A Numerical Confirmation of a Fractional-Order COVID-19 Model’s Efficiency
Abstract
:1. Introduction
- Linear and nonlinear definitions: The mathematical model will be linear if the operators are linear. O.W., it is nonlinear. In different contexts, linearity and nonlinearity are defined differently, and linear models can include nonlinear expressions.
- Static and dynamic definitions: A dynamic model accounts for changes in the system’s status over time. As opposed to this, a static (or steady-state) model, which is time-invariant, estimates the system in equilibrium. Dynamic models are typically represented using differential equations or difference equations.
- Explicit and implicit: If all of the input parameters for the whole model are known, and the output parameters can be calculated using a finite number of computations, the model is said to be explicit. However, there are times when only the output parameters are known, necessitating the use of an iterative strategy such as Newton’s technique or Broyden’s method to solve the corresponding inputs.
- Discrete and continuous: In contrast to a continuous model, which depicts objects as continuous, a discrete model views objects as discrete, such as chemical model particle states or statistical model states.
2. Model Formulation
2.1. Epidemiological Mathematical Model
2.2. Coronavirus Models
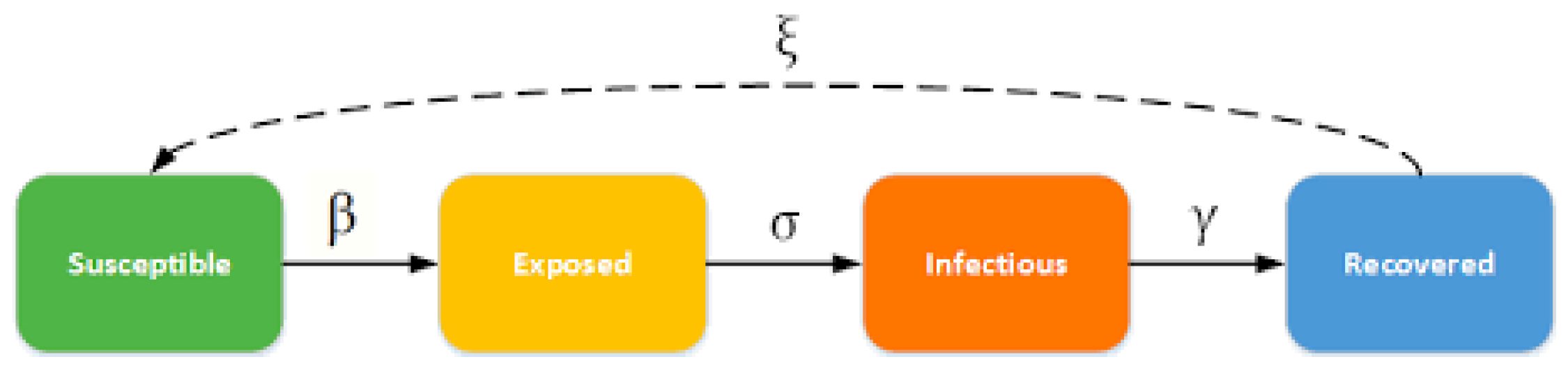
2.3. SEIR Model of COVID-19
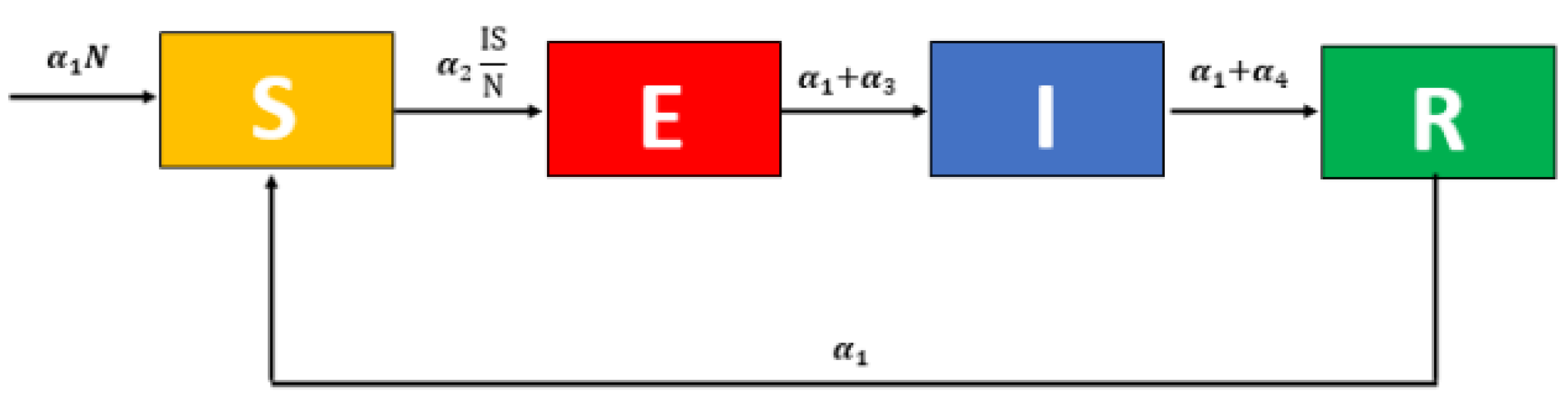
2.4. Fractional-Order COVID-19 Model
2.5. Stability Analysis
2.5.1. The Equilibrium Small Point of the Model
2.5.2. Basic Reproductive Number
3. Solving Fractional-Order COVID-19 Model Using Fractional Euler Method
3.1. Fractional Euler Method
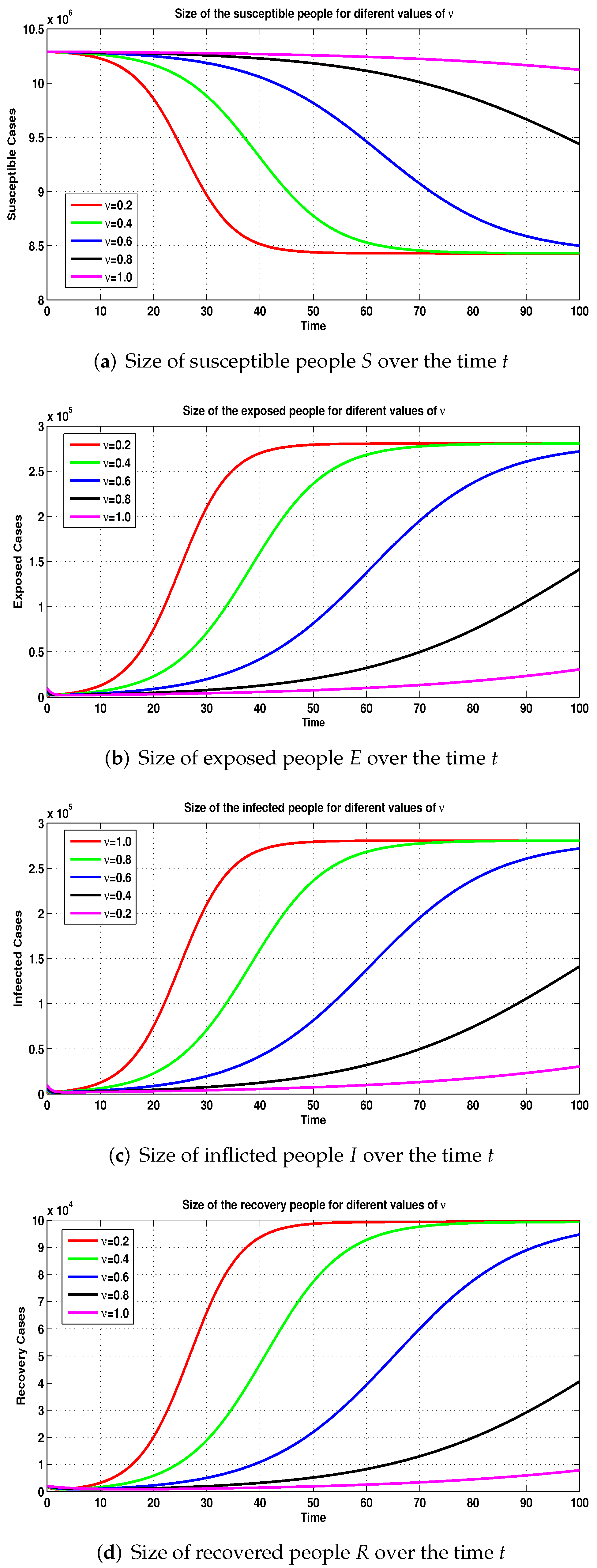
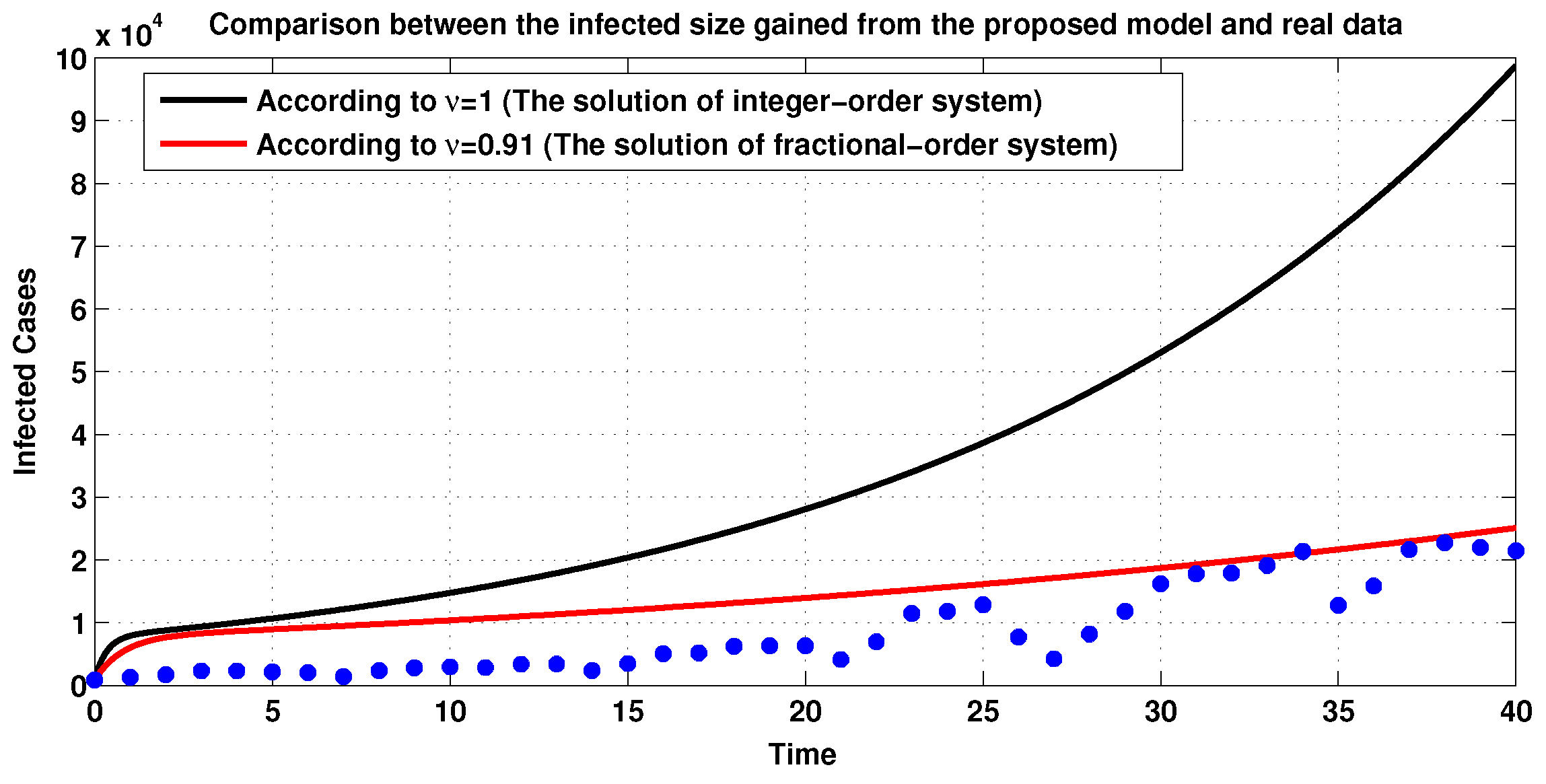
3.2. Simulations of Fractional-Order COVID-19 Model
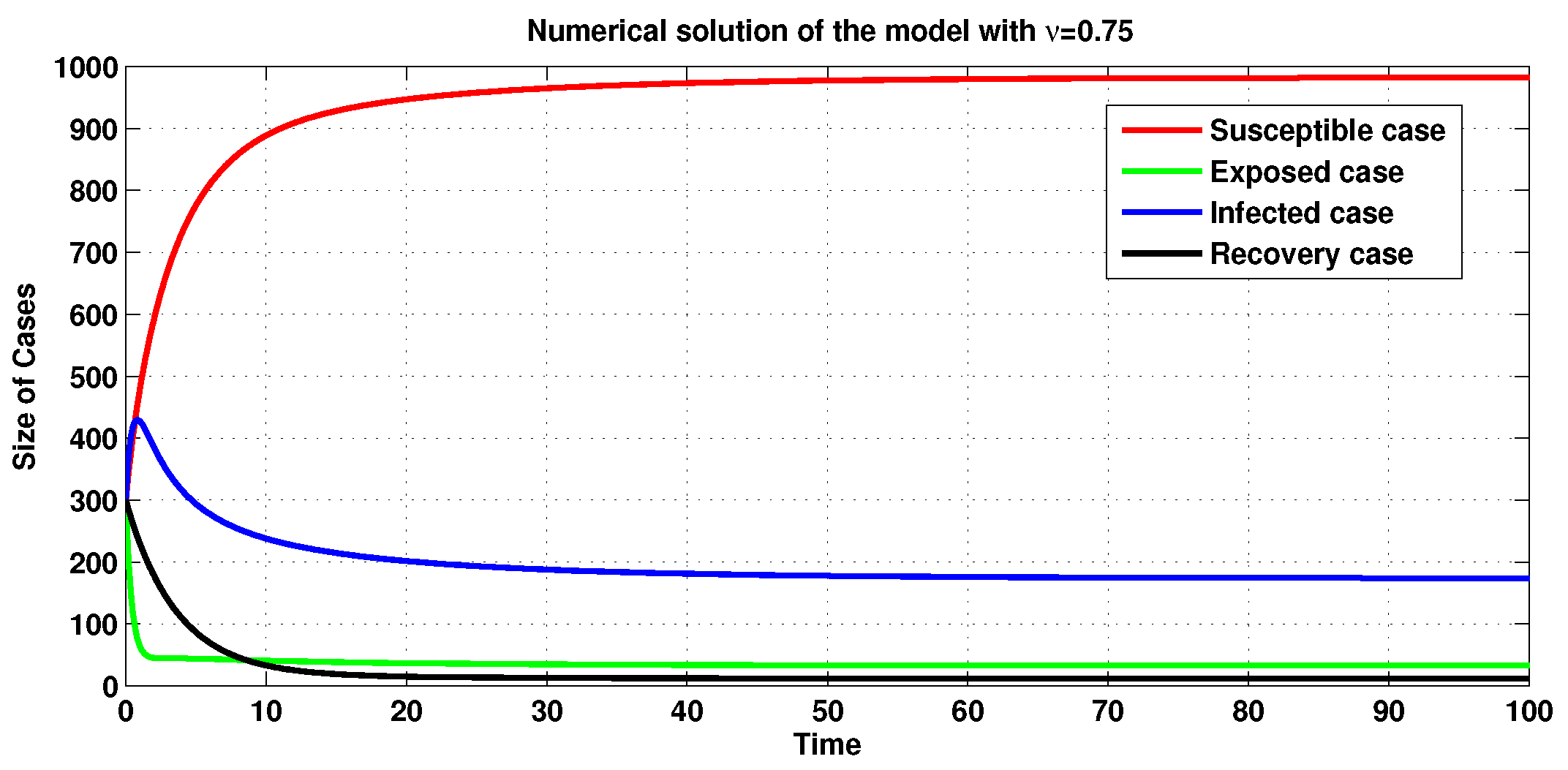
3.3. Numerical Simulations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tymoczko, D. A Geometry of Music: Harmony and Counterpoint in the Extended Common Practice; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Kornai, A. Mathematical Linguistics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mišutka, J.; Galamboš, L. System description: Egomath2 as a tool for mathematical searching on Wikipedia. org. In Proceedings of the International Conference on Intelligent Computer Mathematics, Bertinoro, Italy, 18–23 July 2011; Springer: Berlin/Heidelberg, Germany, 2011; pp. 307–309. [Google Scholar]
- Khan, T.; Ahmad, S.; Ullah, R.; Bonyah, E.; Ansari, K.J. The asymptotic analysis of novel coronavirus disease via fractional-order epidemiological model. AIP Adv. 2022, 12, 035349. [Google Scholar] [CrossRef]
- Ahmad, S.; Owyed, S.; Abdel-Aty, A.H.; Mahmoud, E.E.; Shah, K.; Alrabaiah, H. Mathematical analysis of COVID-19 via new mathematical model. Chaos Solitons Fractals 2021, 143, 110585. [Google Scholar]
- Ullah, A.; Ahmad, S.; ur Rahman, G.; Alqarni, M.M.; Mahmoud, E.E. Impact of pangolin bootleg market on the dynamics of COVID-19 model. Results Phys. 2021, 23, 103913. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rahmamn, M.u.; Ahmad, S.; Baleanu, D.; Anjam, Y.N. A new fractional infectious disease model under the non-singular Mittag–Leffler derivative. Waves Random Complex Media 2022, 1–27. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, R.; Baleanu, D. Mathematical analysis of tuberculosis control model using nonsingular kernel type Caputo derivative. Adv. Differ. Equ. 2021, 2021, 26. [Google Scholar] [CrossRef]
- Bezziou, M.; Dahmani, Z.; Jebril, I.; Kaid, M. Caputo-hadamard approach applications: Solvability for an integro-differential problem of lane and emden type. J. Math. Comput. Sci. 2021, 11, 1629–1649. [Google Scholar]
- Tanimoto, J. Sociophysics Approach to Epidemics; Springer: Singapore, 2021; Volume 23, pp. 153–169. [Google Scholar]
- Omame, A.; Abbas, M.; Onyenegecha, C.P. A fractional-order model for COVID-19 and tuberculosis co-infection using Atangana–Baleanu derivative. Chaos Solitons Fractals 2021, 153, 111486. [Google Scholar] [CrossRef]
- Kermack, W.O.; Mckendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. London. Ser. A Contain. Pap. Math. Phys. Character 1927, 115, 700–721. [Google Scholar]
- Wirkus, S.A.; Swift, R.J. A Course in Ordinary Differential Equations; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Cobb, N.L.; Sathe, N.A.; Duan, K.I.; Seitz, K.P.; Thau, M.R.; Sung, C.C.; Morrell, E.D.; Mikacenic, C.; Kim, H.N.; Liles, W.C.; et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann. Am. Thorac. Soc. 2021, 18, 632–640. [Google Scholar] [CrossRef]
- Eppes, H. A Seir Mathematical Model of SAR-CoV-2. Ph.D. Thesis, Elizabeth City State University, Elizabeth City, NC, USA, 2021. [Google Scholar]
- Wald, E.R.; Schmit, K.M.; Gusland, D.Y. A pediatric infectious disease perspective on COVID-19. Clin. Infect. Dis. 2021, 72, 1660–1666. [Google Scholar] [CrossRef]
- Singh, P.S.K.; Arora, U. Stability of seir model of infectious diseases with human immunity. Glob. J. Pure Appl. Math. 2017, 13, 1811–1819. [Google Scholar]
- Al-Raeei, M. The forecasting of covid-19 with mortality using sird epidemic model for the United States, Russia, China, and the Syrian Arab republic. AIP Adv. 2020, 10, 065325. [Google Scholar] [CrossRef]
- Pengpeng, S.; Shengi, C.; Peihua, F. Seir transmission dynamics model of 2019 nCoV coronavirus with considering the weak infectious ability and changes in latency duration. MedRxiv 2020. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A. On the definition and the computation of the basic reproduction ratio r 0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [Green Version]
- Van den Driessche, P. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Van den Driessche, P. Reproduction numbers of infectious disease models. Infect. Dis. Model. 2017, 2, 288–303. [Google Scholar] [CrossRef]
- Odibat, Z.M.; Shawagfeh, N.T. Generalized taylor’s formula. Appl. Math. Comput. 2007, 186, 286–293. [Google Scholar] [CrossRef]
- Dahmani, Z.; Anber, A.; Jebril, I. Solving Conformable Evolution Equations by an Extended Numerical Method. Jordan J. Math. Stat. 2022, 15, 363–380. [Google Scholar]
- Debbouche, N.; Ouannas, A.; Batiha, I.M.; Grassi, G. Chaotic dynamics in a novel COVID-19 pandemic model described by commensurate and incommensurate fractional-order derivatives. Nonlinear Dyn. 2021, 109, 33–45. [Google Scholar] [CrossRef]
- Albadarneh, R.B.; Batiha, I.M.; Ouannas, A.; Momani, S. Modeling COVID-19 pandemic outbreak using fractional-order systems. Int. J. Math. Comput. Sci. 2021, 16, 1405–1421. [Google Scholar]
- Batiha, I.M.; Momani, S.; Ouannas, A.; Momani, Z.; Hadid, S.B. Fractional-order COVID-19 pandemic outbreak: Modeling and stability analysis. Int. J. Biomath. 2022, 15, 2150090. [Google Scholar] [CrossRef]
- Djenina, N.; Ouannas, A.; Batiha, I.M.; Grassi, G.; Oussaeif, T.E.; Momani, S. A Novel Fractional-Order Discrete SIR Model for Predicting COVID-19 Behavior. Mathematics 2022, 10, 2224. [Google Scholar] [CrossRef]
- Batiha, I.M.; Al-Nana, A.A.; Albadarneh, R.B.; Ouannas, A.; Al-Khasawneh, A.; Momani, S. Fractional-order coronavirus models with vaccination strategies impacted on Saudi Arabia’s infections. AIMS Math. 2022, 7, 12842–12858. [Google Scholar] [CrossRef]
- Batiha, I.M.; Alshorm, S.; Ouannas, A.; Momani, S.; Ababneh, O.Y.; Albdareen, M. Modified Three-Point Fractional Formulas with Richardson Extrapolation. Mathematics 2022, 10, 3489. [Google Scholar] [CrossRef]

| Class | Initial Value |
|---|---|
| S (0) | 10,287,128 |
| E (0) | 10,000 |
| I (0) | 872 |
| R (0) | 2000 |
| Parameter | Value |
|---|---|
| 0.15 | |
| 0.23 | |
| 0.85 | |
| 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batiha, I.M.; Obeidat, A.; Alshorm, S.; Alotaibi, A.; Alsubaie, H.; Momani, S.; Albdareen, M.; Zouidi, F.; Eldin, S.M.; Jahanshahi, H. A Numerical Confirmation of a Fractional-Order COVID-19 Model’s Efficiency. Symmetry 2022, 14, 2583. https://doi.org/10.3390/sym14122583
Batiha IM, Obeidat A, Alshorm S, Alotaibi A, Alsubaie H, Momani S, Albdareen M, Zouidi F, Eldin SM, Jahanshahi H. A Numerical Confirmation of a Fractional-Order COVID-19 Model’s Efficiency. Symmetry. 2022; 14(12):2583. https://doi.org/10.3390/sym14122583
Chicago/Turabian StyleBatiha, Iqbal M., Ahmad Obeidat, Shameseddin Alshorm, Ahmed Alotaibi, Hajid Alsubaie, Shaher Momani, Meaad Albdareen, Ferjeni Zouidi, Sayed M. Eldin, and Hadi Jahanshahi. 2022. "A Numerical Confirmation of a Fractional-Order COVID-19 Model’s Efficiency" Symmetry 14, no. 12: 2583. https://doi.org/10.3390/sym14122583





