Effects of a Targeted Exercise Program on Inter-Leg Asymmetries in Patients with Patellofemoral Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
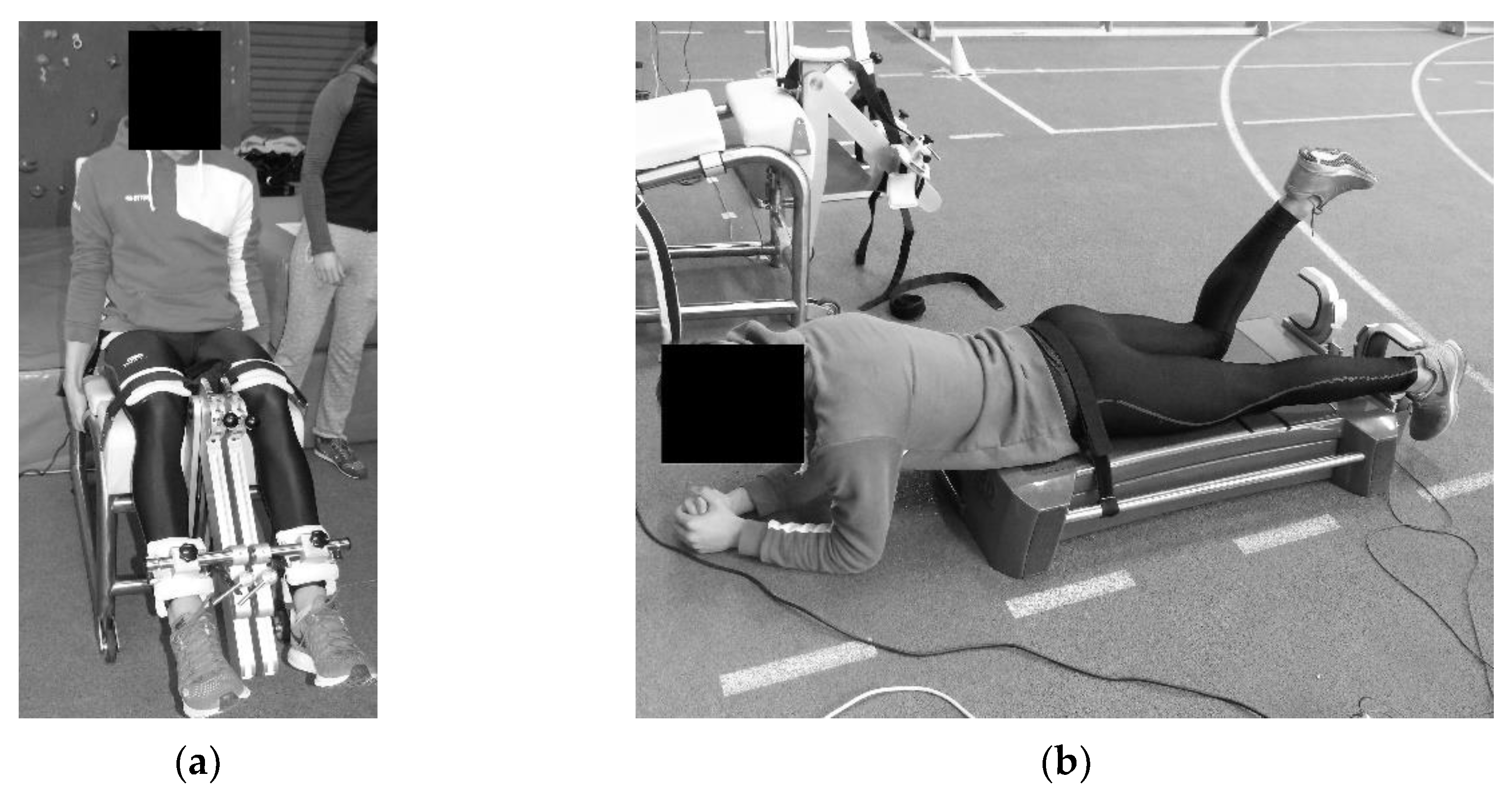
2.2. Study Design, Tasks and Measurement Procedure
2.2.1. Stability Measurements
2.2.2. Strength Measurements
2.2.3. Flexibility Measurements
2.3. Data Processing and Statistical Analysis
2.4. Intervention Procedure
3. Results
3.1. Inter-Leg Asymmetry and Agonist–Antagonist Ratios
3.2. Correlation with VAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pre-Test | Post-Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Affected Leg (Mean ± SD) | Non-Affected Leg (Mean ± SD) | Asymmetry Index (%) | Affected Leg (Mean ± SD) | Non-Affected Leg (Mean ± SD) | Asymmetry Index (%) | Cohen’s d | Degrees of Freedom | p-Value | |
| Body Sway (mm) | 1145.29 ± 237.07 | 1151.58 ± 236.89 | 9.85 ± 6.12 | 1158.87 ± 281.75 * | 1194.22 ± 273.45 * | 8.86 ± 9.31 | 0.13 | 17 | 0.667 |
| Weight—standing (%) | 51.14 ± 2.35 | 48.86 ± 2.35 | 3.77 ± 3.67 | 49.94 ± 1.71 | 50.06 ± 1.71 | 3.05 ± 1.67 | 0.25 | 17 | 0.499 |
| Weight—semi-squat (%) | 50.98 ± 2.37 | 49.02 ± 2.37 | 4.70 ± 2.23 | 50.05 ± 1.76 * | 49.95 ± 1.76 * | 3.48 ± 1.77 | 0.61 | 17 | 0.020 * |
| Weight—squat (%) | 51.14 ± 2.35 | 48.86 ± 2.35 | 4.85 ± 2.89 | 49.94 ± 1.71 | 50.06 ± 1.71 | 3.95 ± 2.23 | 0.35 | 17 | 0.489 |
| Knee Ext (Nm/kg) | 2.21 ± 0.52 | 2.35 ± 0.57 | 6.17 ± 7.91 | 2.73 ± 0.84 | 2.79 ± 0.85 * | 2.27 ± 7.23 | 0.51 | 16 | 0.604 |
| Knee Flex (Nm/kg) | 1.20 ± 0.34 | 1.12 ± 0.27 | 5.90 ± 11.50 | 1.44 ± 0.33 * | 1.37 ± 0.30 * | 4.90 ± 5.96 | 0.11 | 16 | 0.091 |
| Ankle PlantFlex (Nm/kg) | 1.68 ± 0.73 | 1.89 ± 0.74 | 11.17 ± 24.48 | 1.85 ± 0.36 * | 1.95 ± 0.45 | 5.37 ± 6.67 | 0.32 | 17 | 0.003 * |
| Ankle DorsiFlex (Nm/kg) | 0.82 ± 0.34 | 0.70 ± 0.31 | 14.73 ± 16.17 | 0.59 ± 0.13 * | 0.54 ± 0.10 | 8.53 ± 9.96 | 0.46 | 17 | 0.000 * |
| Trunk LatFlex (Nm/kg) | 3.76 ± 1.77 | 3.87 ± 1.77 | 2.82 ± 7.14 | 5.08 ± 0.92 * | 5.25 ± 1.00 | 3.20 ± 5.54 | 0.06 | 16 | 0.236 |
| Hip Abd (Nm/kg) | 0.82 ± 0.15 | 0.82 ± 0.15 | 0.89 ± 2.56 | 0.89 ± 0.13 * | 0.88 ± 0.13 * | 0.62 ± 2.07 | 0.12 | 16 | 0.113 |
| Hip Add (Nm/kg) | 0.77 ± 0.14 | 0.76 ± 0.13 | 0.75 ± 2.68 | 0.80 ± 0.12 * | 0.81 ± 0.12 * | 0.74 ± 3.49 | 0.01 | 16 | 0.308 |
| Hip ExtRot (Nm/kg) | 0.78 ± 0.17 | 0.79 ± 0.18 | 0.66 ± 4.58 | 0.86 ± 0.20 * | 0.88 ± 0.19 * | 2.13 ± 4.18 | 0.34 | 16 | 0.557 |
| Hip IntRot (Nm/kg) | 0.94 ± 0.27 | 0.88 ± 0.20 | 6.36 ± 7.34 | 1.08 ± 0.24 * | 1.03 ± 0.26 | 4.55 ± 6.08 | 0.27 | 16 | 0.310 |
| Hip Ext (Nm/kg) | 1.97 ± 0.52 | 1.87 ± 0.45 | 4.84 ± 8.13 | 1.99 ± 0.35 * | 1.97 ± 0.33 * | 1.09 ± 7.59 | 0.48 | 16 | 0.271 |
| Hip Flex (Nm/kg) | 1.62 ± 0.39 | 1.65 ± 0.34 | 1.68 ± 5.26 | 1.87 ± 0.34 * | 1.85 ± 0.35 * | 1.15 ± 5.62 | 0.10 | 16 | 0.049 * |
| Nordic Hamstring (Nm/kg) | 1.42 ± 0.33 | 1.44 ± 0.33 | 2.76 ± 4.91 | 1.51 ± 0.35 * | 1.52 ± 0.34 * | 1.34 ± 5.39 | 0.28 | 16 | 0.010 * |
| Hip Add (°) | 28.61 ± 5.83 | 27.94 ± 5.77 | 12.64 ± 10.39 | 26.65 ± 5.85 | 26.76 ± 5.11 | 7.24 ± 5.72 | 0.64 | 17 | 0.119 |
| Hip Abd (°) | 42.28 ± 8.64 | 43.89 ± 11.51 | 8.85 ± 5.37 | 39.18 ± 8.16 * | 42.12 ± 8.38 * | 8.49 ± 5.34 | 0.07 | 17 | 0.885 |
| Hip Flex (°) | 145.34 ± 8.44 | 146.39 ± 7.81 | 3.38 ± 2.06 | 149.49 ± 9.54 | 146.97 ± 11.55 | 4.70 ± 3.22 | 0.49 | 17 | 0.271 |
| Hip Ext (°) | 29.57 ± 11.01 | 30.21 ± 10.60 | 10.60 ± 9.09 | 20.59 ± 5.71 * | 22.01 ± 5.91 | 18.96 ± 13.24 | 0.74 | 17 | 0.099 |
| Hip IntRot (°) | 56.38 ± 13.42 | 56.18 ± 15.76 | 11.61 ± 8.52 | 60.38 ± 11.59 | 57.70 ± 13.80 | 10.80 ± 8.72 | 0.09 | 17 | 0.679 |
| Hip ExtRot (°) | 57.68 ± 15.01 | 60.48 ± 12.35 | 14.44 ± 12.91 | 64.64 ± 15.16 * | 67.52 ± 10.33 | 11.72 ± 7.20 | 0.26 | 17 | 0.362 |
| Knee Flex (°) | 148.78 ± 6.92 | 149.78 ± 7.41 | 1.67 ± 2.23 | 149.65 ± 4.86 | 149.41 ± 5.04 | 1.25 ± 1.37 | 0.23 | 17 | 0.507 |
| Knee Ext (°) | 3.78 ± 3.92 | 3.61 ± 4.29 | 32.37 ± 37.84 | 3.29 ± 3.53 | 3.53 ± 3.78 | 36.27 ± 36.79 | 0.11 | 17 | 0.691 |
| Ankle PlantFlex (°) | 85.50 ± 10.21 | 86.33 ± 8.43 | 4.06 ± 3.25 | 91.06 ± 11.16 | 89.76 ± 6.89 | 5.53 ± 4.83 | 0.36 | 17 | 0.329 |
| Ankle DorsiFlex (°) | 13.50 ± 7.41 | 10.17 ± 9.98 | 50.01 ± 42.44 | 18.70 ± 9.35 | 18.35 ± 7.98 | 21.61 ± 23.10 | 0.83 | 17 | 0.025 * |
References
- Crossley, K.M.; Middelkoop, M.V.; Barton, C.J.; Culvenor, A.G. Best Practice & Research Clinical Rheumatology Rethinking patellofemoral pain: Prevention, management and long-term consequences. Best Pract. Res. Clin. Rheumatol. 2019, 33, 48e65. [Google Scholar] [CrossRef]
- de Oliveira Silva, D.; Pazzinatto, M.F.; del Priore, L.B.; Ferreira, A.S.; Briani, R.V.; Ferrari, D.; Bazett-Jones, D.; de Azevedo, F.M. Knee crepitus is prevalent in women with patellofemoral pain, but is not related with function, physical activity and pain. Phys. Ther. Sport 2018, 33, 7–11. [Google Scholar] [CrossRef]
- Liew, B.X.W.; Abichandani, D.; De Nunzio, A.M. Individuals with patellofemoral pain syndrome have altered inter-leg force coordination. Gait Posture 2020, 79, 65–70. [Google Scholar] [CrossRef]
- Van Cant, J.; Pineux, C.; Pitance, L.; Feipel, V. Hip muscle strength and endurance in females with patellofemoral pain: A systematic review with meta-analysis. Int. J. Sports Phys. Ther. 2014, 9, 564–582. [Google Scholar]
- Creaby, M.W.; Bennell, K.L.; Hunt, M.A. Gait differs between unilateral and bilateral knee osteoarthritis. Arch. Phys. Med. Rehabil. 2012, 93, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Mokha, M.; Sprague, P.A.; Gatens, D.R. Predicting Musculoskeletal Injury in National Collegiate Athletic Association Division II Athletes from Asymmetries and Individual-Test Versus Composite Functional Movement Screen Scores. J. Athl. Train. 2016, 51, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.; Payne, J.; Larkin, T.A. Patellofemoral Pain Syndrome and Pain Severity Is Associated with Asymmetry of Gluteus Medius Muscle Activation Measured Via Ultrasound. Am. J. Phys. Med. Rehabil. 2020, 99, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, E.; Fukuda, T.Y.; Sacramento, S.N.; Forgas, A.; Cohen, M.; Abdalla, R.J. A comparison of hip strength between sedentary females with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2010, 40, 641–647. [Google Scholar] [CrossRef]
- Plastaras, C.; McCormick, Z.; Nguyen, C.; Rho, M.; Nack, S.H.; Roth, D.; Casey, E.; Carneiro, K.; Cucchiara, A.; Press, J.; et al. Is Hip Abduction Strength Asymmetry Present in Female Runners in the Early Stages of Patellofemoral Pain Syndrome? Am. J. Sports Med. 2016, 44, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Boling, M.C.; Nguyen, A.D.; Padua, D.A.; Cameron, K.L.; Beutler, A.; Marshall, S.W. Gender-Specific Risk Factor Profiles for Patellofemoral Pain. Clin. J. Sport Med. 2021, 31, 49–56. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; de Scorpio, G.; Indino, A.; Iona, T.; Ammendolia, A. Late activation of the vastus medialis in determining the risk of anterior cruciate ligament injury in soccer players. J. Sport Rehabil. 2020, 29, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Demeco, A.; Moggio, L.; Isabello, L.; Iona, T.; Ammendolia Prof, A. Correlation between dynamic knee valgus and quadriceps activation time in female athletes. J. Phys. Educ. Sport 2020, 20, 2508–2512. [Google Scholar] [CrossRef]
- de Sire, A.; Demeco, A.; Marotta, N.; Moggio, L.; Palumbo, A.; Iona, T.; Ammendolia, A. Anterior Cruciate Ligament Injury Prevention Exercises: Could a Neuromuscular Warm-Up Improve Muscle Pre-Activation before a Soccer Game? A Proof-of-Principle Study on Professional Football Players. Appl. Sci. 2021, 11, 4958. [Google Scholar] [CrossRef]
- Baldon, R.D.M.; Nakagawa, T.H.; Muniz, T.B.; Amorim, C.F.; Maciel, C.D.; Serrão, F.V. Eccentric hip muscle function in females with and without patellofemoral pain syndrome. J. Athl. Train. 2009, 44, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M. The Influence of Altered Lower-Extremity Kinematics on Patellofemoral Joint Dysfunction: A Theoretical Perspective. J. Orthop. Sports Phys. Ther. 2003, 33, 639–646. [Google Scholar] [CrossRef]
- Chevidikunnan, M.F.; Saif, A.A.; Gaowgzeh, R.A.; Mamdouh, K.A. Effectiveness of core muscle strengthening for improving pain and dynamic balance among female patients with patellofemoral pain syndrome. J. Phys. Ther. Sci. 2016, 28, 1518–1523. [Google Scholar] [CrossRef]
- Tyler, T.F.; Nicholas, S.J.; Mullaney, M.J.; McHugh, M.P. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am. J. Sports Med. 2006, 34, 630–636. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Bissolotti, L.; Touche, R.L.; Pedersini, P.; Negrini, S. Effect of muscle strengthening on perceived pain and static knee angles in young subjects with patellofemoral pain syndrome. J. Exerc. Rehabil. 2019, 15, 454–459. [Google Scholar] [CrossRef]
- Keays, S.L.; Mason, M.; Newcombe, P.A. Individualized physiotherapy in the treatment of patellofemoral pain. Physiother. Res. Int. 2015, 20, 22–36. [Google Scholar] [CrossRef]
- Yosmaoğlu, H.; Sonmezer, E.; Ozkoslu, M.; Sahin, E.; Çerezci, S.; Richards, J.; Selfe, J.; Janssen, J. Targeted Treatment Protocol in Patellofemoral Pain (TIPPs): Does Treatment Designed According to Subgroups Improve Clinical Outcomes in Patients Unresponsive to Multimodal Treatment? Sports Health 2019, 12, 170–180. [Google Scholar] [CrossRef]
- Dolak, K.L.; Silkman, C.; Mckeon, J.M.; Hosey, R.G.; Lattermann, C.; Uhl, T.L. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2011, 41, 560–570. [Google Scholar] [CrossRef]
- Nakagawa, T.H.; Moriya, E.T.U.; MacIel, C.D.; Serrão, F.V. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single-leg squat in males and females with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2012, 42, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Boling, M.C.; Bolgla, L.A.; Mattacola, C.G.; Uhl, T.L.; Hosey, R.G. Outcomes of a Weight-Bearing Rehabilitation Program for Patients Diagnosed With Patellofemoral Pain Syndrome. Arch. Phys. Med. Rehabil. 2006, 87, 1428–1435. [Google Scholar] [CrossRef]
- Kozinc, Ž.; Smajla, D.; Šarabon, N. Relationship between hip abductor strength, rate of torque development scaling factor and medio-lateral stability in older adults. Gait Posture 2020. [Google Scholar] [CrossRef]
- Nakagawa, T.H.; Maciel, C.D.; Serrão, F.V. Trunk biomechanics and its association with hip and knee kinematics in patients with and without patellofemoral pain. Man. Ther. 2015, 20, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Fraeulin, L.; Holzgreve, F.; Brinkbäumer, M.; Dziuba, A.; Friebe, D.; Klemz, S.; Schmitt, M.; Anna-Lena Theis, A.; Tenberg, S.; van Mark, A.; et al. Intra- And inter-rater reliability of joint range of motion tests using tape measure, digital inclinometer and inertial motion capturing. PLoS ONE 2020, 15, e0243646. [Google Scholar] [CrossRef]
- Piriyaprasarth, P.; Morris, M.E.; Winter, A.; Bialocerkowski, A.E. The reliability of knee joint position testing using electrogoniometry. BMC Musculoskelet. Disord. 2008, 9, 6. [Google Scholar] [CrossRef]
- Norkin, C.; White, J.D. Measurement Of Joint Motion: A Guide To Goniometry. Available online: https://books.google.hr/books?hl=sl&lr=&id=TSluDQAAQBAJ&oi=fnd&pg=PR1&ots=2h2Qy7BdE_&sig=LJEEU65O1rtE4GiYmR2ZL1y7mwE&redir_esc=y#v=onepage&q&f=false (accessed on 27 January 2021).
- Kozinc, Ž.; Šarabon, N. Inter-limb asymmetries in volleyball players: Differences between testing approaches and association with performance. J. Sport. Sci. Med. 2020, 19, 745–752. [Google Scholar]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Associations of lower limb joint asymmetry with fatigue and disability in people with multiple sclerosis. Clin. Biomech. 2020, 75, 104989. [Google Scholar] [CrossRef]
- Proessl, F.; Ketelhut, N.B.; Rudroff, T. No association of leg strength asymmetry with walking ability, fatigability, and fatigue in multiple sclerosis. Int. J. Rehabil. Res. 2018, 41, 267–269. [Google Scholar] [CrossRef]
- Impellizzeri, F.M.; Rampinini, E.; Maffiuletti, N.; Marcora, S.M. A vertical jump force test for assessing bilateral strength asymmetry in athletes. Med. Sci. Sports Exerc. 2007, 39, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Croisier, J.L.; Reveillon, V.; Ferret, J.M.; Cotte, T.; Genty, M.; Popovich, N.; Filho, M.; Faryniuk, J.E.; Ganteaume, S.; Crielaard, J.M. Isokinetic assessment of knee flexors and extensors in professional soccer players. Isokinet. Exerc. Sci. 2003, 11, 61–62. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Available online: https://books.google.si/books?id=2v9zDAsLvA0C&pg=PP1&redir_esc=y#v=onepage&q&f=false (accessed on 10 May 2021).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Ferber, R.; Bolgla, L.; Earl-Boehm, J.E.; Emery, C.; Hamstra-Wright, K. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: A multicenter randomized controlled trial. J. Athl. Train. 2015, 50, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Gamaleldein, M.H.; Hassa, K.A. Closed Kinetic Chain exercises with or without additional hip strengthening exercises in management of Patellofemoral pain syndrome: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2013, 49, 687–698. [Google Scholar] [PubMed]
- Crowe, M.A.; Bampouras, T.M.; Walker-Small, K.; Howe, L.P. Restricted Unilateral Ankle Dorsiflexion Movement Increases Interlimb Vertical Force Asymmetries in Bilateral Bodyweight Squatting. J. Strength Cond. Res. 2020, 34, 332–336. [Google Scholar] [CrossRef]
- Macrum, E.; Bell, D.R.; Boling, M.; Lewek, M.; Padua, D. Effect of limiting ankle-dorsiflexion range of motion on lower extremity kinematics and muscle-activation patterns during a squat. J. Sport Rehabil. 2012, 21, 144–150. [Google Scholar] [CrossRef]
- Nakagawa, T.H.; dos Santos, A.F.; Lessi, G.C.; Petersen, R.S.; Silva, R.S. Y-Balance Test Asymmetry and Frontal Plane Knee Projection Angle during Single-Leg Squat as Predictors of Patellofemoral Pain in Male Military Recruits. Phys. Ther. Sport 2020, 44, 121–127. [Google Scholar] [CrossRef]
- Brechter, J.H.; Powers, C.M. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait Posture 2002, 16, 115–123. [Google Scholar] [CrossRef]
- Noehren, B.; Scholz, J.; Davis, I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br. J. Sports Med. 2011, 45, 691–696. [Google Scholar] [CrossRef]
- Rabelo, N.D.D.A.; Lima, B.; Reis, A.C.D.; Bley, A.S.; Yi, L.C.; Fukuda, T.Y.; Costa, L.O.P.; Lucareli, P.R.G. Neuromuscular training and muscle strengthening in patients with patellofemoral pain syndrome: A protocol of randomized controlled trial. BMC Musculoskelet. Disord. 2014, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.C.; de Vasconcelos, R.A.; de Mancinelli, L.V.; de Munno, M.S.; Liporaci, R.F.; Grossi, D.B. Is hip strengthening the best treatment option for females with patellofemoral pain? A randomized controlled trial of three different types of exercises. Braz. J. Phys. Ther. 2018, 22, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J.R.; Edwards, P.; Preez, L.D.; Furzer, B.; Joss, B. Knee extensor strength, hop performance, patient-reported outcome and inter-test correlation in patients 9–12 months after anterior cruciate ligament reconstruction. Knee 2021, 30, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Piva, S.R.; Fitzgerald, G.K.; Irrgang, J.J.; Fritz, J.M.; Wisniewski, S.; McGinty, G.T.; Childs, J.D.; Domenech, M.A.; Jones, S.; Delitto, A. Associates of Physical Function and Pain in Patients with Patellofemoral Pain Syndrome. Arch. Phys. Med. Rehabil. 2009, 90, 285–295. [Google Scholar] [CrossRef]
- Magalhães, E.; Silva, A.P.; Sacramento, S.N.; Martin, R.L. FT Isometric strength ratios of the hip musculature in females with patellofemoral pain: A comparison to pain-free controls. J. Strength Cond. Res. 2013, 27, 2165–2170. [Google Scholar] [CrossRef]
- Şahin, M.; Ayhan, F.F.; Borman, P.; Atasoy, H. The effect of hip and knee exercises on pain, function, and strength in patients with patellofemoral pain syndrome: A randomized controlled trial. Turk. J. Med. Sci. 2016, 46, 265–277. [Google Scholar] [CrossRef]
- Ferber, R.; Kendall, K.D.; Farr, L. Changes in Knee Biomechanics after a Hip-Abductor Strengthening Protocol for Runners with Patellofemoral Pain Syndrome. J. Athl. Train. 2011, 46, 142–149. [Google Scholar] [CrossRef]
- Barton, C.J.; Lack, S.; Malliaras, P.; Morrissey, D. Gluteal muscle activity and patellofemoral pain syndrome: A systematic review. Br. J. Sports Med. 2013, 47, 207–214. [Google Scholar] [CrossRef]
- Rathleff, M.S.; Richter, C.; Brushøj, C.; Bencke, J.; Bandholm, T.; Hölmich, P.; Thorborg, K. Increased medial foot loading during drop jump in subjects with patellofemoral pain. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 2301–2307. [Google Scholar] [CrossRef]
- Mølgaard, C.M.; Rathleff, M.S.; Andreasen, J.; Christensen, M.; Lundbye-Christensen, S.; Simonsen, O.; Kaalund, S. Foot exercises and foot orthoses are more effective than knee focused exercises in individuals with patellofemoral pain. J. Sci. Med. Sport 2018, 21, 10–15. [Google Scholar] [CrossRef]
- Barton, C.J.; Lack, S.; Hemmings, S.; Tufail, S.; Morrissey, D. The “Best Practice Guide to Conservative Management of Patellofemoral Pain”: Incorporating level 1 evidence with expert clinical reasoning. Br. J. Sport. Med. 2015, 923–934. [Google Scholar] [CrossRef] [PubMed]
| Females | Males | All | |
|---|---|---|---|
| N | 13 | 5 | 18 |
| Age (years) | 19.11 ± 8.42 | 37.43 ± 12.31 | 24.17 ± 12.52 |
| Body height (cm) | 167.52 ± 7.07 | 181.04 ± 11.38 | 171.22 ± 10.24 |
| Body mass (kg) | 57.94 ± 6.67 | 84.21 ± 19.71 | 65.22 ± 16.42 |
| Body mass index | 20.63 ± 1.74 | 25.58 ± 4.77 | 22.41 ± 3.56 |
| Pre-Test Asymmetry Index (%) | Post-Test Asymmetry Index (%) | Cohen’s d | Degrees of Freedom | p-Value | |
|---|---|---|---|---|---|
| Knee Ext/Flex (%) | 45.30 ± 12.11 | 47.18 ± 11.39 | 0.16 | 16 | 0.941 |
| Ankle PlantFlex/DorsiFlex (%) | 45. 87 ± 23.11 | 67.92 ± 10.03 | 1.24 | 17 | 0.036 * |
| Trunk Ext/Flex (%) | 29.53 ± 15.10 | 28.03 ± 17.74 | 0.09 | 16 | 0.836 |
| Hip Abd/Add (%) | 5.74 ± 9.86 | 9.86 ± 9.30 | 0.43 | 16 | 0.472 |
| Hip ExtRot/IntRot (%) | 16.63 ± 13.05 | 19.91 ± 13.06 | 0.25 | 16 | 0.106 |
| Hip Ext/Flex (%) | 17.70 ± 14.73 | 6.15 ± 9.37 | 0.94 | 16 | 0.031 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manojlović, D.; Zorko, M.; Spudić, D.; Šarabon, N. Effects of a Targeted Exercise Program on Inter-Leg Asymmetries in Patients with Patellofemoral Pain. Symmetry 2021, 13, 1075. https://doi.org/10.3390/sym13061075
Manojlović D, Zorko M, Spudić D, Šarabon N. Effects of a Targeted Exercise Program on Inter-Leg Asymmetries in Patients with Patellofemoral Pain. Symmetry. 2021; 13(6):1075. https://doi.org/10.3390/sym13061075
Chicago/Turabian StyleManojlović, Denisa, Martin Zorko, Darjan Spudić, and Nejc Šarabon. 2021. "Effects of a Targeted Exercise Program on Inter-Leg Asymmetries in Patients with Patellofemoral Pain" Symmetry 13, no. 6: 1075. https://doi.org/10.3390/sym13061075
APA StyleManojlović, D., Zorko, M., Spudić, D., & Šarabon, N. (2021). Effects of a Targeted Exercise Program on Inter-Leg Asymmetries in Patients with Patellofemoral Pain. Symmetry, 13(6), 1075. https://doi.org/10.3390/sym13061075







