Design Control of Copper-Doped Titania–Zirconia Catalysts for Methanol Decomposition and Total Oxidation of Ethyl Acetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Characterization
2.3. Catalytic Tests
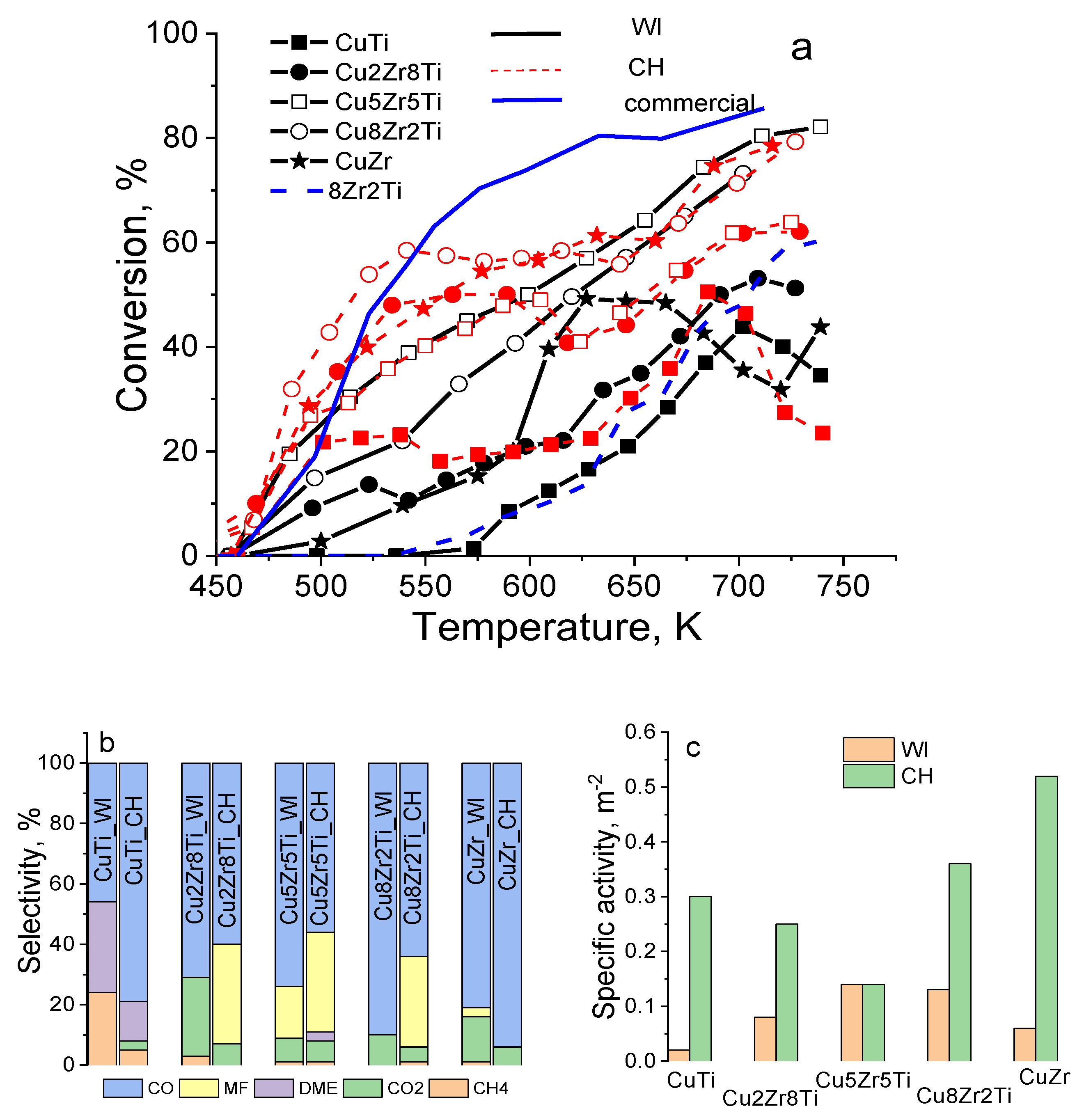
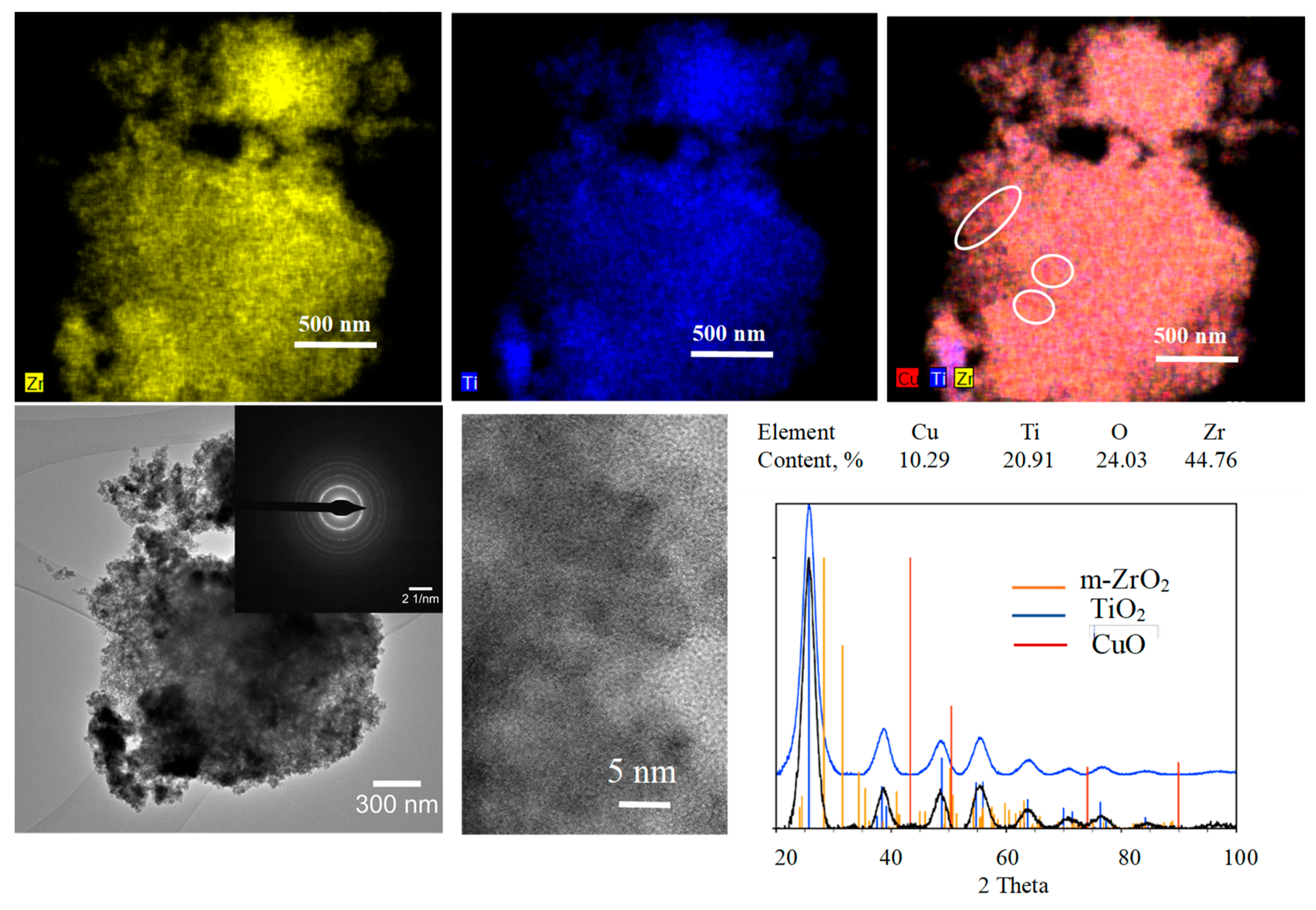
3. Results and Discussion
3.1. Copper-Zirconia Composites
3.2. Copper-Titania Composites
3.3. Copper–Zirconia–Titania Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 12 December 2015).
- Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 17 May 2021).
- Rana, J.; Sahoo, S.T.; Dawv, D. Homogeneous first-row transition metal catalyst for sustainable hydrogen production and organic transformation from methanol, formic acid, and bio-alcohols. Tetrahedron 2021, 99, 132473. [Google Scholar] [CrossRef]
- Li, H.; Ma, C.; Zou, X.; Li, A.; Huang, Z.; Zhu, L. On-board methanol catalytic reforming for hydrogen Production—A review. Int. J. Hydrogen Energy 2021, 46, 22303–22327. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Enhanced production of hydrogen from methanol using spark discharge generated in a small portable reactor. Energy Rep. 2022, 8, 183–191. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Xu, C.; Jin, F.; Ye, F.; Li, X. Distinct facets to enhance the process of hydrogen production via methanol steam reforming—A review. Energy Storage Sav. 2022, 1, 53–69. [Google Scholar] [CrossRef]
- Hakandai, C.; Pramono, H.S.; Aziz, M. Conversion of municipal solid waste to hydrogen and its storage to methanol. Sustain. Energy Technol. Assess. 2022, 51, 101968. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation. Appl. Catal. A Gen. 2018, 554, 44–53. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kwon, E.E.; Kim, K.H. Is mass-scale electrocatalysis of aqueous methanol an energetically and economically viable option for hydrogen production? J. Ind. Eng. Chem. 2022, 105, 58–62. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, P.; Dhar, A. Performance enhancement of methanol reforming reactor through finned surfaces and diffused entry for on-board hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 7478–7490. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, D.; Chen, Q.; Tang, Z. Techno-economic analysis of green methanol plant with optimal design of renewable hydrogen production: A case study in China. Int. J. Hydrogen Energy 2022, 47, 5085–5100. [Google Scholar] [CrossRef]
- McKinlay, C.J.; Turnock, S.R.; Hudson, D.A. Route to zero emission shipping: Hydrogen, ammonia or methanol? Int. J. Hydrogen Energy 2021, 46, 28282–28297. [Google Scholar] [CrossRef]
- Available online: https://www.state.gov/key-topics-office-of-environmental-quality-and-transboundary-issues/convention-on-long-range-transboundary-air-pollution/ (accessed on 8 June 2021).
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs): A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Manzoli, M.; Chiorino, A.; Boccuzzi, F. Decomposition and combined reforming of methanol to hydrogen: A FTIR and QMS study on Cu and Au catalysts supported on ZnO and TiO2. Appl. Catal. B Environ. 2004, 57, 201–209. [Google Scholar] [CrossRef]
- Aguila, G.; Gracia, F.; Cortes, J.; Araya, P. Effect of copper species and the presence of reaction products on the activity of methane oxidation on supported CuO catalysts. Appl. Catal. B Environ. 2008, 77, 325–338. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, J.; Lv, Y.; Qi, L.; Liu, B.; Gao, F.; Sun, K.; Dong, L.; Chen, Y. Dispersion, reduction and catalytic performance of CuO supported on ZrO2-doped TiO2 for NO removal by CO. Appl. Catal. B Environ. 2011, 103, 206–220. [Google Scholar] [CrossRef]
- Tian, J.; Ke, Y.; Kong, G.; Tan, M.; Wang, Y.; Lin, J.; Zhou, W.; Wan, S. A novel structured PdZnAl/Cu fiber catalyst for methanol steam reforming in micro reactor. Renew. Energy 2017, 113, 30–42. [Google Scholar] [CrossRef]
- Ahmadi, F.; Haghighi, M.; Ajamein, H. Sonochemically coprecipitation synthesis of CuO/ZnO/ZrO2/Al2O3 nanocatalyst for fuel cell grade hydrogen production via steam methanol reforming. J. Mol. Catal. A Chem. 2016, 421, 196–208. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on copper and palladium-based catalysts for methanol steam reforming to produce hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Lei, Y.; Luo, Y.; Li, X.; Lu, J.; Mei, Z.; Peng, W.; Chen, R.; Chen, K.; Chen, D.; He, D. The role of samarium on Cu/Al2O3 catalyst in the methanol steam reforming for hydrogen production. Catal. Today 2018, 307, 162–168. [Google Scholar] [CrossRef]
- Tong, W.; Cheung, K.; West, A.; Yu, K.M.; Tsang, S.C. Direct methanol steam reforming to hydrogen over CuZnGaOx catalysts without CO post-treatment: Mechanistic considerations. Phys. Chem. Chem. Phys. 2013, 15, 7240–7248. [Google Scholar] [CrossRef] [PubMed]
- Mosińska, M.; Stepińska, N.; Maniukiewicz, W.; Rogowski, J.; Mierczynska-Vasilev, A.; Vasilev, K.; Szynkowska, M.I.; Mierczyński, P. Hydrogen production on Cu-Ni catalysts via the oxy-steam reforming of methanol. Catalysts 2020, 10, 273. [Google Scholar] [CrossRef] [Green Version]
- Cordi, E.M.; O’Neill, P.J.; Falconer, J.L. Transient oxidation of volatile organic compounds on a CuO/Al2O3 catalyst. Appl. Catal. B Environ. 1997, 14, 23–36. [Google Scholar] [CrossRef]
- Deze, E.G.; Papavasiliou, A.; Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Romanos, G.E.; Boukos, N.; Xin, Q.; Nyalosaso, J.L.; Cool, P. Metal loaded nanoporous silicas with tailor-made properties through hyper branched polymer assisted templating approaches. Microporous Mesoporous Mater. 2016, 235, 107–119. [Google Scholar] [CrossRef]
- Yu, X.F.; Wu, N.Z.; Xie, Y.C.; Tang, Y.Q. A monolayer dispersion study of titania supported copper oxide. J. Mater. Chem. 2000, 10, 1629–1634. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Klissurski, D.G. Surface chemistry of titania (anatase) and titania supported catalysts. Chem. Soc. Rev. 1996, 25, 61–69. [Google Scholar] [CrossRef]
- Bagherzadeh, S.B.; Haghighi, M. Plasma-enhanced comparative hydrothermal and co-precipitation preparation of CuO/ZnO/Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming. Energy Convers. Manag. 2017, 142, 452–465. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, T.; Shen, M.; Zhu, H.; Wei, S.; Hong, X.; Ding, W.; Dong, L.; Chen, Y. Influence of titanium oxide on the surface interactions of MO (M = Cu and Ni)/γ-Al2O3 catalysts. J. Solid State Chem. 2003, 170, 58–67. [Google Scholar] [CrossRef]
- Larsson, P.O.; Andersson, A.; Wallenberg, L.R.; Svensson, B. Combustion of CO and toluene: Characterization of copper oxide supported on titania and activity comparisons with supported cobalt, iron and manganese oxides. J. Catal. 1996, 163, 279–293. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Lopez, T.; Moreno, A.; Cadús, L.E. Evaluation and characterization of Mn–Cu mixed oxide catalysts supported on TiO2 and ZrO2 for ethanol total oxidation. Fuel 2009, 88, 2122–2129. [Google Scholar] [CrossRef]
- Fang, F.; Liu, Y.; Sun, X.; Fu, C.; Bhoi, Y.P.; Xiong, W.; Huang, W. TiO2 Facet-dependent reconstruction and photocatalysis of CuOx/TiO2 photocatalysts in CO2 photo reduction. Appl. Surf. Sci. 2021, 564, 150407. [Google Scholar] [CrossRef]
- Okamoto, Y.; Gotoh, H.; Hishida, K.; Aritani, H.; Tanaka, T.; Yoshida, S. Surface copper-TiO2 interaction species for NO-CO reactions. Appl. Surf. Sci. 1997, 121–122, 509–512. [Google Scholar] [CrossRef]
- Araque, D.G.; Pena, P.A.; Ortega, D.R.; Calderon, H.A.; Gomez, R. Charge transfer processes involved in photocatalytic hydrogen production over CuO/ZrO2-TiO2 materials. Int. J. Hydrogen Energy 2017, 42, 9744–9753. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, D.; Wang, G.; Guo, X.; Yu, J. CO2 hydrogenation to methanol over CuO-ZnO-TiO2-ZrO2 catalyst prepared by a facile solid-state route: The significant influence of assistant complexing agents. Int. J. Hydrogen Energy 2019, 44, 14831–14841. [Google Scholar] [CrossRef]
- Jiang, X.; Ding, G.; Lou, L.; Chen, Y.; Zheng, X. Effect of ZrO2 addition on CuO/TiO2 activity in the NO + CO reaction. Catal. Today 2004, 93–95, 811–818. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Shen, F.; Cheng, H.; Dai, J.; Long, Y. Theoretical study of stability and reaction mechanism of CuO supported on ZrO2 during chemical looping combustion. Appl. Surf. Sci. 2016, 367, 485–492. [Google Scholar] [CrossRef]
- Guo, X.; Mao, D.; Lu, G.; Wang, S.; Wu, G. Glycine–nitrate combustion synthesis of CuO–ZnO–ZrO2 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2010, 271, 178–185. [Google Scholar] [CrossRef]
- Hertl, W. Surface chemistry of zirconia polymorphs. Langmuir 1989, 5, 96–100. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Gazque, J.L. Oxygen vacancy model in strong metal-support interaction. J. Catal. 1987, 104, 120–135. [Google Scholar] [CrossRef]
- Bianchi, D.; Chafik, T.; Khalfallah, M.; Teichner, S.J. Intermediate species on zirconia supported methanol aerogel catalysts V. Adsorption of methanol. Appl. Catal. A Gen. 1995, 123, 89–110. [Google Scholar] [CrossRef]
- Kundakovic, L.; Flytzani-Stephanopoulos, M. Reduction characteristics of copper oxide in cerium and zirconium oxide systems. Appl. Catal. A Gen. 1998, 171, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Xie, J.; Fang, D.; Liu, X.; He, F.; Li, F. Novel heterogeneous denitrification catalyst over a wide temperature range: Synergy between CeO2, ZrO2 and TiO2. Chem. Eng. J. 2019, 356, 598–608. [Google Scholar] [CrossRef]
- Chen, H.R.; Shi, J.L.; Yu, J.; Wang, L.Z.; Yan, D.S. Synthesis of titanium-doped ordered porous zirconium oxide with high-surface-area. Microporous Mesoporous Mater. 2000, 39, 171–176. [Google Scholar] [CrossRef]
- Sekuli, J.; Magraso, A.; Elshof, J.E.; Blank, D.H.A. Influence of ZrO2 addition on microstructure and liquid permeability of mesoporous TiO2 membranes. Microporous Mesoporous Mater. 2004, 72, 49–57. [Google Scholar] [CrossRef]
- Anzures, F.M.; Rivas, F.C.; Ventura, J.H.; Hernández, P.S.; Berlier, G.; Tlacuatl, G.Z. Spectroscopic characterization of CuOx/TiO2–ZrO2 catalysts prepared by a-step sol–gel method. Appl. Catal. A Gen. 2015, 489, 218–225. [Google Scholar] [CrossRef]
- Kikugawa, M.; Yamazaki, K.; Shinjoh, H. Characterization and catalytic activity of CuO/TiO2-ZrO2 for low temperature CO oxidation. Appl. Catal. A Gen. 2017, 547, 199–204. [Google Scholar] [CrossRef]
- Wang, S.; Mao, D.; Guo, X.; Wu, G.; Lu, G. Dimethyl ether synthesis via CO2 hydrogenation over CuO–TiO2–ZrO2/HZSM-5 bifunctional catalysts. Catal. Commun. 2009, 10, 1367–1370. [Google Scholar] [CrossRef]
- Nomura, N.; Tagawa, T.; Goto, S. In situ FTIR study on hydrogenation of carbon dioxide over titania-supported copper catalysts. Appl. Catal. A Gen. 1998, 166, 321–326. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Mileva, A.; Issa, G.; Dimitrov, M.; Kovacheva, D.; Henych, J.; Kormunda, M.; Scotti, N.; Slušná, M.; Tolasz, J.; et al. Titania and zirconia binary oxides as catalysts for total oxidation of ethyl acetate and methanol decomposition. J. Environ. Chem. Eng. 2018, 6, 2540–2550. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Mileva, A.; Issa, G.; Henych, J.; Tolasz, J.; Dimitrov, M.; Kovacheva, D.; Atanasova, G.; Štengl, V. Mesoporous copper-ceria-titania ternary oxides as catalysts for environmental protection: Impact of Ce/Ti ratio and preparation procedure. Appl. Catal. A Gen. 2020, 595, 117487. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, W.; Dong, L. Electron Spin Resonance Studies of CuO Supported on Tetragonal ZrO2. J. Catal. 1997, 172, 243–246. [Google Scholar] [CrossRef]
- Kurpaska, L.; Jasinski, J.; Wyszkowska, E.; Nowakowska-Langier, K.; Sitarz, M. Influence of Ar-ion implantation on the structural and mechanical properties of zirconia as studied by Raman spectroscopy and nano indentation techniques, Spectrochim. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.; Zhang, M.; Tao, K. A comparison of surface acidic features between tetragonal and monoclinic nanostructured zirconia. Catal. Commun. 2002, 3, 239–245. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Rokicińska, A.; Natkański, P.; Michalik, M.; Kuśtrowski, P. Novel CuO-containing catalysts based on ZrO2 hollow spheres for total oxidation of toluene. Microporous Mesoporous Mater. 2019, 279, 446–455. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Zhao, L.; Zhang, J.; Li, S.; Zeng, G. The catalytic performance and characterization of ZrO2 support modification on CuO-CeO2/TiO2 catalyst for the simultaneous removal of Hg0 and NO. Appl. Surf. Sci. 2017, 400, 227–237. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Li, D.; Wang, M.; Zhang, Y.; Huang, W. CuOx/TiO2 photocatalysts via TiO2 morphology engineering. Appl. Surf. Sci. 2019, 473, 500–510. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, H.; Chen, Y.; Cui, X.; Zhu, Y.; Zhou, X.; Shi, J. A Cu/Mn co-loaded mesoporous ZrO2–TiO2 composite and its CO catalytic oxidation property. Microporous Mesoporous Mater. 2013, 173, 112–120. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef] [Green Version]
- Stefani, G.; Musi, S.; Ivanda, M. Effect of Cu2+ ion incorporation on the phase development of ZrO2-type solid solutions during the thermal treatments. J. Alloys Comp. 2010, 491, 536–544. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface Oxidation and Reduction of CuO and Cu2O Studied Using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Rui, Z.; Huang, Y.; Zheng, Y.; Ji, H.; Yu, X. Effect of titania polymorph on the properties of CuO/TiO2 catalysts for trace methane combustion. J. Mol. Catal. A Chem. 2013, 372, 128–136. [Google Scholar] [CrossRef]
- Shimokawabe, M.; Asakawa, H.; Takezawa, N. Characterization of copper/zirconia catalysts prepared by an impregnation method. Appl. Catal. 1990, 59, 45–58. [Google Scholar] [CrossRef]
- Anzures, F.M.; Hernández, P.S.; Gutiérrezc, C.O.; Moralesd, F.J.T.; Hernández, R.P. Synthesis by the sol-gel method and characterization of Pt-promoted CuO/TiO2-ZrO2 catalysts for decomposition of 2-propanol. Catal. Today 2020, 349, 228–234. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Genova, I.; Dimitrov, M.; Sarcadi-Priboczki, E.; Venezia, A.M.; Kovacheva, D.; Scotti, N.; Dal Santo, V. Nanostructured copper-zirconia composites as catalysts for methanol decomposition. Appl. Catal. B Environ. 2015, 165, 599–610. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Rosmini, C.; Mihaylov, M.; Henych, J.; Chakarova, K.; Velinov, N.; Kovacheva, D.; Nemecková, Z.; Kormunda, M.; Ivanova, R.; et al. Nickel-Decorated Mesoporous Iron−Cerium Mixed Oxides: Microstructure and Catalytic Activity in Methanol Decomposition. ACS Appl. Mater. Interfaces 2022, 14, 873–890. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Everbroeck, T.V.; Blonda, C.; Oliani, B.; Sakellis, E.; Cool, P.; Canu, P.; Katsaros, F.K. Mesoporous CuO/TiO2 catalysts prepared by the ammonia driven deposition precipitation method for CO preferential oxidation: Effect of metal loading. Fuel 2022, 311, 122491. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mayer, J.; Garfunkel, E.; Madey, T.E.; Diebold, U. Titanium and reduced titania over layers on titanium dioxide (110). J. Electron Spectrosc. Relat. Phenom. 1995, 73, 1–11. [Google Scholar] [CrossRef]
- Souto, R.M.; Mareci, D. Effect of acidic fluoride solution on the corrosion resistance of ZrTi alloys for dental implant application. Corros. Sci. 2014, 87, 334–343. [Google Scholar]
- Deshmukh, S.B.; Bari, R.H. Synthesis and characterization of CuO doped ZrO2 hollow sphere for gas sensing application. Mater. Today Proc. 2016, 3, 216–223. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Liu, D.; Zhang, J.; Hao, Y.; Zhang, W. Multi-layer and open three-dimensionally ordered macroporous TiO2–ZrO2 composite: Diversified design and the comparison of multiple mode photocatalytic performance. Mater. Des. 2015, 86, 818–828. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Sanjines, R.; Nadtochenko, V.; Lavanchy, G.C.; Kiwi, J. Preparation and mechanism of Cu-decorated TiO2–ZrO2 films showing accelerated bacterial inactivation. ACS Appl. Mater. Interfaces 2015, 7, 12832–12839. [Google Scholar] [CrossRef] [PubMed]
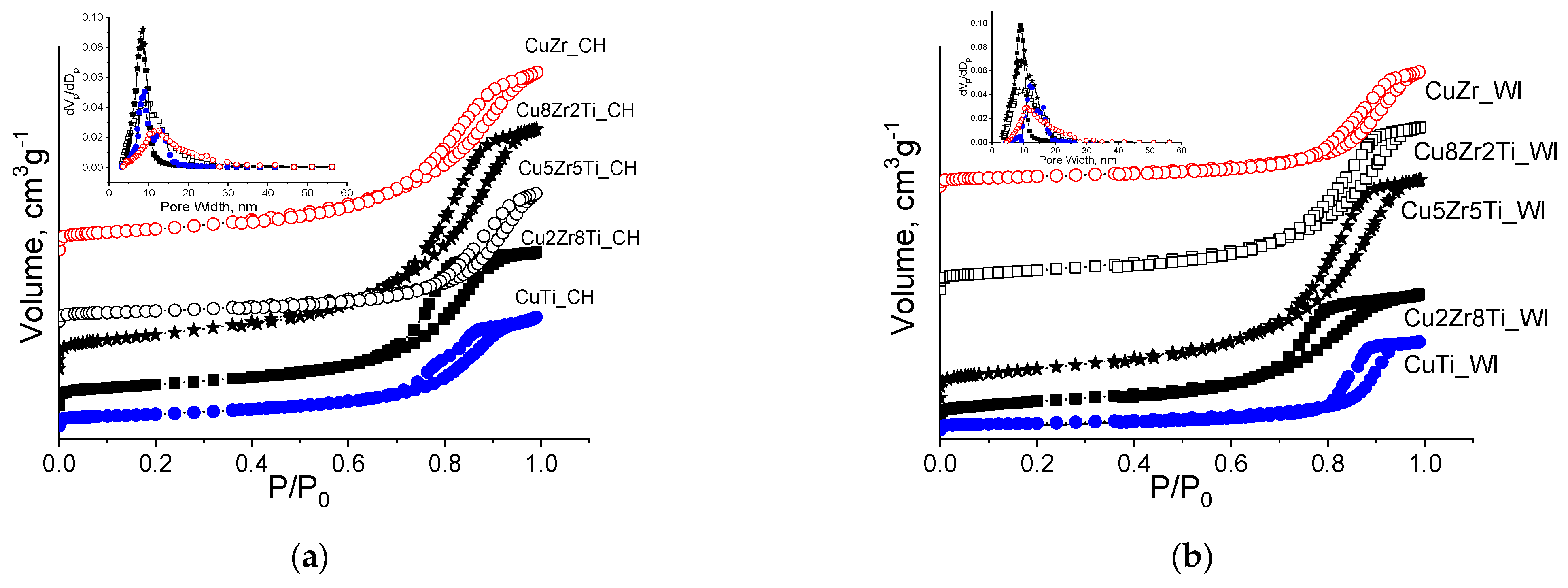


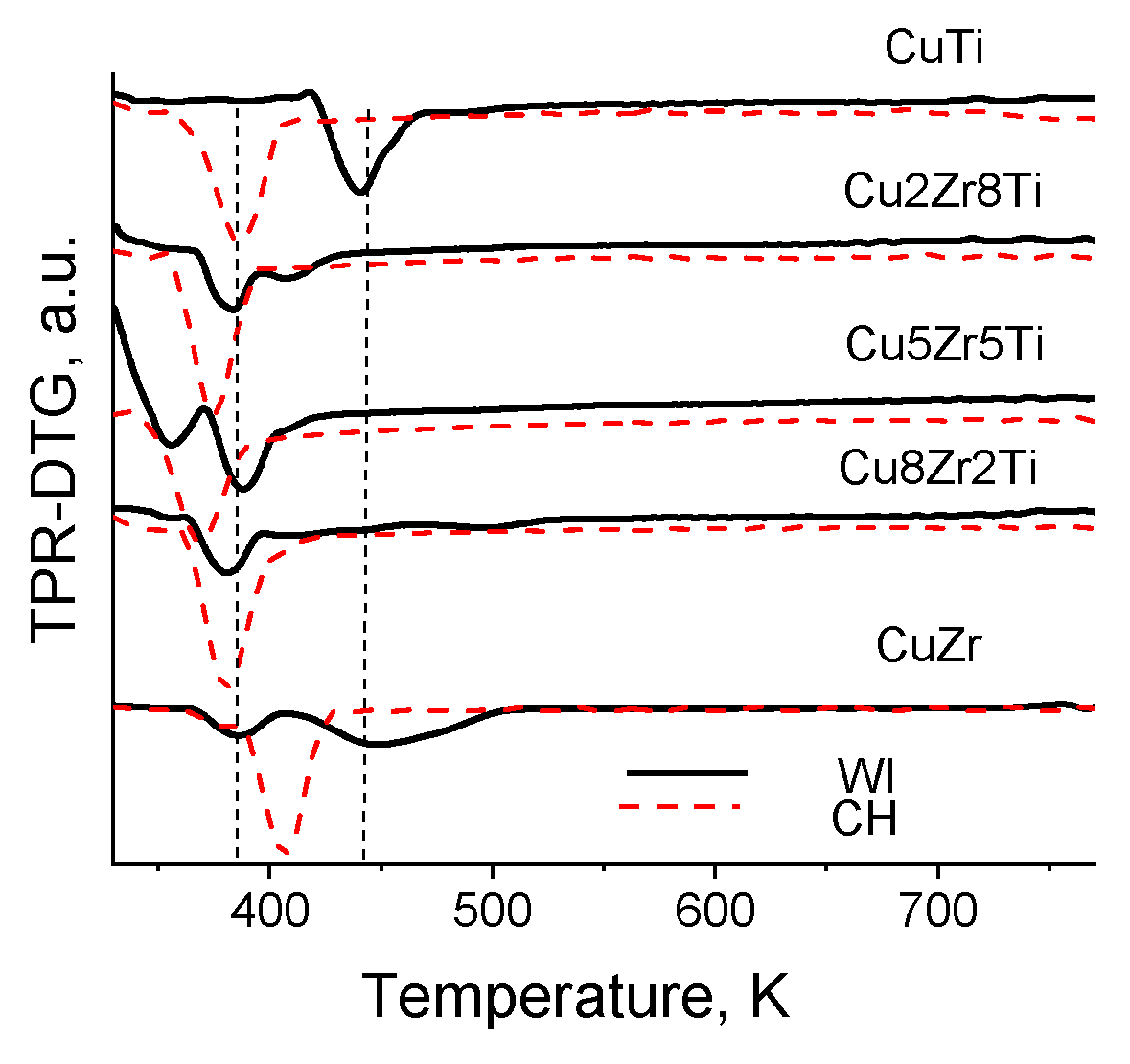
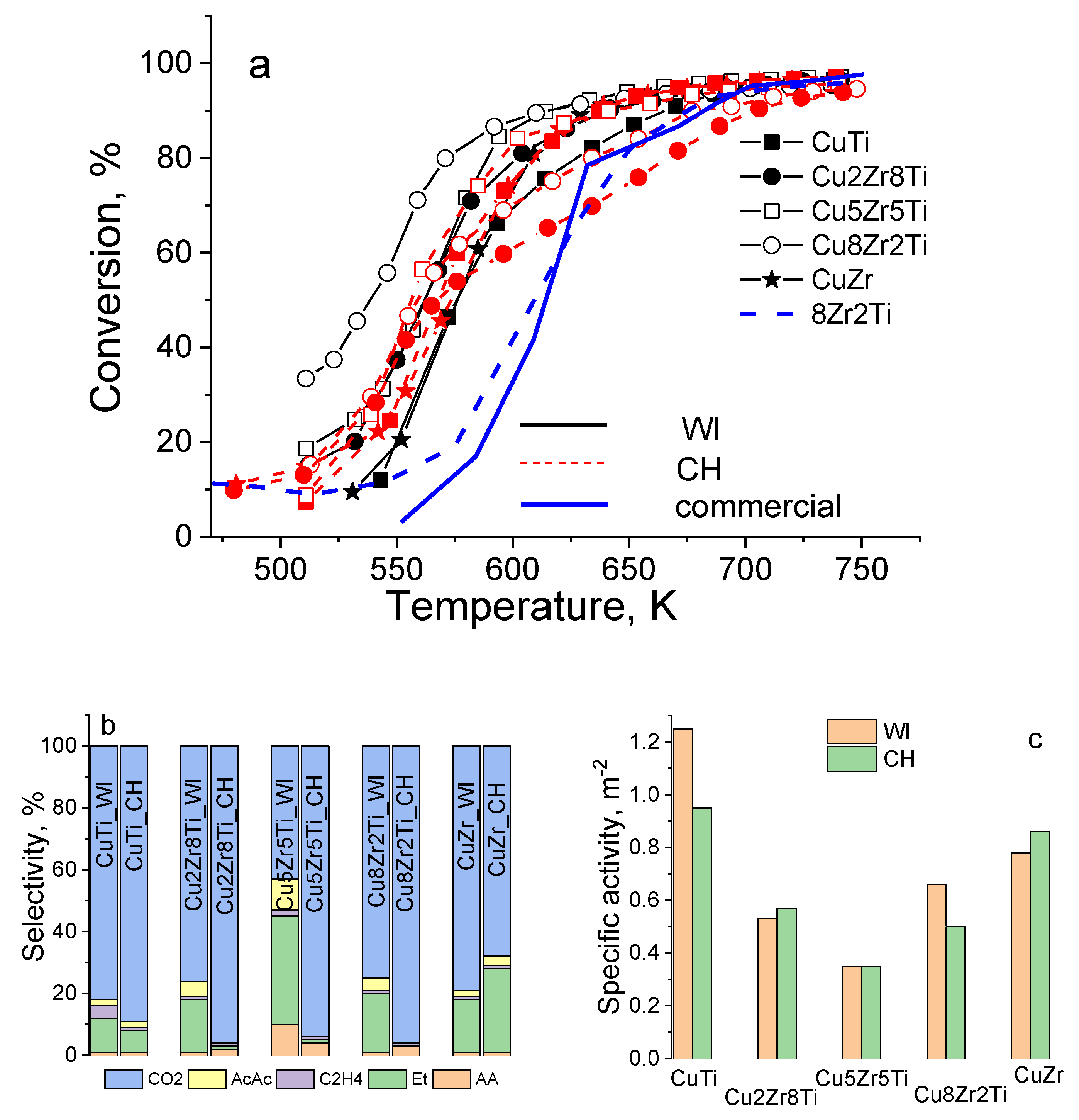

| Sample | ZrTi Support | CuZrTi_WI | CuZrTi_CH | |||
|---|---|---|---|---|---|---|
| SBET m2.g−1 | Vp mL.g−1 | SBET m2.g−1 | Vp mL.g−1 | SBET m2.g−1 | Vp mL.g−1 | |
| TiO2 | 85 | 0.29 | 40 | 0.24 | 66 | 0.26 |
| 2Zr8Ti | 157 | 0.44 | 119 | 0.37 | 117 | 0.35 |
| 5Zr5Ti | 248 | 0.69 | 187 | 0.60 | 193 | 0.59 |
| 8Zr2Ti | 157 | 0.48 | 122 | 0.44 | 116 | 0.43 |
| ZrO2 | 67 | 0.32 | 61 | 0.31 | 58 | 0.31 |
| Sample | Incipient Wetness Impregnation (WI) | Chemisorption–Hydrolysis (CH) | ||||
|---|---|---|---|---|---|---|
| Preparation Technique | Phase Composition | Unit Cell Parameters, Å | Crystallite Size, nm | Phase Composition | Unit Cell Parameters, Å | Crystallite Size, nm |
| CuTi | Anatase Tenorite | 3.7861(2) 9.4881(9) 4.691(3) 3.419(1) 5.138(4) 99.59(4) | 20.8(1) 36(4) | Anatase Tenorite | 3.7871(2) 9.487(1) 4.683(7) 3.417(4) 5.145(9) 99.67(9) | 17.5(1) 14(1) |
| Cu2Zr8Ti | Anatase | 3.7979(9) 9.540(2) | 18 | Anatase | 3.7958(9) 9.535(2) | 18 |
| Cu5Zr5Ti | amorphous | amorphous | ||||
| Cu8Zr2Ti | ZrO2-tetragonal | 3.558(2) 5.243(4) | 20 fix | ZrO2-tetragonal | 3.579(2) 5.243(4) | 20 fix |
| CuZr | ZrO2-tetragonal ZrO2-monoclinic Tenorite | 3.600(1) 5.203(7) 5.327(5) 5.150(4) 5.230(5) Beta-99.21(2) 4.703(4) 3.443(3) 5.120(4) 99.49(7) | 13 14 57(25) | ZrO2-tetragonal ZrO2-monoclinic Tenorite | 3.598(2) 5.19(1) 5.321(6) 5.151(5) 5.219(6) Beta-99.17(3) 4.691(4) 3.433(6) 5.122(6) 99.56(7) | 13 14 32(7) |
| Sample | Zr4+ (m–ZrO2) at% | Zr4+ (t–ZrO2) at% | Zr4+ (TiOZr) at% | Cu1+ at% | Cu2+ at% | Ti4+ (TiO2) at% | Ti4+ (TiOZr) at% | Ti3+ at% | O, at% |
|---|---|---|---|---|---|---|---|---|---|
| ZrO2 | 12.8 | 19.1 | 0 | 68.1 | |||||
| CuZr_WI | 20.9 | 8.3 | 0 | 3.4 | 4.0 | 63.4 | |||
| CuZr_CH | 20.1 | 1.9 | 0 | 1.7 | 14.2 | 62.1 | |||
| 5Zr5Ti | 10.4 | 5.3 | 1.1 | 9.9 | 1.9 | 0.6 | 70.7 | ||
| Cu5Zr5Ti_WI | 6.6 | 3.4 | 4.4 | 3.1 | 2.4 | 7.4 | 3.0 | 2.6 | 67.1 |
| Cu5Zr5Ti_CH | 5.5 | 7.0 | 2.5 | 3.9 | 3.1 | 9.3 | 2.4 | 1.1 | 65.2 |
| TiO2 | 32.2 | 0 | 1.4 | 66.4 | |||||
| CuTi_WI | 3.0 | 7.9 | 20.7 | 0 | 2.3 | 66.1 | |||
| CuTi_CH | 2.0 | 7.7 | 22.4 | 0 | 1.3 | 66.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoncheva, T.; Issa, G.; Ivanova, R.; Dimitrov, M.; Kovacheva, D.; Atanasova, G.; Henych, J. Design Control of Copper-Doped Titania–Zirconia Catalysts for Methanol Decomposition and Total Oxidation of Ethyl Acetate. Symmetry 2022, 14, 751. https://doi.org/10.3390/sym14040751
Tsoncheva T, Issa G, Ivanova R, Dimitrov M, Kovacheva D, Atanasova G, Henych J. Design Control of Copper-Doped Titania–Zirconia Catalysts for Methanol Decomposition and Total Oxidation of Ethyl Acetate. Symmetry. 2022; 14(4):751. https://doi.org/10.3390/sym14040751
Chicago/Turabian StyleTsoncheva, Tanya, Gloria Issa, Radostina Ivanova, Momtchil Dimitrov, Daniela Kovacheva, Genoveva Atanasova, and Jiří Henych. 2022. "Design Control of Copper-Doped Titania–Zirconia Catalysts for Methanol Decomposition and Total Oxidation of Ethyl Acetate" Symmetry 14, no. 4: 751. https://doi.org/10.3390/sym14040751
APA StyleTsoncheva, T., Issa, G., Ivanova, R., Dimitrov, M., Kovacheva, D., Atanasova, G., & Henych, J. (2022). Design Control of Copper-Doped Titania–Zirconia Catalysts for Methanol Decomposition and Total Oxidation of Ethyl Acetate. Symmetry, 14(4), 751. https://doi.org/10.3390/sym14040751







