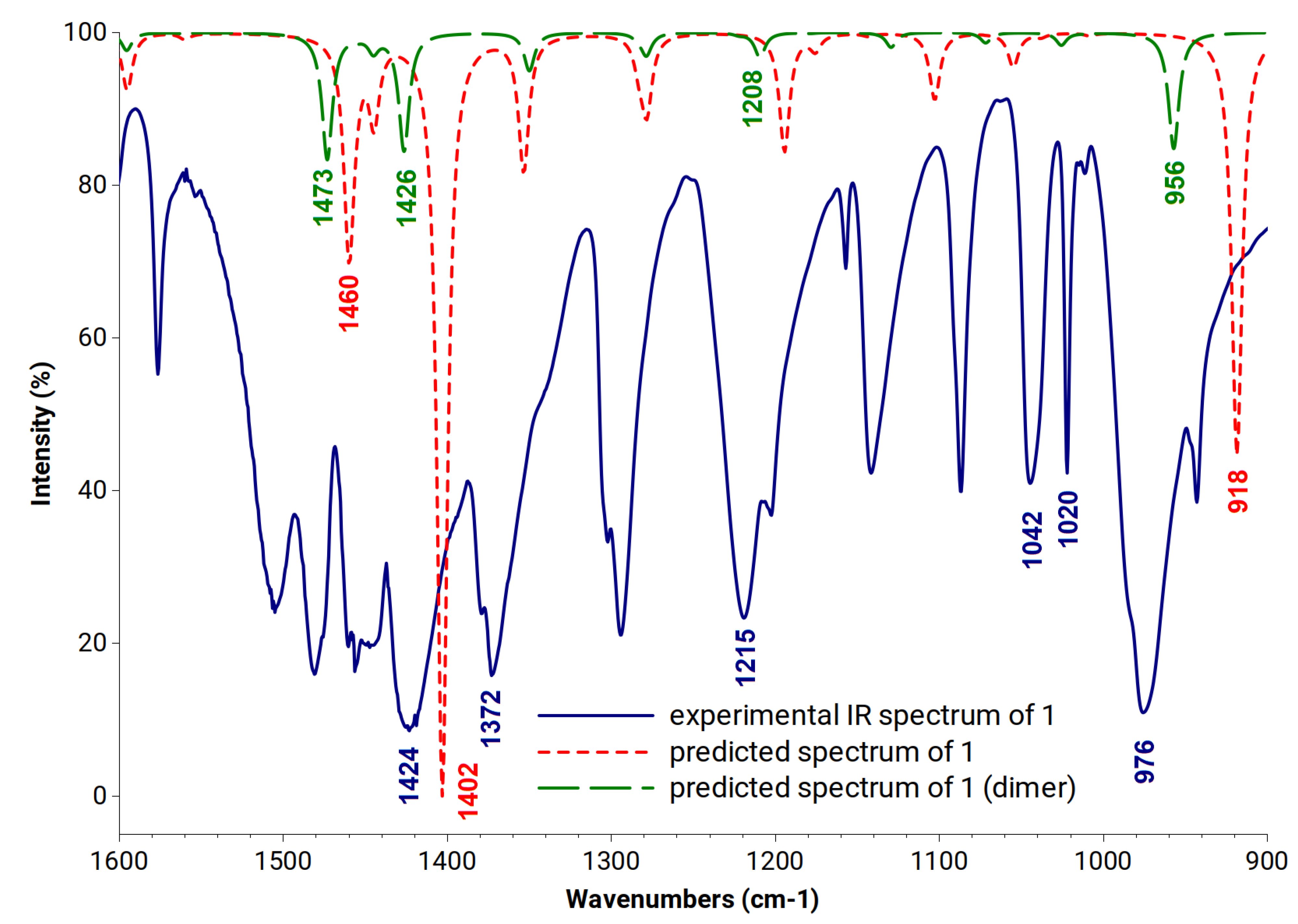

| No | Vibrations Based on DFT Calculations | 1 | Dimer of 1 | Experimental |
|---|---|---|---|---|
| 1 | B-OH stretching + ring stretching + CH in plane bending (aromatic) | 1460 | 1473 | 1424 (IR) |
| 2 | B-OH stretching + CH in plane bending (aromatic) | 1402 | 1426 | 1372 (IR) |
| 3 | B-OH in plane bending | 918 | 1208 | 1215 (IR) |
| 4 | BOC out of phase asymmetric stretching | ---- | 1025 | 1042 (IR) |
| 5 | Ring breathing | 1008 | 1009 | 1020 (R) |
| 6 | C-O stretching + BO2 in plane bending | ---- | 956 | 976 (IR) |
| 7 | Ring deformation | 815 | 819 | 838 (R) |
| 8 | B-OH out of plane bending | 523 | 767 | 768 (IR) |
| 9 | Ring deformation + borole ring symmetric stretching | 709 | 715 | 721 (IR) 723 (R) |
| 10 | BO2 out of plane bending | 633 | 623 | 626 (IR) |
| 11 | BO2 in plane bending + aromatic ring in plane bending | 500 | 521 | 524 (R) |
| Vibration | B-OH Stretching + Ring Stretching + CH in Plane Bending (Aromatic) | B-OH Stretching + CH in Plane Deformations | C-O Stretching (Borole Ring) | ||
|---|---|---|---|---|---|
| method | DFT | Exp. | DFT | Exp | DFT |
| AN2690 | 1469 | 1418 | 1402 | 1366 | 919 and 1041 |
| 1 | 1460 | 1424 | 1402 | 1371 | 1038 |
| 2 | 1461 | 1413 [39] | 1410 | 1347 [39] | 1024 |
| 3 | 1462 | ---- | 1410 | ---- | 918 and 1026 |
| 4 | 1461 | ---- | 1407 | ---- | 913 |
| 5 | 1460 | 1427 | 1407 | 1352 | 1023 |
| 6 | 1460 | 1414 | 1407 | 1346 | 998 |
| 7 | 1460 | 1414 | 1406 | 1372 | 854 |
| 8 | 1460 | 1429 | 1406 | 1346 [10] | 854 |
| 9 | 1464 | 1414 | ---- | 1332 | 965 |
| 10 | 1464 | 1414 | ---- | 1349 | 978 |
| 11 | 1469 | ---- | 1416 | ---- | 853 |
| 12 | 1469 | ---- | 1417 | ---- | 852 |
| 13 | 1469 | ---- | 1413 | ---- | 932 |
| 14 | 1467 | 1446 | 1416 | 1371 | 1021 |
| 15 | 1468 | 1431 | 1420 | 1377 | 882 |
| Ligand | The Lowest Binding Energy (kcal/mol) | Number of Structures | Mean Binding Energy (kcal/mol) | Inhibition Constant (nM) | Number of Hydrogen Bonds |
|---|---|---|---|---|---|
| AN2690-AMP | −11.89 | 17 | −10.80 | 1.93 | 5 [49] |
| 1-2-AMP * | −11.51 | 17 | −10.60 | 3.63 | 5 |
| 2-S-2-AMP * | −12.02 | 3 | −10.49 | 1.53 | 3 |
| 3-R-1-AMP * | −13.04 | 2 | −12.48 | 0.28 | 3 |
| 4-R-1-AMP * | −12.86 | 4 | −12.29 | 0.38 | 4 |
| 5-R-2-AMP | −12.21 | 13 | −11.16 | 1.12 | 4 |
| 6-R-1-AMP | −12.73 | 20 | −11.93 | 0.47 | 2 |
| 7-R-2-AMP | −12.24 | 27 | −11.65 | 1.07 | 3 |
| 8-R-1-c-AMP | −12.20 | 29 | −11.37 | 1.15 | 3 |
| 9-R-1-AMP | −12.87 | 26 | −12.00 | 0.36 | 3 |
| 10-R-1-AMP * | −12.17 | 1 | −12.17 | 1.21 | 4 |
| 11-R-1-AMP | −11.20 | 11 | −10.67 | 6.15 | 2 |
| 12-R-1-AMP | −11.68 | 19 | −11.08 | 2.76 | 4 |
| 13-R-1-AMP | −11.85 | 24 | −11.25 | 2.05 | 2 |
| 14-R-1-AMP | −11.26 | 12 | −10.52 | 5.57 | 2 |
| 15-R-2-AMP | −12.92 | 18 | −11.71 | 0.34 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczorowska, E.; Adamczyk-Woźniak, A.; Żukowska, G.Z.; Kostecka, P.; Sporzyński, A. Vibrational Properties of Benzoxaboroles and Their Interactions with Candida albicans’ LeuRS. Symmetry 2021, 13, 1845. https://doi.org/10.3390/sym13101845
Kaczorowska E, Adamczyk-Woźniak A, Żukowska GZ, Kostecka P, Sporzyński A. Vibrational Properties of Benzoxaboroles and Their Interactions with Candida albicans’ LeuRS. Symmetry. 2021; 13(10):1845. https://doi.org/10.3390/sym13101845
Chicago/Turabian StyleKaczorowska, Ewa, Agnieszka Adamczyk-Woźniak, Grażyna Zofia Żukowska, Paulina Kostecka, and Andrzej Sporzyński. 2021. "Vibrational Properties of Benzoxaboroles and Their Interactions with Candida albicans’ LeuRS" Symmetry 13, no. 10: 1845. https://doi.org/10.3390/sym13101845






