Optimization of SOX2 Expression for Enhanced Glioblastoma Stem Cell Virotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model
- is the population of GSCs;
- is the population of GSCs infected by ZIKV and the subpopulation of
- is the free Zika viruses;
- The term describes the logistic growth rate of GSCs;
- is the normalized rate of SOX2 expression level in a GSC, which ranges between 0 and 1;
- The constant value represents the strength of infectivity of the Zika virus in the GSCs;
- The term describes the rate of infected cells by free virus
- b is the bursting size of free virus particles;
- represents the death rate of infected GSCs after the cell oncolysis;
- is the clearance rate of the virus.
2.2. Analysis and Stability of Equilibrium
- If , then and from the second and the third equations in Equation (5). Therefore, we have an equilibrium point .
- If and , we get from the second equation, which results in from the first equation in Equation (5). Then, . Thus, we have an equilibrium point .
- If , then, from the second and third equations in Equation (5), . Since , we have . From the first and second equation in Equation (5), . Then, . From the third equation in Equation (5), . Then, Thus, we have an equilibrium point , where , and .
2.3. Basic Reproduction Number
2.4. Stability of Equilibrium Points
2.5. Estimation of Parameters
2.6. Sensitivity Analysis
3. Results
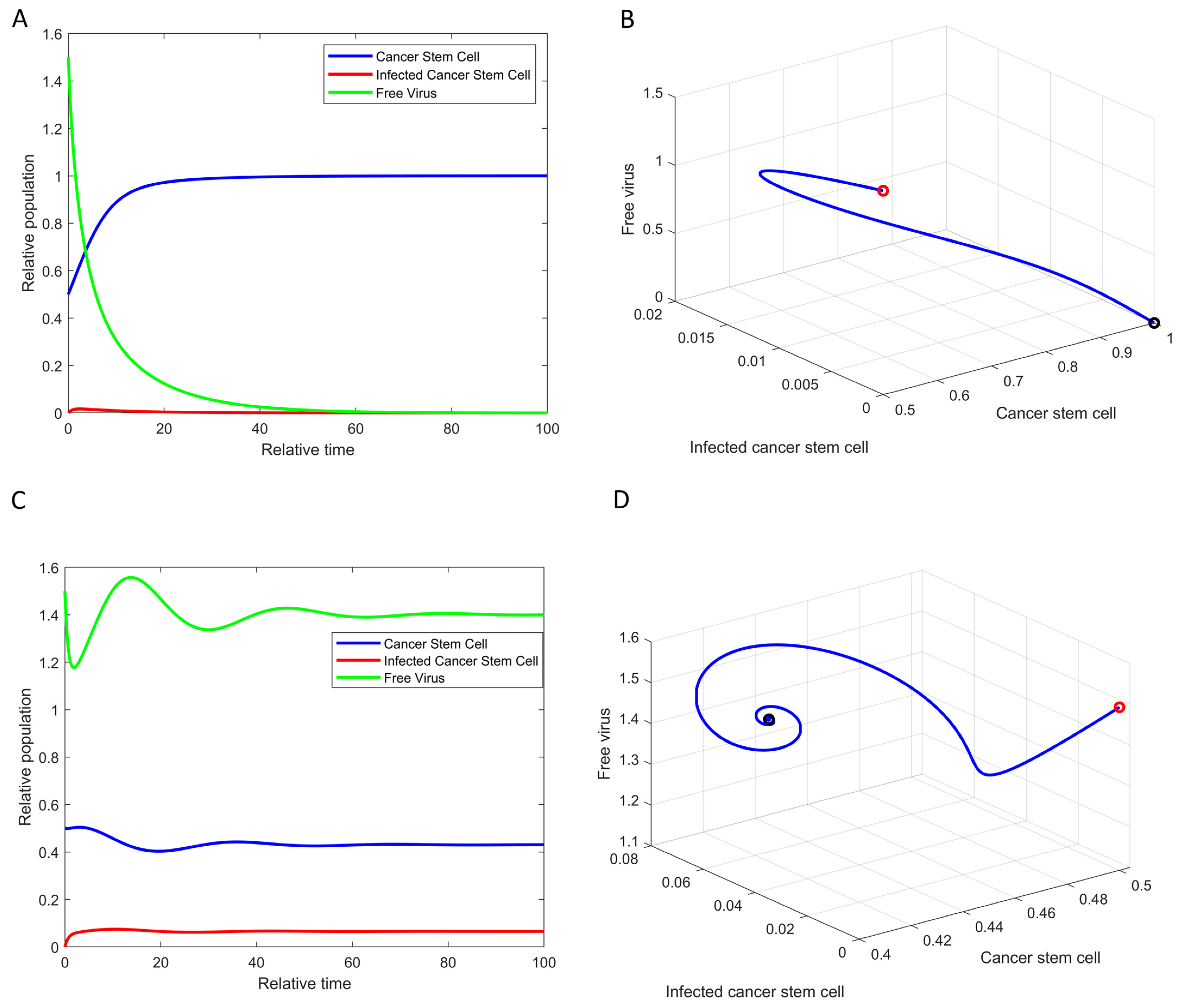
3.1. Existence and Stability of the Equilibrium Points
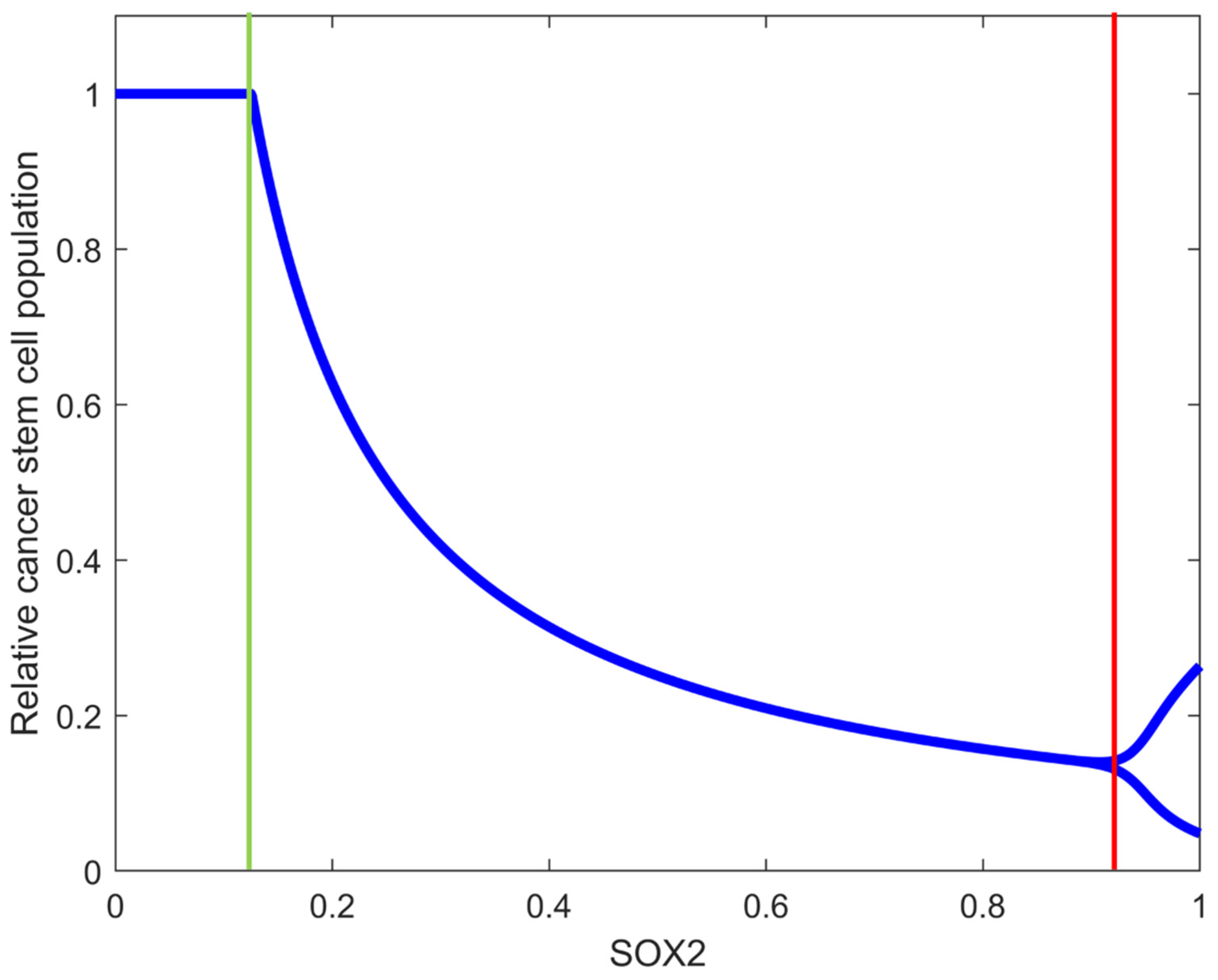
3.2. SOX2 Expression Level Changes the Structure of GSC Dynamics
3.3. Interplay between SOX2 Expression Level and Bursting Size Affects the Dynamic of OVT
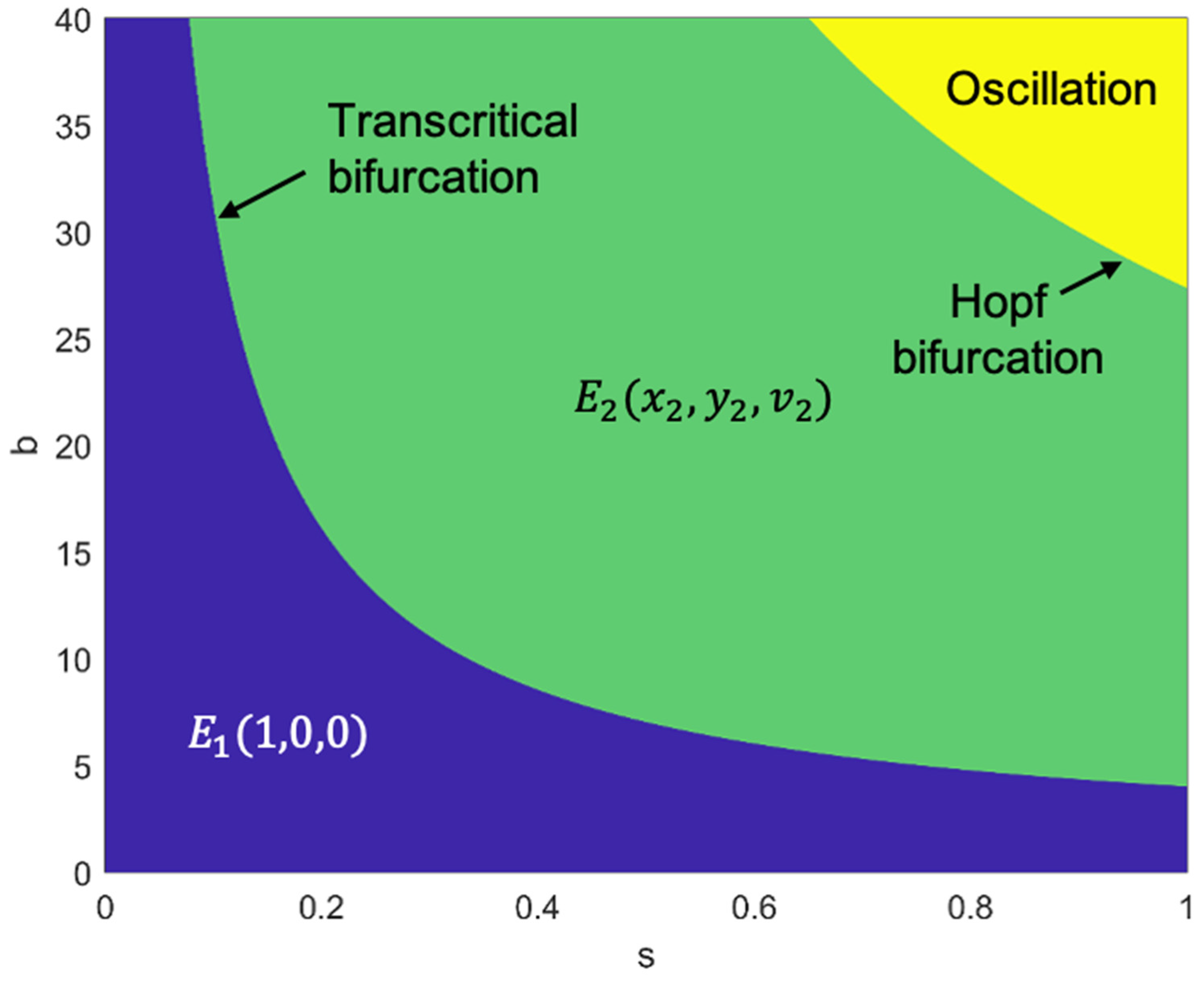
3.4. Stability Regions for Equilibrium Points in Two-Dimensional Parameters: SOX2 Expression Level and Bursting Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Koscinski, J.; Graczyk, P.; Halas, T.; Lewandowska, A.M.; et al. Glioblastoma Multiforme: The Latest Diagnostics and Treatment Techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee Sh, U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, M.; Boselli, C.; Schinelli, S. Glioblastoma under Siege: An Overview of Current Therapeutic Strategies. Brain Sci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Shagufta, B.I.; Abbas, S.Q.; Patel, S.; Khan, I.; Shah, S.A.A.; Akhter, N.; Hassan, S.S.U. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed. Pharmacother. 2017, 92, 681–689. [Google Scholar] [CrossRef]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [CrossRef]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 206. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes. Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Henriksson, R.; Asklund, T.; Poulsen, H.S. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: A review. J. Neuro-Oncol. 2011, 104, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.J.; Hsieh, J.K.; Manjila, S.; Lathia, J.D.; Sloan, A. The role of cancer stem cells in glioblastoma. Neurosurg. Focus 2014, 37, E6. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, M.; Huang, Y. Oncolytic therapy and gene therapy for cancer: Recent advances in antitumor effects of Newcastle disease virus. Discov. Med. 2020, 30, 39–48. [Google Scholar] [PubMed]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Lam, S.S.; Peng, W.; Aghbashlo, M.; Tabatabaei, M.; Guillemin, G.J. Oncolytic viruses as a promising therapeutic strategy against the detrimental health impacts of air pollution: The case of glioblastoma multiforme. Semin. Cancer Biol. 2022, 86, 1122–1142. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.H.; Hwang, T.H.; O’Gorman, W.E.; Bartlett, D.L.; Sei, S.; Kanji, F.; Brown, C.; Werier, J.; Cho, J.H.; Lee, D.E.; et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Investig. 2007, 117, 3350–3358. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- de Noronha, L.; Zanluca, C.; Burger, M.; Suzukawa, A.A.; Azevedo, M.; Rebutini, P.Z.; Novadzki, I.M.; Tanabe, L.S.; Presibella, M.M.; Duarte Dos Santos, C.N. Zika Virus Infection at Different Pregnancy Stages: Anatomopathological Findings, Target Cells and Viral Persistence in Placental Tissues. Front. Microbiol. 2018, 9, 2266. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Barros-Aragao, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; de Sousa, V.L.; Souza, A.S.; et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef]
- Halani, S.; Tombindo, P.E.; O’Reilly, R.; Miranda, R.N.; Erdman, L.K.; Whitehead, C.; Bielecki, J.M.; Ramsay, L.; Ximenes, R.; Boyle, J.; et al. Clinical manifestations and health outcomes associated with Zika virus infections in adults: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009516. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Y.; Huang, J.; Feng, Y.; Zhang, Z.; Zhong, K.; Chen, Y.; Wang, Z.; Huang, C.; Yang, H.; et al. Zika virus NS5 protein inhibits cell growth and invasion of glioma. Biochem. Biophys. Res. Commun. 2019, 516, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Q.; Zhou, X.; Zhao, H.; Wang, K.; Niu, H.; Wang, Y. Gospel of malignant Glioma: Oncolytic virus therapy. Gene 2022, 818, 146217. [Google Scholar] [CrossRef] [PubMed]
- Mazar, J.; Brooks, J.K.; Peloquin, M.; Rosario, R.; Sutton, E.; Longo, M.; Drehner, D.; Westmoreland, T.J. The Oncolytic Activity of Zika Viral Therapy in Human Neuroblastoma In Vivo Models Confers a Major Survival Advantage in a CD24-dependent Manner. Cancer Res. Commun. 2024, 4, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Mazar, J.; Li, Y.; Rosado, A.; Phelan, P.; Kedarinath, K.; Parks, G.D.; Alexander, K.A.; Westmoreland, T.J. Zika virus as an oncolytic treatment of human neuroblastoma cells requires CD24. PLoS ONE 2018, 13, e0200358. [Google Scholar] [CrossRef]
- Francipane, M.G.; Douradinha, B.; Chinnici, C.M.; Russelli, G.; Conaldi, P.G.; Iannolo, G. Zika Virus: A New Therapeutic Candidate for Glioblastoma Treatment. Int. J. Mol. Sci. 2021, 22, 10996. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Tiwari, S.K.; Lichinchi, G.; Yau, E.H.; Hui, H.; Li, W.; Furnari, F.; Rana, T.M. Integrin αvβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy. Cell Rep. 2020, 30, 969–983.e4. [Google Scholar] [CrossRef]
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017, 214, 2843–2857. [Google Scholar] [CrossRef]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Wettersten, H.I.; Beck, S.; Clark, A.E.; Wu, Q.; et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin α(v)β(5) Axis. Cell Stem Cell 2020, 26, 187–204.e10. [Google Scholar] [CrossRef]
- Al-Tuwairqi, S.M.; Al-Johani, N.O.; Simbawa, E.A. Modeling dynamics of cancer virotherapy with immune response. Adv. Differ. Equ. 2020, 2020, 438. [Google Scholar] [CrossRef]
- Kim, P.S.; Crivelli, J.J.; Choi, I.K.; Yun, C.O.; Wares, J.R. Quantitative impact of immunomodulation versus oncolysis with cytokine-expressing virus therapeutics. Math. Biosci. Eng. 2015, 12, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.P. The replicability of oncolytic virus: Defining conditions in tumor virotherapy. Math. Biosci. Eng. 2011, 8, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, D. Computational modeling approaches to the dynamics of oncolytic viruses. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M.; Al Agha, A.D. Analysis of a delayed and diffusive oncolytic M1 virotherapy model with immune response. Nonlinear Anal. Real. World Appl. 2020, 55, 103116. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, J.P. Spatial Model for Oncolytic Virotherapy with Lytic Cycle Delay. Bull. Math. Biol. 2019, 81, 2396–2427. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.P.; Wei, J. Lytic cycle: A defining process in oncolytic virotherapy. Appl. Math. Model. 2013, 37, 5962–5978. [Google Scholar] [CrossRef]
- Kim, D.; Shin, D.-H.; Sung, C.K. The Optimal Balance between Oncolytic Viruses and Natural Killer Cells: A Mathematical Approach. Mathematics 2022, 10, 3370. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, J.Y.; Lee, T.J.; Liu, J.; Yu, J.; Caligiuri, M.A.; Kaur, B.; Friedman, A. Complex role of NK cells in regulation of oncolytic virus-bortezomib therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 4927–4932. [Google Scholar] [CrossRef]
- Phan, T.A.; Tian, J.P. The Role of the Innate Immune System in Oncolytic Virotherapy. Comput. Math. Methods Med. 2017, 2017, 6587258. [Google Scholar] [CrossRef]
- Senekal, N.S.; Mahasa, K.J.; Eladdadi, A.; de Pillis, L.; Ouifki, R. Natural Killer Cells Recruitment in Oncolytic Virotherapy: A Mathematical Model. Bull. Math. Biol. 2021, 83, 75. [Google Scholar] [CrossRef]
- Alvarado, A.G.; Thiagarajan, P.S.; Mulkearns-Hubert, E.E.; Silver, D.J.; Hale, J.S.; Alban, T.J.; Turaga, S.M.; Jarrar, A.; Reizes, O.; Longworth, M.S.; et al. Glioblastoma Cancer Stem Cells Evade Innate Immune Suppression of Self-Renewal through Reduced TLR4 Expression. Cell Stem Cell 2017, 20, 450–461.E4. [Google Scholar] [CrossRef] [PubMed]
- Beards, C.F. 5—Automatic control systems. In Engineering Vibration Analysis with Application to Control Systems; Beards, C.F., Ed.; Butterworth-Heinemann: London, UK, 1995; pp. 171–279. [Google Scholar]
- Harpold, H.L.; Alvord, E.C., Jr.; Swanson, K.R. The evolution of mathematical modeling of glioma proliferation and invasion. J. Neuropathol. Exp. Neurol. 2007, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jordao, G.; Tavares, J.N. Mathematical models in cancer therapy. Biosystems 2017, 162, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, V.; Weaver, A.M.; Cummings, P.T.; Anderson, A.R. Mathematical modeling of cancer: The future of prognosis and treatment. Clin. Chim. Acta 2005, 357, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, C.; Chen, Q.; Shang, J.; Liu, Z.; Guo, Y.; Li, C.; Wang, H.; Ye, Q.; Li, X.; et al. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol. Ther. Oncolytics 2022, 24, 522–534. [Google Scholar] [CrossRef]
- Garcia, G., Jr.; Chakravarty, N.; Paiola, S.; Urena, E.; Gyani, P.; Tse, C.; French, S.W.; Danielpour, M.; Breunig, J.J.; Nathanson, D.A.; et al. Differential Susceptibility of Ex Vivo Primary Glioblastoma Tumors to Oncolytic Effect of Modified Zika Virus. Cells 2023, 12, 2384. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Puig, A.; Rabiei, F.; Hawkins, E.J.; Hernandez, T.F.; Sung, C.K. Optimization of SOX2 Expression for Enhanced Glioblastoma Stem Cell Virotherapy. Symmetry 2024, 16, 1186. https://doi.org/10.3390/sym16091186
Kim D, Puig A, Rabiei F, Hawkins EJ, Hernandez TF, Sung CK. Optimization of SOX2 Expression for Enhanced Glioblastoma Stem Cell Virotherapy. Symmetry. 2024; 16(9):1186. https://doi.org/10.3390/sym16091186
Chicago/Turabian StyleKim, Dongwook, Abraham Puig, Faranak Rabiei, Erial J. Hawkins, Talia F. Hernandez, and Chang K. Sung. 2024. "Optimization of SOX2 Expression for Enhanced Glioblastoma Stem Cell Virotherapy" Symmetry 16, no. 9: 1186. https://doi.org/10.3390/sym16091186







