Biopolymer and Biomaterial Conjugated Iron Oxide Nanomaterials as Prostate Cancer Theranostic Agents: A Comprehensive Review
Abstract
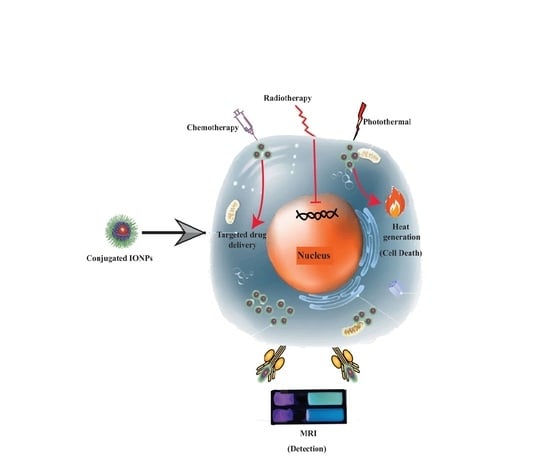
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Inclusion Criteria
2.4. Exclusion Criteria
3. Prostate Cancer
4. Diagnosis and Detection of Prostate Tumor Cells
4.1. Detection of Overexpressed Receptors
4.2. Antibody and Polypeptide-Based Detection
4.3. Detection of Physiological Metabolite
4.4. Detection of Metastases
4.5. Others
| Types of IONPs | Coating/Hybridizing Material | Test Specimen | Target Receptor | Imaging Technique | Detection (Lower) Limit | Ref. |
|---|---|---|---|---|---|---|
| IONP | PTX, LHRH peptide, AE105 peptide | RC77T/E and RC77N/E | LHRH-R and uPAR | MRI | NR | [26] |
| SPION | PBA | PC-3 | GRP-R | MRI | 103 cells | [56] |
| IONP | FA | RWPE-1 and PC-3 | FA-R | MRI | NR | [60] |
| FLUSPION | FM | LnCap tumor xenografts, HUVEC | Rf-R | MRI | 2 × 106 cells | [61] |
| SPION | anti-PSCA Ab | PC-3 cells | PSCA-R | ELISA and WB | 70 µg/mL | [64] |
| IONP | muJ591-Ab | LNCaP and DU145 | PSMA | MRI | NR | [71] |
| SPION | J591-Ab | LNCaP, DU145 | PSMA | MRI | NR | [72] |
| IONP | J591-Ab | LNCaP, PC-3, DU145, and SCID mice | PSMA | MRI | NR | [73] |
| SPION | CQKHHNYLC | LNCaP, PC-3 and LNCaP tumor-bearing mice | PSMA | MRI | 0.240 mg/mL | [74] |
| SPION | scAbPSCA-PEI-g-PEG | PC-3, PC3M, and NIH3T3 | PSCA | MRI | 40 μg/mL | [75] |
| IONP | Anti-PSA Ab | Human Serum | PSA-R | TEM | 0.001 µg/L PSA | [76] |
| IONP | Anti-PSA Ab | Human Serum | PSA-R | IDE surface | 1.9 pg/mL (PSA) | [77] |
| SPION | CS, SOX | Yeast cell, RBCs | NR | SEM | NR | [81] |
| SPION | NR | 20 PCa patients | SLN | MRI | 9.84 ng/mL (PSA) | [84] |
| SPION | NR | 50 PCa patients | SLN | MRI | >10 ng/mL (PSA) | [85] |
| USPION | NR | NR | PSA | MRI | 18.8 ± 30.5 ng/mL (PSA) | [86] |
| USPION | NR | NR | PLN | MRI | 2.6 mg iron/kg body weight of USPION | [87] |
| USPION | NR | NR | Lymph nodes/PSA | MRI | NR | [88] |
| SPION | NR | CT1258 | NR | MRI | 103 and 104 cells per tube | [27] |
| SPION | Cdex, PLL | PC-3 | NR | NR | 2 × 105 cells/mL | [89] |
| IONP | PIAA | PC-3 and LNCaP | R11-R | MRI | 5000 cells/well | [91] |
5. IONP-Based Treatment of Prostate Cancer
5.1. Photothermal Therapy
5.2. Radiotherapy
5.3. Chemotherapy
5.4. Hyperthermia
5.5. Phytochemical-Based Therapy
5.6. Others
| Treatment | IONPs Shape | Size | Coating Materials | Drug/Radiation Therapy Used | Cell Line | In Vivo | Treatment Period | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PTT | Nano flower | 27 ± 4 nm | NR | NR | PC-3 | NR | 30 min | Induced apoptosis and necrosis for PC-3 cell destruction | [92] |
| RT | Nanocapsule | 67 nm | PLGA | 5-FU and x-ray Radiation | DU145 | NR | 24 h | An effective drug delivery vehicle for 5-FU that can penetrate the cells | [93] |
| CT | Nanosphere | 15.74 ± 0.44 nm | PTX, LHRH peptide, AE105 peptide | PTX | RC77T/E and RC77N/E | NR | NR | Reduced the viability of PC-3 cells two times, lowers the toxicity of PTX, enhances pharmacokinetic efficiency with better patient outcomes | [26] |
| Nanosphere | 8–10 nm | DTX and J591 | DTX | C4-2 and PC-3 | NR | NR | Reduced the levels of MDR1 proteins that help to reduce chemoresistance of drugs | [109] | |
| NR | ~100 nm | miRNA | DTX | C4-2 and PC-3 | NR | 8 h | The number of C4-2 and PC-3 cell colonies declined by approximately 40% compared to the non-treated and DTX alone | [107] | |
| core-shell | 9.0 ± 4.0 nm | GSH-pDA | NR | PC-3 | NR | 24 h | In response to the pH and chemicals, the drug is released to the site of action | [104] | |
| HT | Hexagonal | 13.97 ± 3.63 nm | Zn and Mn | NR | DU145 and HEK-293 | Mouse Model of human PCa | 24 h | ZnMn-IONPs killed more than 90% of PCa cells safely without noticeable toxicity | [114] |
| Irregular shape | ~12 nm | ms-silica shell | Silica | LNCaP-Pro5 | Male nude mice | 24 h | Efficient heating capability made it suitable for PCa theranostic | [116] | |
| PTC-Based | Regular spherical | 58.5 ± 4 nm | mPEG-b-PLGA | Eu | DU-145, LNCaP, and HUVECs | NR | 200 h | Induced apoptosis and decreased the rate of necrosis at a lower dose | [94] |
| Others | Core-shell | 154.3 nm | Wy5a aptamer (Apt) | DTX | PC-3 | Mice Model | 4 weeks | Found enhanced efficacy of CRPC theranostic and low toxicity to the circulation both in vivo and in vitro | [120] |
| Spherical shape | 146.9 ± 8.6 nm | scAbPSCA | DTX | PC-3 | NR | 24, 48, and 72 h | Showed antiproliferative activity against PC3 as well as MRI contrast agent | [121] | |
| NR | ~5-nm | Dextran | NR | HUVEC | Xenografts of human prostate tissue | 30 days | Bound to the primary xenografts of human prostate tissue on ADT-damaged human microvasculature and prevention of angiogenesis to the PCa cells | [122] | |
| NR | ~65 ± 12 nm | PSMA conjugated TCL | DOX | LNCaP | Male nude athymic mice | 50 h | Selectively deliver anticancer drugs and monitor the therapeutic response | [123] |
6. Toxicity
6.1. In Vitro Toxicity
6.2. In Vivo Toxicity
7. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandhi, J.; Afridi, A.; Vatsia, S.; Joshi, G.; Joshi, G.; Kaplan, S.A.; Smith, N.L.; Khan, S.A. The molecular biology of prostate cancer: Current understanding and clinical implications. Prostate Cancer Prostatic Dis. 2018, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Rana, Z.; Tyndall, J.D.; Hanif, M.; Hartinger, C.G.; Rosengren, R.J.J.P. Cytostatic Action of Novel Histone Deacetylase Inhibitors in Androgen Receptor-Null Prostate Cancer Cells. Pharm 2021, 14, 103. [Google Scholar] [CrossRef]
- Printz, C. Prostate cancer mortality projections reach a new high: With prostate cancer deaths projected to rise to their highest level in 20 years, some experts worry that changes to screening guidelines made in 2012 could be a factor. Cancer 2020, 126, 3893–3894. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Reda, I.; Khalil, A.; Elmogy, M.; Abou El-Fetouh, A.; Shalaby, A.; Abou El-Ghar, M.; Elmaghraby, A.; Ghazal, M.; El-Baz, A. Deep Learning Role in Early Diagnosis of Prostate Cancer. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Kohaar, I.; Petrovics, G.; Srivastava, S. A Rich Array of Prostate Cancer Molecular Biomarkers: Opportunities and Challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [CrossRef]
- Oh, S.W.; Cheon, G.J. Prostate-Specific Membrane Antigen PET Imaging in Prostate Cancer: Opportunities and Challenges. Korean J. Radiol. 2018, 19, 819–831. [Google Scholar] [CrossRef]
- Pan, L.H.; Kuo, S.H.; Lin, T.Y.; Lin, C.W.; Fang, P.Y.; Yang, H.W. An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosens. Bioelectron. 2017, 89, 598–605. [Google Scholar] [CrossRef]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- Sushentsev, N.; Kaggie, J.D.; Buonincontri, G.; Schulte, R.F.; Graves, M.J.; Gnanapragasam, V.J.; Barrett, T. The effect of gadolinium-based contrast agent administration on magnetic resonance fingerprinting-based T(1) relaxometry in patients with prostate cancer. Sci. Rep. 2020, 10, 20475. [Google Scholar] [CrossRef]
- Helfand, B.T.; Glaser, A.P.; Rimar, K.; Zargaroff, S.; Hedges, J.; McGuire, B.B.; Catalona, W.J.; McVary, K.T. Prostate cancer diagnosis is associated with an increased risk of erectile dysfunction after prostate biopsy. BJU Int. 2013, 111, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.S.; Hasan, M.A.; Uddin, M.F.; Islam, A.; Nahar, A.; Das, H.; Khan, M.N.I.; Dey, B.P.; Rokeya, B.; Hoque, S.M.J.A.A.B.M. In Vivo Toxicity Studies of Chitosan-Coated Cobalt Ferrite Nanocomplex for Its Application as MRI Contrast Dye. ACS Appl. Bio Mater. 2020, 3, 7952–7964. [Google Scholar] [CrossRef]
- Soares, S.C.M.; de Camargo Cancela, M.; Migowski, A.; de Souza, D.L.B. Digital rectal examination and its associated factors in the early detection of prostate cancer: A cross-sectional population-based study. BMC Public Health 2019, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Druskin, S.C.; Andreas, D.; Mullane, P.; Chappidi, M.; Joo, S.; Ghabili, K.; Mamawala, M.; Agostino, J.; Carter, H.B.; et al. Prostate Health Index density improves detection of clinically significant prostate cancer. BJU Int. 2017, 120, 793–798. [Google Scholar] [CrossRef]
- Wilt, T.J.; Ahmed, H.U. Prostate cancer screening and the management of clinically localized disease. BMJ Clin. Res. Ed. 2013, 346, f325. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Koyama, T.; Fan, K.H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9, S3. [Google Scholar]
- Chaussy, C.G.; Thüroff, S. High-Intensity Focused Ultrasound for the Treatment of Prostate Cancer: A Review. J. Endourol. 2017, 31, S30–S37. [Google Scholar] [CrossRef]
- Chennupati, S.K.; Pelizzari, C.A.; Kunnavakkam, R.; Liauw, S.L. Late toxicity and quality of life after definitive treatment of prostate cancer: Redefining optimal rectal sparing constraints for intensity-modulated radiation therapy. Cancer Med. 2014, 3, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Kurup Sasikala, A.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.U.; Salam, A.B.; Yates, C.; Willian, K.; Jaynes, J.; Turner, T.; Abdalla, M.O. Double-receptor-targeting multifunctional iron oxide nanoparticles drug delivery system for the treatment and imaging of prostate cancer. Int. J. Nanomed. 2017, 12, 6973–6984. [Google Scholar] [CrossRef] [PubMed]
- Sterenczak, K.A.; Meier, M.; Glage, S.; Meyer, M.; Willenbrock, S.; Wefstaedt, P.; Dorsch, M.; Bullerdiek, J.; Murua Escobar, H.; Hedrich, H.; et al. Longitudinal MRI contrast enhanced monitoring of early tumour development with manganese chloride (MnCl2) and superparamagnetic iron oxide nanoparticles (SPIOs) in a CT1258 based in vivo model of prostate cancer. BMC Cancer 2012, 12, 284. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.S.; Hasan, M.A.; Sarker, S.R. Iron Oxide Nanoparticles for Breast Cancer Theranostics. Curr. Drug Metab. 2019, 20, 446–456. [Google Scholar] [CrossRef]
- Inamura, K. Prostatic cancers: Understanding their molecular pathology and the 2016 WHO classification. Oncotarget 2018, 9, 14723–14737. [Google Scholar] [CrossRef]
- Mazaris, E.; Tsiotras, A.J.N.-u.m. Molecular pathways in prostate cancer. Nephro-urol. Mon. 2013, 5, 792. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C.; Favero, F.; Risch, T.; Simon, R.; Feuerbach, L.; Assenov, Y.; Heckmann, D.; Sidiropoulos, N.; Waszak, S.M.; Hübschmann, D.; et al. Molecular Evolution of Early-Onset Prostate Cancer Identifies Molecular Risk Markers and Clinical Trajectories. Cancer Cell 2018, 34, 996–1011.e1018. [Google Scholar] [CrossRef] [PubMed]
- Granlund, K.L.; Tee, S.S.; Vargas, H.A.; Lyashchenko, S.K.; Reznik, E.; Fine, S.; Laudone, V.; Eastham, J.A.; Touijer, K.A.; Reuter, V.E.; et al. Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab. 2020, 31, 105–114.e103. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; Etzioni, R.; Bolouri, H.; Montgomery, B.; White, T.; et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Hieronymus, H.; Schultz, N.; Gopalan, A.; Carver, B.S.; Chang, M.T.; Xiao, Y.; Heguy, A.; Huberman, K.; Bernstein, M.; Assel, M.; et al. Copy number alteration burden predicts prostate cancer relapse. Proc. Natl. Acad. Sci. USA 2014, 111, 11139–11144. [Google Scholar] [CrossRef] [PubMed]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e296. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- cBioPortal for Cancer Genomics. The Metastatic Prostate Cancer Project (Provisional, November 2019). Available online: https://bit.ly/32YuZ6J (accessed on 15 March 2021).
- cBioPortal for Cancer Genomics. Prostate Cancer. Available online: https://bit.ly/3cRgaqS (accessed on 15 March 2021).
- Shoag, J.; Barbieri, C.E. Clinical variability and molecular heterogeneity in prostate cancer. Asian J. Androl. 2016, 18, 543–548. [Google Scholar] [CrossRef]
- Yadav, S.S.; Stockert, J.A.; Hackert, V.; Yadav, K.K.; Tewari, A.K. Intratumor heterogeneity in prostate cancer. Urol. Oncol. 2018, 36, 349–360. [Google Scholar] [CrossRef]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Ahmad, M.F.; Shadab, G.; Shadab, G.G.H.A.; Siddique, H.R. Superparamagnetic iron oxide nanoparticles based cancer theranostics: A double edge sword to fight against cancer. J. Drug Deliv. Sci. Technol. 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Q.; Jiang, J.; Luo, Y.; Sun, Z. A conjugate of methotrexate and an analog of luteinizing hormone releasing hormone shows increased efficacy against prostate cancer. Sci. Rep. 2016, 6, 33894. [Google Scholar] [CrossRef]
- Popovics, P.; Schally, A.V.; Szalontay, L.; Block, N.L.; Rick, F.G. Targeted cytotoxic analog of luteinizing hormone-releasing hormone (LHRH), AEZS-108 (AN-152), inhibits the growth of DU-145 human castration-resistant prostate cancer in vivo and in vitro through elevating p21 and ROS levels. Oncotarget 2014, 5, 4567–4578. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.; Shulkes, A.; Baldwin, G.S. Gastrin-releasing peptide and cancer. Biochim. et Biophys. Acta 2006, 1766, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.L.; Hickey, J.L.; Ablack, A.L.; Lewis, J.D.; Luyt, L.G.; Gillies, E.R. Synthesis of bombesin-functionalized iron oxide nanoparticles and their specific uptake in prostate cancer cells. J. Nanoparticle Res. Interdiscip. Forum Nanoscale Sci. Technol. 2009, 12, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.C.; Keeney, G.L.; Lingle, W.L.; Christianson, T.J.; Varghese, B.; Hillman, D.; Oberg, A.L.; Low, P.S. Folate receptor overexpression is associated with poor outcome in breast cancer. Int. J. Cancer 2007, 121, 938–942. [Google Scholar] [CrossRef]
- Lee, R.J.; Low, P.S. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim. Et Biophys. Acta 1995, 1233, 134–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.M.; Yan, X.P. Self-assembly of folate onto polyethyleneimine-coated CdS/ZnS quantum dots for targeted turn-on fluorescence imaging of folate receptor overexpressed cancer cells. Anal. Chem. 2013, 85, 228–234. [Google Scholar] [CrossRef]
- Bonvin, D.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Mionić Ebersold, M. Folic acid on iron oxide nanoparticles: Platform with high potential for simultaneous targeting, MRI detection and hyperthermia treatment of lymph node metastases of prostate cancer. Dalton Trans. 2017, 46, 12692–12704. [Google Scholar] [CrossRef]
- Jayapaul, J.; Arns, S.; Bunker, M.; Weiler, M.; Rutherford, S.; Comba, P.; Kiessling, F. In vivo evaluation of riboflavin receptor targeted fluorescent USPIO in mice with prostate cancer xenografts. Nano Res. 2016, 9, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Saeki, N.; Gu, J.; Yoshida, T.; Wu, X. Prostate stem cell antigen: A Jekyll and Hyde molecule? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 3533–3538. [Google Scholar] [CrossRef]
- Patra, P.; Bhattacharya, M.; Sharma, A.R.; Ghosh, P.; Sharma, G.; Patra, B.C.; Mallick, B.; Lee, S.S.; Chakraborty, C. Identification and Design of a Next-Generation Multi Epitopes Bases Peptide Vaccine Candidate Against Prostate Cancer: An In Silico Approach. Cell Biochem. Biophys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shahrabi Farahani, M.; Mohsenzadegan, M.; Taeb, J.; Farajollahi, M.M. In-vitro prostate cancer biomarker detection by directed conjugation of anti-PSCA antibody to super paramagnetic iron oxide nanoparticless. Med. J. Islamic Repub. Iran 2019, 33, 16. [Google Scholar] [CrossRef]
- Reiter, R.E.; Sato, I.; Thomas, G.; Qian, J.; Gu, Z.; Watabe, T.; Loda, M.; Jenkins, R.B. Coamplification of prostate stem cell antigen (PSCA) and MYC in locally advanced prostate cancer. Geneschromosomes Cancer 2000, 27, 95–103. [Google Scholar] [CrossRef]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N.J.E.u. Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. J. Drug Deliv. Sci. Technol. 2016, 70, 926–937. [Google Scholar] [CrossRef]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef]
- Bander, N.H.; Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 4591–4601. [Google Scholar] [CrossRef]
- Bander, N.H.; Trabulsi, E.J.; Kostakoglu, L.; Yao, D.; Vallabhajosula, S.; Smith-Jones, P.; Joyce, M.A.; Milowsky, M.; Nanus, D.M.; Goldsmith, S.J. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J. Urol. 2003, 170, 1717–1721. [Google Scholar] [CrossRef]
- Bates, D.; Abraham, S.; Campbell, M.; Zehbe, I.; Curiel, L. Development and characterization of an antibody-labeled super-paramagnetic iron oxide contrast agent targeting prostate cancer cells for magnetic resonance imaging. PLoS ONE 2014, 9, e97220. [Google Scholar] [CrossRef]
- Abdolahi, M.; Shahbazi-Gahrouei, D.; Laurent, S.; Sermeus, C.; Firozian, F.; Allen, B.J.; Boutry, S.; Muller, R.N. Synthesis and in vitro evaluation of MR molecular imaging probes using J591 mAb-conjugated SPIONs for specific detection of prostate cancer. Contrast Media Mol. Imaging 2013, 8, 175–184. [Google Scholar] [CrossRef]
- Tse, B.W.; Cowin, G.J.; Soekmadji, C.; Jovanovic, L.; Vasireddy, R.S.; Ling, M.T.; Khatri, A.; Liu, T.; Thierry, B.; Russell, P.J. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomed. Lond. Engl. 2015, 10, 375–386. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Chen, Y.; Liu, W.; Jiang, J.; Guan, W.; Zhang, Z.; Duan, Y. In Vivo Molecular MRI Imaging of Prostate Cancer by Targeting PSMA with Polypeptide-Labeled Superparamagnetic Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 9573–9587. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, L.; Wang, W.; Pang, J.; Zou, Y.; Shuai, X.; Gao, X.J.C.S.B. Prostate cancer targeted MRI nanoprobe based on superparamagnetic iron oxide and copolymer of poly (ethylene glycol) and polyethyleneimin. Chin. Sci. Bull. 2009, 54, 3137–3146. [Google Scholar] [CrossRef]
- Farshchi, F.; Hasanzadeh, M.; Mokhtarzadeh, A. A novel electroconductive interface based on Fe3O4 magnetic nanoparticle and cysteamine functionalized AuNPs: Preparation and application as signal amplification element to minoring of antigen-antibody immunocomplex and biosensing of prostate cancer. J. Mol. Recognit. 2020, 33, e2825. [Google Scholar] [CrossRef]
- Zhang, W.; Li, K.; Guo, J.; Ma, T.; Wang, D.; Shi, S.; Gopinath, S.C.B.; Gu, D. Sensitive identification of prostate-specific antigen by iron oxide nanoparticle antibody conjugates on the gap-finger electrode surface. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a potential prostate cancer biomarker—A review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, R.B.; Gemelli, T.; Rojas, D.B.; Dutra-Filho, C.S.; Wannmacher, C.M. Chemically induced acute model of sarcosinemia in wistar rats. Metab. Brain Dis. 2016, 31, 363–368. [Google Scholar] [CrossRef]
- Khan, A.P.; Rajendiran, T.M.; Ateeq, B.; Asangani, I.A.; Athanikar, J.N.; Yocum, A.K.; Mehra, R.; Siddiqui, J.; Palapattu, G.; Wei, J.T.; et al. The role of sarcosine metabolism in prostate cancer progression. Neoplasia 2013, 15, 491–501. [Google Scholar] [CrossRef]
- Uhlirova, D.; Stankova, M.; Docekalova, M.; Hosnedlova, B.; Kepinska, M.; Ruttkay-Nedecky, B.; Ruzicka, J.; Fernandez, C.; Milnerowicz, H.; Kizek, R. A Rapid Method for the Detection of Sarcosine Using SPIONs/Au/CS/SOX/NPs for Prostate Cancer Sensing. Int. J. Mol. Sci. 2018, 19, 3722. [Google Scholar] [CrossRef]
- Garcia-Uribe, A.; Erpelding, T.N.; Krumholz, A.; Ke, H.; Maslov, K.; Appleton, C.; Margenthaler, J.A.; Wang, L.V. Dual-Modality Photoacoustic and Ultrasound Imaging System for Noninvasive Sentinel Lymph Node Detection in Patients with Breast Cancer. Sci. Rep. 2015, 5, 15748. [Google Scholar] [CrossRef]
- How, J.; Lau, S.; Press, J.; Ferenczy, A.; Pelmus, M.; Stern, J.; Probst, S.; Brin, S.; Drummond, N.; Gotlieb, W. Accuracy of sentinel lymph node detection following intra-operative cervical injection for endometrial cancer: A prospective study. Gynecol. Oncol. 2012, 127, 332–337. [Google Scholar] [CrossRef]
- Winter, A.; Woenkhaus, J.; Wawroschek, F. A novel method for intraoperative sentinel lymph node detection in prostate cancer patients using superparamagnetic iron oxide nanoparticles and a handheld magnetometer: The initial clinical experience. Ann. Surg. Oncol. 2014, 21, 4390–4396. [Google Scholar] [CrossRef]
- Winter, A.; Kowald, T.; Paulo, T.S.; Goos, P.; Engels, S.; Gerullis, H.; Schiffmann, J.; Chavan, A.; Wawroschek, F. Magnetic resonance sentinel lymph node imaging and magnetometer-guided intraoperative detection in prostate cancer using superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 6689–6698. [Google Scholar] [CrossRef]
- Li, C.S.; Harisinghani, M.G.; Lin, W.C.; Braschi, M.; Hahn, P.F.; Mueller, P.R. Enhancement characteristics of ultrasmall superparamagnetic iron oxide particle within the prostate gland in patients with primary prostate cancer. J. Comput. Assist. Tomogr. 2008, 32, 523–528. [Google Scholar] [CrossRef]
- Triantafyllou, M.; Studer, U.E.; Birkhäuser, F.D.; Fleischmann, A.; Bains, L.J.; Petralia, G.; Christe, A.; Froehlich, J.M.; Thoeny, H.C. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur. J. Cancer 2013, 49, 616–624. [Google Scholar] [CrossRef]
- Li, Y.R.; Dattoli, M.J.; Barentsz, J.; Roach, M. Radiotherapy (RT) guided by ultra-small superparamagnetic iron oxide (USPIO)-contrast MRI staging for patients with advanced or recurrent prostate cancer. J. Clin. Oncol. 2020, 38, 218. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Zhu, Y.; Yao, X.; Qi, J. Efficient in vitro labeling of human prostate cancer cells with superparamagnetic iron oxide nanoparticles. Cancer Biother. Radiopharm. 2011, 26, 461–467. [Google Scholar] [CrossRef]
- Ding, C.; Wu, K.; Wang, W.; Guan, Z.; Wang, L.; Wang, X.; Wang, R.; Liu, L.; Fan, J. Synthesis of a cell penetrating peptide modified superparamagnetic iron oxide and MRI detection of bladder cancer. Oncotarget 2017, 8, 4718–4729. [Google Scholar] [CrossRef] [PubMed]
- Wadajkar, A.S.; Menon, J.U.; Tsai, Y.S.; Gore, C.; Dobin, T.; Gandee, L.; Kangasniemi, K.; Takahashi, M.; Manandhar, B.; Ahn, J.M.; et al. Prostate cancer-specific thermo-responsive polymer-coated iron oxide nanoparticles. Biomaterials 2013, 34, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Cabana, S.; Curcio, A.; Michel, A.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Mediated Photothermal Therapy in the Second Biological Window: A Comparative Study between Magnetite/Maghemite Nanospheres and Nanoflowers. Nanomaterials 2020, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Hajikarimi, Z.; Khoei, S.; Khoee, S.; Mahdavi, S.R. Evaluation of the cytotoxic effects of PLGA coated iron oxide nanoparticles as a carrier of 5-fluorouracil and mega-voltage X-ray radiation in DU145 prostate cancer cell line. IEEE Trans. Nanobiosci. 2014, 13, 403–408. [Google Scholar] [CrossRef]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.; Delphi, L.; Mansourizadeh, F.J.J.o.P.A. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNcaP human prostate cancer cell lines. J. Pharm. Anal. 2020, 11, 108–121. [Google Scholar] [CrossRef]
- Nomura, S.; Morimoto, Y.; Tsujimoto, H.; Arake, M.; Harada, M.; Saitoh, D.; Hara, I.; Ozeki, E.; Satoh, A.; Takayama, E.; et al. Highly reliable, targeted photothermal cancer therapy combined with thermal dosimetry using a near-infrared absorbent. Sci. Rep. 2020, 10, 9765. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.A.; Krauss, D.J. Adjuvant androgen deprivation therapy for prostate cancer treated with radiation therapy. Transl. Androl. Urol. 2018, 7, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; Sartor, O.; Patel, M.P.; Bahary, J.P.; Zietman, A.L.; et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N. Engl. J. Med. 2017, 376, 417–428. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Z.; He, S.; Fan, T.; Jin, Y.; Zhu, X.; Chen, C.; Zhang, Z.R.; Huang, Y. Treatment of prostate carcinoma with (galectin-3)-targeted HPMA copolymer-(G3-C12)-5-Fluorouracil conjugates. Biomaterials 2012, 33, 2260–2271. [Google Scholar] [CrossRef]
- Evans, A.J. Treatment effects in prostate cancer. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2018, 31, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, M. Chemotherapy in Prostate Cancer. Curr. Oncol. Rep. 2015, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Andriguetti, N.B.; Raymundo, S.; Antunes, M.V.; Perassolo, M.S.; Verza, S.G.; Suyenaga, E.S.; Linden, R. Pharmacogenetic and Pharmacokinetic Dose Individualization of the Taxane Chemotherapeutic Drugs Paclitaxel and Docetaxel. Curr. Med. Chem. 2017, 24, 3559–3582. [Google Scholar] [CrossRef]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine Modified Superparamagnetic Iron Oxide Nanoparticles as Multifunctional Nanocarrier for Targeted Prostate Cancer Treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Boya, V.K.N.; Kashyap, V.K.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy. Cancers 2018, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Figg, W.D.; Woo, S.; Zhu, W.; Chen, X.; Ajiboye, A.S.; Steinberg, S.M.; Price, D.K.; Wright, J.J.; Parnes, H.L.; Arlen, P.M.; et al. A phase I clinical study of high dose ketoconazole plus weekly docetaxel for metastatic castration resistant prostate cancer. J. Urol. 2010, 183, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Johnson, N.R.; Boya, V.K.N.; Chowdhury, P.; Othman, S.F.; Khalilzad-Sharghi, V.; Hafeez, B.B.; Ganju, A.; Khan, S.; Behrman, S.W.; et al. PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surf. B Biointerfaces 2016, 144, 8–20. [Google Scholar] [CrossRef]
- Matin, F.; Jeet, V.; Clements, J.A.; Yousef, G.M.; Batra, J. MicroRNA Theranostics in Prostate Cancer Precision Medicine. Clin. Chem. 2016, 62, 1318–1333. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Li, X.; Padi, S.K.; Zhang, Q.; Tang, M.S.; Guo, B. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010, 1, e105. [Google Scholar] [CrossRef]
- Bettaieb, A.; Wrzal, P.K.; Averill-Bates, D.A. Hyperthermia: Cancer treatment and beyond. In Cancer Treatment—Conventional and Innovative Approaches; Rangel, L., Ed.; InTech Open: London, UK, 2013; pp. 257–283. [Google Scholar]
- Nagaraju, G.P.; Srivani, G.; Dariya, B.; Chalikonda, G.; Farran, B.; Behera, S.K.; Alam, A.; Kamal, M.A. Nanoparticles guided drug delivery and imaging in gastric cancer. Semin. Cancer Biol. 2021, 69, 69–76. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.R.; Ring, H.L.; Etheridge, M.; Zhang, J.; Gao, Z.; Shao, Q.; Klein, N.D.; Szlag, V.M.; Chung, C.; Reineke, T.M.; et al. Predictable Heating and Positive MRI Contrast from a Mesoporous Silica-Coated Iron Oxide Nanoparticle. Mol. Pharm. 2016, 13, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Z.; Zhou, C.; Zhu, J.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef]
- Saad, F.; Hotte, S.J. Guidelines for the management of castrate-resistant prostate cancer. J. De L’association Des Urol. Du Can. 2010, 4, 380–384. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, S.; Yang, F.; Situ, J.; Lin, S.; Luo, Y. Aptamer-Conjugated Multifunctional Polymeric Nanoparticles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems for Treatment of Castration-Resistant Prostate Cancer. Biomed Res. Int. 2020, 2020, 9186583. [Google Scholar] [CrossRef]
- Ling, Y.; Wei, K.; Luo, Y.; Gao, X.; Zhong, S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 7139–7150. [Google Scholar] [CrossRef]
- Montecinos, V.P.; Morales, C.H.; Fischer, T.H.; Burns, S.; San Francisco, I.F.; Godoy, A.S.; Smith, G.J. Selective targeting of bioengineered platelets to prostate cancer vasculature: New paradigm for therapeutic modalities. J. Cell. Mol. Med. 2015, 19, 1530–1537. [Google Scholar] [CrossRef]
- Yu, M.K.; Kim, D.; Lee, I.H.; So, J.S.; Jeong, Y.Y.; Jon, S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small Weinh. Der Bergstr. Ger. 2011, 7, 2241–2249. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small Weinh. Der Bergstr. Ger. 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Teh, J.; Tripathi, M.; Reichel, D.; Sagong, B.; Montoya, R.; Zhang, Y.; Wagner, S.; Saouaf, R.; Chung, L.W.K.; Perez, J.M. Intraoperative assessment and postsurgical treatment of prostate cancer tumors using tumor-targeted nanoprobes. Nanotheranostics 2021, 5, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.R.; Hsiao, C.D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]



| Serial Number | Prostate Cancer Types | Frequency | Frequently Mutated Top 3 Genes | Age (Years) |
|---|---|---|---|---|
| 1 | Prostate Adenocarcinoma | 94.9% | TP53 (26.5%), SPOP (9.6%), FOXA1 (8.5%) | 61–65 |
| 2 | Castration-Resistant Prostate Cancer | 2.7% | TP53 (17.1%), TTN (15.7%), SPOP (15.7%) | NR |
| 3 | Prostate Neuroendocrine Carcinoma | 2.1% | TP53 (33.3%), FOXA1 (16.7%), RB1 (16.7%) | NR |
| 4 | Prostate Small Cell Carcinoma | 0.3% | RB1 (57.1%), APC (42.9%), BRCA2 (42.9%) | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayhan, M.A.; Hossen, M.S.; Niloy, M.S.; Bhuiyan, M.H.; Paul, S.; Shakil, M.S. Biopolymer and Biomaterial Conjugated Iron Oxide Nanomaterials as Prostate Cancer Theranostic Agents: A Comprehensive Review. Symmetry 2021, 13, 974. https://doi.org/10.3390/sym13060974
Rayhan MA, Hossen MS, Niloy MS, Bhuiyan MH, Paul S, Shakil MS. Biopolymer and Biomaterial Conjugated Iron Oxide Nanomaterials as Prostate Cancer Theranostic Agents: A Comprehensive Review. Symmetry. 2021; 13(6):974. https://doi.org/10.3390/sym13060974
Chicago/Turabian StyleRayhan, Md. Abu, Md. Sakib Hossen, Mahruba Sultana Niloy, Mozammel Haque Bhuiyan, Sudip Paul, and Md. Salman Shakil. 2021. "Biopolymer and Biomaterial Conjugated Iron Oxide Nanomaterials as Prostate Cancer Theranostic Agents: A Comprehensive Review" Symmetry 13, no. 6: 974. https://doi.org/10.3390/sym13060974
APA StyleRayhan, M. A., Hossen, M. S., Niloy, M. S., Bhuiyan, M. H., Paul, S., & Shakil, M. S. (2021). Biopolymer and Biomaterial Conjugated Iron Oxide Nanomaterials as Prostate Cancer Theranostic Agents: A Comprehensive Review. Symmetry, 13(6), 974. https://doi.org/10.3390/sym13060974