Prediction of Large Second Harmonic Generation in the Metal-Oxide/Organic Hybrid Compound CuMoO3(p2c)
Abstract
:1. Introduction
2. Materials and Methods
Computational Details
3. Results and Discussion
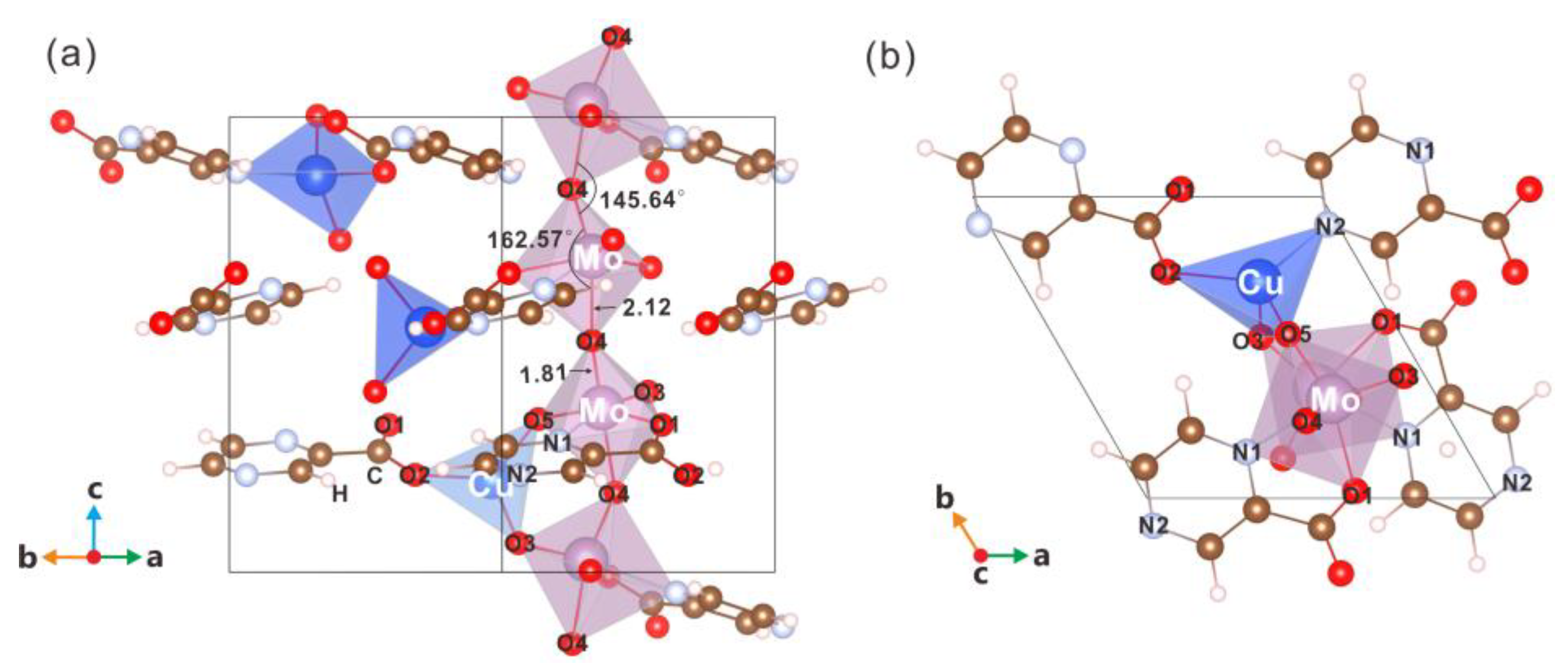
3.1. Structure
3.2. Electronic Structures
3.3. Optical Properties
3.4. Atom Response Theory Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.R. The Principles of Nonlinear Optics; Wiley-Interscience: New York, NY, USA, 1984. [Google Scholar]
- Chen, C.T.; Sasaki, T.; Li, R.; Wu, Y.; Lin, Z.; Mori, Y.; Kaneda, Y. Nonlinear Optical Borate Crystals; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Chung, I.; Kanatzidis, M.G. Metal Chalcogenides: A Rich Source of Nonlinear Optical Materials. Chem. Mater. 2014, 26, 849–869. [Google Scholar] [CrossRef]
- Hu, C.-L.; Mao, J.-G. Recent Advances on Second-Order NLO Materials Based on Metal Iodates. Coor. Chem. Rev. 2015, 288, 1–17. [Google Scholar] [CrossRef]
- Ok, K.M. Toward the Rational Design of Novel Noncentrosymmetric Materials: Factors Influencing the Framework Structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Evans, O.R.; Lin, W. Crystal Engineering of NLO Materials Based on Metal-Organic Coordination Networks. Acc. Chem. Res. 2002, 35, 511–522. [Google Scholar] [CrossRef]
- Maury, O.; Bozec, H.L. Molecular Engineering of Octupolar NLO Molecules and Materials Based on Bipyridyl Metal Complexes. Acc. Chem. Res. 2005, 38, 691–704. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Lin, W. Rational Synthesis of Noncentrosymmetric Metal-Organic Frameworks for Second-Order Nonlinear Optics. Chem. Rev. 2012, 112, 1084–1104. [Google Scholar] [CrossRef]
- Mingabudinova, L.R.; Vinogradov, V.V.; Milichko, V.A.; Hey-Hawkins, E.; Vinogradov, A.V. Metal-Organic Frameworks as Competitive Materials for Non-Linear Optics. Chem. Soc. Rev. 2016, 45, 5408–5431. [Google Scholar] [CrossRef] [Green Version]
- Medishetty, R.; Zareba, J.K.; Mayer, D.; Samoc, M.; Fischer, R.A. Nonlinear Optical Properties, Upconversion and Lasing in Metal-Organic Frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.C.; Kitagawa, S. Metal-Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Evans, O.R.; Lin, W. Rational Design of Nonlinear Optical Materials Based on 2D Coordination Networks. Chem. Mater. 2001, 13, 3009–3017. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Z.; Ma, L. A Novel Octupolar Metalorganic NLO Material Based on a Chiral 2D Coordination Network. J. Am. Chem. Soc. 1999, 121, 11249–11250. [Google Scholar] [CrossRef]
- Ye, Q.; Li, Y.H.; Song, Y.M.; Huang, X.F.; Xiong, R.G.; Xue, Z. A Second-Order Nonlinear Optical Material Prepared Through in situ Hydrothermal Ligand Synthesis. Inorg. Chem. 2005, 44, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cui, Y.; Wu, C.; Yang, Y.; Wang, Z.; O’Keeffe, M.; Chen, B.; Qian, G. Second-Order Nonlinear Optical Activity Induced by Ordered Dipolar Chromophores Confined in the Pores of an Anionic Metal-Organic Framework. Angew. Chem. Int. Ed. 2012, 51, 10542–10545. [Google Scholar] [CrossRef]
- Maggard, P.A.; Stern, C.L.; Poeppelmeier, K.R. Understanding the Role of Helical Chains in the Formation of Noncentrosymmetric Solids. J. Am. Chem. Soc. 2001, 123, 7742–7743. [Google Scholar] [CrossRef]
- Maggard, P.A.; Kopf, A.L.; Stern, C.L.; Poeppelmeier, K.R. From Linear Inorganic Chains to Helices: Chirality in the M(pyz)(H2O)2MoO2F4 (M)=Zn, Cd) Compounds. Inorg. Chem. 2002, 41, 4852–4858. [Google Scholar] [CrossRef]
- Yan, B.; Capracotta, M.D.; Maggard, P.A. Structural Origin of Chirality and Properties of a Remarkable Helically Pillared Solid. Inorg. Chem. 2005, 44, 6509–6511. [Google Scholar] [CrossRef]
- Ahmed, B.; Jo, H.; Oh, S.J.; Ok, K.M. Variable Asymmetric Chains in Transition Metal Oxyfluorides: Structure-Second-Harmonic-Generation Property Relationships. Inorg. Chem. 2018, 57, 6702–6709. [Google Scholar] [CrossRef]
- Muller, E.A.; Cannon, R.J.; Sarjeant, A.N.; Ok, K.M.; Halasyamani, P.S.; Norquist, A.J. Directed Synthesis of Noncentrosymmetric Molybdates. Cryst. Growth Des. 2005, 5, 1913–1917. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Y.; Maggard, P.A. Rare Example of Chiral and Achiral Polymorphs of a Metal-Oxide/Organic Hybrid Compound. J. Solid State Chem. 2020, 287, 121358. [Google Scholar] [CrossRef]
- Cheng, X.; Whangbo, M.-H.; Guo, G.-C.; Hong, M.; Deng, S. Large Second Harmonic Generation of LiCs2PO4 Caused by the Metal-Cation-Centered Groups. Angew. Chem. Int. Ed. 2018, 57, 3933–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mat. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for ab initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Aversa, C.; Sipe, J.E. Nonlinear Optical Susceptibilities of Semiconductors: Results with a Length-Gauge Analysis. Phys. Rev. B 1995, 52, 14636–14645. [Google Scholar] [CrossRef] [PubMed]
- Rashkeev, S.N.; Lambrecht, W.R.; Segall, B. Efficient ab initio Method for the Calculation of Frequency-Dependent Second-Order Optical Response in Semiconductors. Phys. Rev. B 1998, 57, 3905–3919. [Google Scholar] [CrossRef]
- Sharma, S.; Ambrosch-Draxl, C. Second-Harmonic Optical Response from First Principles. Phys. Scripta 2004, T109, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Rashkeev, S.N.; Lambrecht, W.R.L. Second-Harmonic Generation of I-III-VI2 Chalcopyrite Semiconductors: Effects of Chemical Substitutions. Phys. Rev. B 2001, 63, 165212. [Google Scholar] [CrossRef]
- Sharma, S.; Dewhurst, J.K.; Ambrosch-Draxl, C. Linear and Second-Order Optical Response of III-V Monolayer Superlattices. Phys. Rev. B 2003, 67, 165332. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Whangbo, M.-H.; Hong, M.; Deng, S. Atom Response Theory of Nonlinear Optical Responses and Its Applications. Chinese J. Struct. Chem. 2020, 39, 2172–2181. [Google Scholar] [CrossRef]
- Sioncke, S.; Verbiest, T.; Persoons, A. Second-Order Nonlinear Optical Properties of Chiral Materials. Mater. Sci. Eng. R 2003, 42, 115–155. [Google Scholar] [CrossRef]
- Gonze, X.; Lee, C. Dynamical Matrices, Born Effective Charges, Dielectric Permittivity Tensors, and Interatomic Force Constants from Density-Functional Perturbation Theory. Phys. Rev. B 1997, 55, 10355–10368. [Google Scholar] [CrossRef]
- Veithen, M.; Gonze, X.; Ghosez, P. Nonlinear Optical Susceptibilities, Raman Efficiencies, and Electro-Optic Tensors from First-Principles Density Functional Perturbation Theory. Phys. Rev. B 2005, 71, 125107. [Google Scholar] [CrossRef] [Green Version]
- Djani, H.; Hermet, P.; Ghosez, P. First-Principles Characterization of the P21ab Ferroelectric Phase of Aurivillius Bi2WO6. J. Phys. Chem. C 2014, 118, 13514–13524. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis as Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.K.; Perry, T.T. A Powder Technique for the Evaluation of Nonlinear Optical Materials. J. Appl. Phys. 1968, 39, 3798–3813. [Google Scholar] [CrossRef]
- Cyvin, S.J.; Rauch, J.E.; Decius, J.C. Theory of Hyper-Raman Effects (Nonlinear Inelastic Light Scattering): Selection Rules and Depolarization Ratios for the Second-Order Polarizability. J. Chem. Phys. 1965, 43, 4083–4095. [Google Scholar] [CrossRef]
- Midwinter, J.E.; Warner, J. The Effects of Phase Matching Method and of Uniaxial Crystal Symmetry on the Polar Distribution of Second-Order Non-Linear Optical Polarization. Br. J. Appl. Phys. 1965, 16, 1135–1142. [Google Scholar] [CrossRef]
- Astill, A.G. Material Figures of Merit for Non-Linear Optics. Thin Solid Films 1991, 204, 1–17. [Google Scholar] [CrossRef]
- Ye, Q.; Tang, Y.Z.; Wang, X.S.; Xiong, R.G. Strong Enhancement of Second-Harmonic Generation (SHG) Response Through Multi-Chiral Centers and Metal-Coordination. Dalton Trans. 2005, 9, 1570–1573. [Google Scholar] [CrossRef]
- Lin, W.; Evans, O.R.; Xiong, R.G.; Wang, Z. Supramolecular Engineering of Chiral and Acentric 2d Networks. Synthesis, Structures, and Second-Order Nonlinear Optical Properties of Bis(nicotinato)zinc and Bis{3-[2-(4-pyridyl)ethenyl]benzoatocadmium. J. Am. Chem. Soc. 1998, 120, 13272–13273. [Google Scholar] [CrossRef]
- Liu, B.W.; Jiang, X.M.; Li, B.X.; Zeng, H.Y.; Guo, G.C. Li[LiCs2Cl][Ga3S6]: A Nanoporous Framework of GaS4 Tetrahedra with Excellent Nonlinear Optical Performance. Angew. Chem. Int. Ed. 2020, 59, 4856–4859. [Google Scholar] [CrossRef]
- Li, R.A.; Zhou, Z.; Lian, Y.K.; Jia, F.; Jiang, X.; Tang, M.C.; Wu, L.M.; Sun, J.; Chen, L. A2SnS5: A Structural Incommensurate Modulation Exhibiting Strong Second-Harmonic Generation and a High Laser-Induced Damage Threshold (A = Ba, Sr). Angew. Chem. Int. Ed. 2020, 59, 11861–11865. [Google Scholar] [CrossRef]
- Ye, R.; Cheng, X.; Liu, B.-W.; Jiang, X.-M.; Yang, L.-Q.; Deng, S.; Guo, G.-C. Strong Nonlinear Optical Effect Attained by Atom-Response-Theory Aided Design in the Na2MIIMIV2Q6 (MII = Zn, Cd; MIV = Ge, Sn; Q = S, Se) Chalcogenide System. J. Mater. Chem. C 2020, 8, 1244–1247. [Google Scholar] [CrossRef]
- Guo, S.-P.; Cheng, X.Y.; Sun, Z.D.; Chi, Y.; Liu, B.W.; Jiang, X.M.; Li, S.F.; Xue, H.G.; Deng, S.; Duppel, V.; et al. Large SHG Effect and High LIDT Observed Coexisting in Gallium Selenide: A Simple but Perfect Case. Angew. Chem. Int. Ed. 2019, 131, 8171–8175. [Google Scholar] [CrossRef]
- Cai, Z.; Cheng, X.; Whangbo, M.-H.; Hong, M.; Deng, S. The Partition Principles for Atom Scale Structure and its Physical Property: Application to the Nonlinear Optical Crystal Material KBe2BO3F2. Phys. Chem. Chem. Phys. 2020, 22, 19299–19306. [Google Scholar] [CrossRef]
- Lin, H.; Maggard, P.A. Copper(i)-rhenate hybrids: Syntheses, structures, and optical properties. Inorg. Chem. 2007, 46, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Gautier, R.; Poeppelmeier, K. Packing of Helices: Is Chirality the Highest Crystallographic Symmetry? Crystals 2016, 6, 106. [Google Scholar] [CrossRef] [Green Version]



| Atom | NA | |||||
|---|---|---|---|---|---|---|
| Cu | 3 | 11.72 | 10.69 | 1.03 | 35.2 | 3.99 |
| Mo | 3 | 6.97 | 2.85 | 4.12 | 20.9 | 2.38 |
| O | 15 | 1.6 | 1.14 | 0.46 | 24.1 | 0.55 |
| N | 6 | 1.23 | 0.51 | 0.72 | 7.4 | 0.42 |
| C | 15 | 0.74 | 0.27 | 0.47 | 11.1 | 0.25 |
| H | 9 | 0.15 | 0.05 | 0.1 | 1.4 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Huang, X.; Cheng, X.; Maggard, P.A.; Whangbo, M.-H.; Luan, C.; Deng, S. Prediction of Large Second Harmonic Generation in the Metal-Oxide/Organic Hybrid Compound CuMoO3(p2c). Symmetry 2022, 14, 824. https://doi.org/10.3390/sym14040824
Yang T, Huang X, Cheng X, Maggard PA, Whangbo M-H, Luan C, Deng S. Prediction of Large Second Harmonic Generation in the Metal-Oxide/Organic Hybrid Compound CuMoO3(p2c). Symmetry. 2022; 14(4):824. https://doi.org/10.3390/sym14040824
Chicago/Turabian StyleYang, Tingting, Xueli Huang, Xiyue Cheng, Paul A. Maggard, Myung-Hwan Whangbo, Chengkai Luan, and Shuiquan Deng. 2022. "Prediction of Large Second Harmonic Generation in the Metal-Oxide/Organic Hybrid Compound CuMoO3(p2c)" Symmetry 14, no. 4: 824. https://doi.org/10.3390/sym14040824







